Metronomic Chemotherapy for Advanced Prostate Cancer: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Statistical Analysis
3. Results
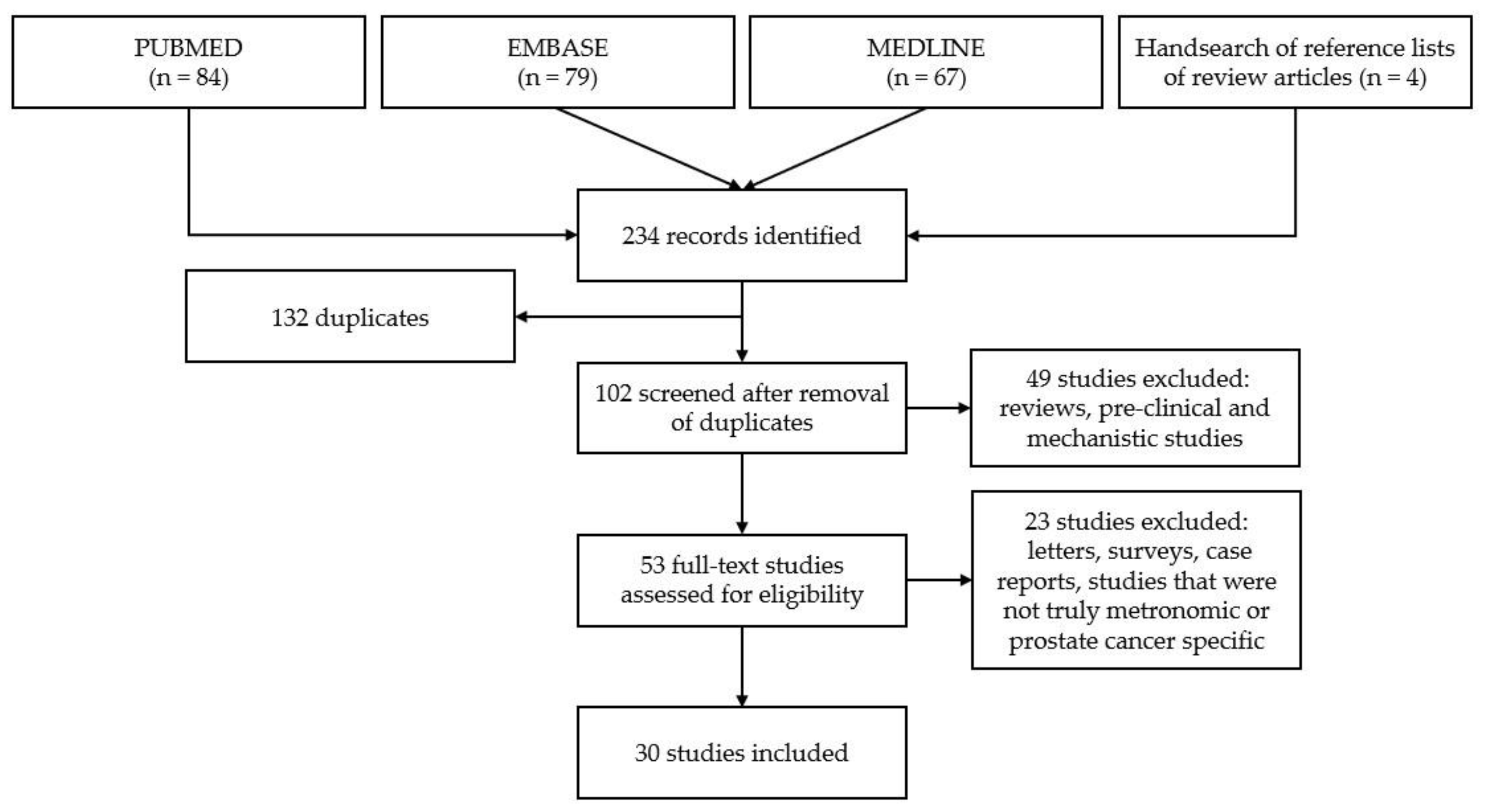
3.1. Study Selection
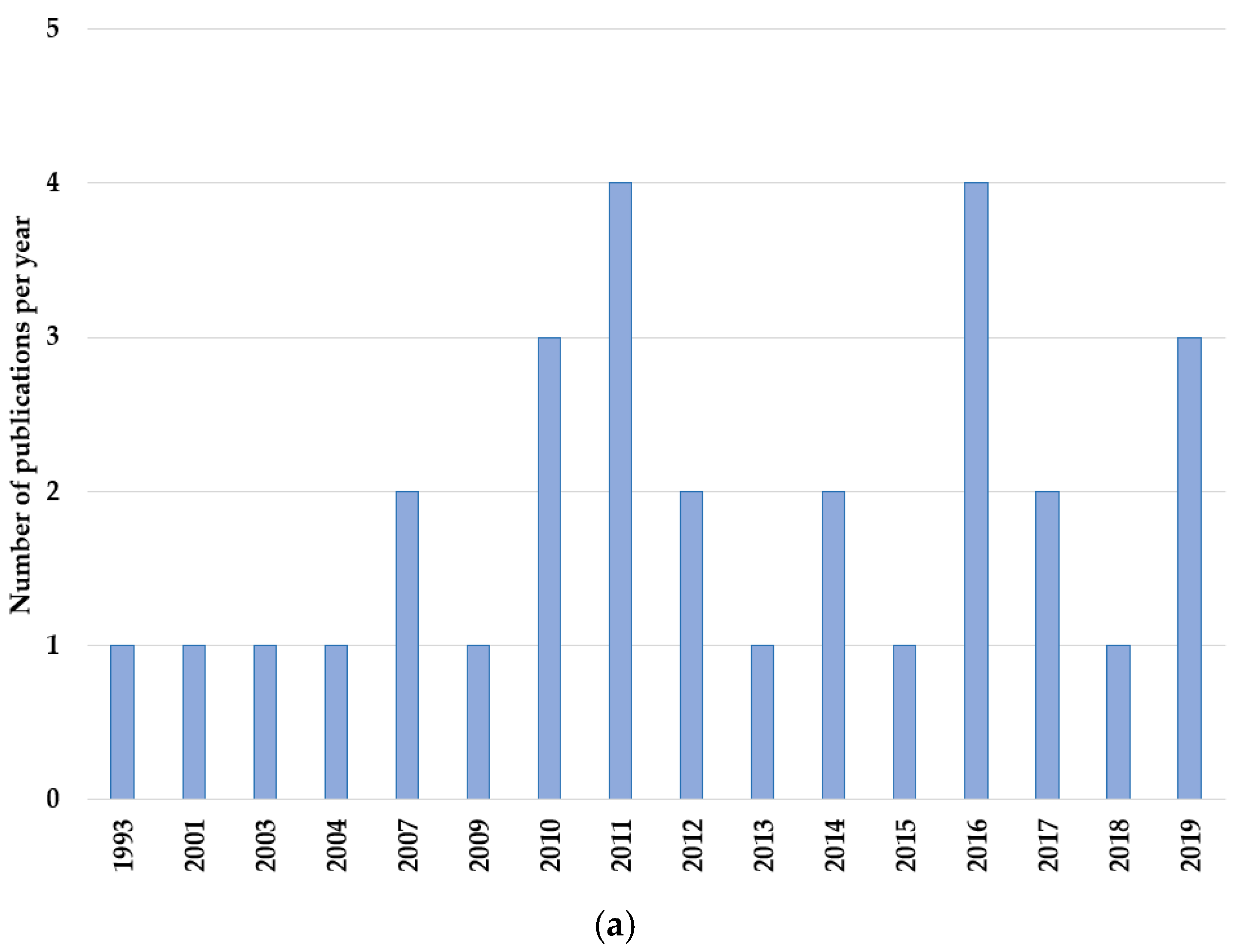
3.2. Study and Patient Characteristics
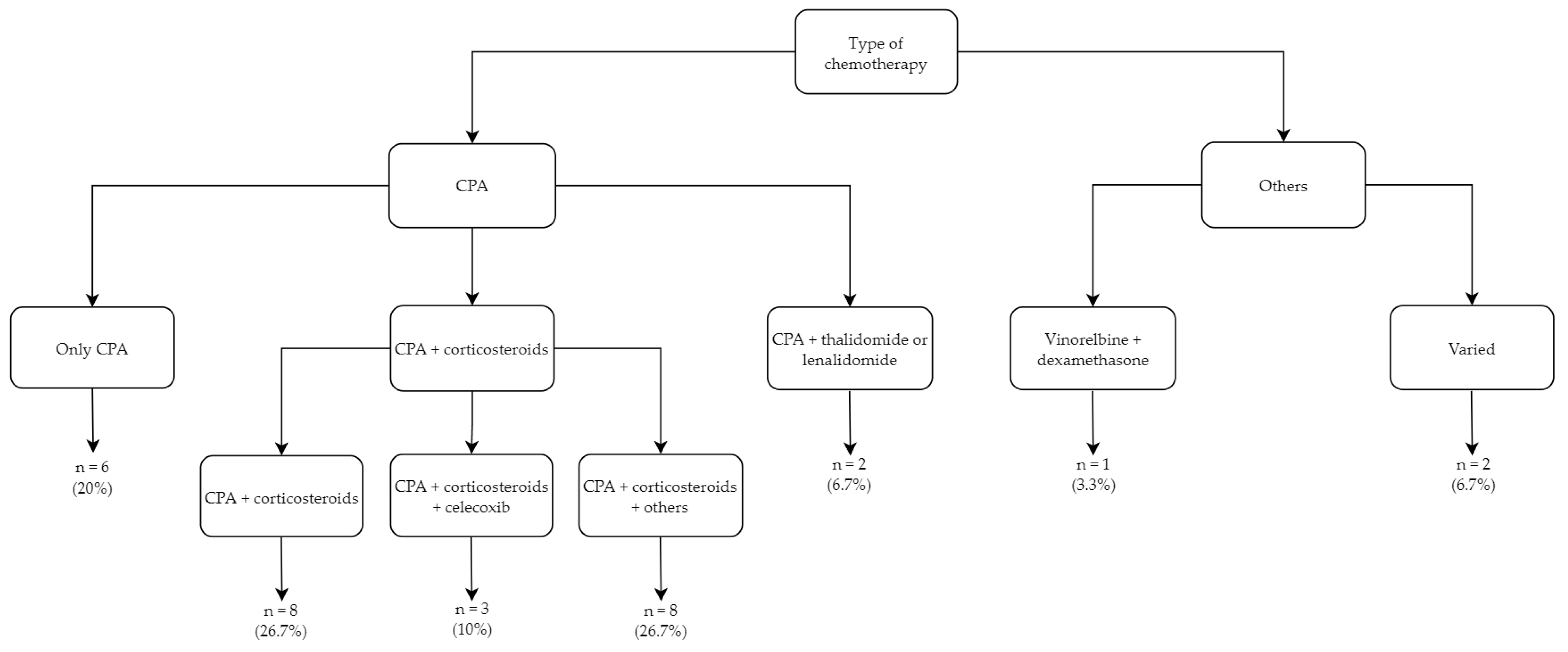
3.3. Metronomic Treatment Regimens
3.4. Outcomes
3.5. Toxicities and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prager, M.G.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E.; Sonke, G.; Martínez, G.A.; Frenel, J.-S.; Karamouzis, M.; et al. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; De Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 378, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Boyle, H.; Alibhai, S.; Decoster, L.; Efstathiou, E.; Fizazi, K.; Mottet, N.; Oudard, S.; Payne, H.; Prentice, M.; Puts, M.; et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur. J. Cancer 2019, 116, 116–136. [Google Scholar] [CrossRef]
- Schulte, B.; Morgans, A.K.; Shore, N.D.; Pezaro, C. Sorting Through the Maze of Treatment Options for Metastatic Castration-Sensitive Prostate Cancer. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 198–207. [Google Scholar] [CrossRef]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Lissbrant, I.F.; Garmo, H.; Widmark, A.; Stattin, P. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013, 52, 1593–1601. [Google Scholar] [CrossRef]
- Leibowitz-Amit, R.; Templeton, A.J.; Alibhai, S.M.; Knox, J.J.; Sridhar, S.S.; Tannock, I.F.; Joshua, A.M. Efficacy and toxicity of abiraterone and docetaxel in octogenarians with metastatic castration-resistant prostate cancer. J. Geriatr. Oncol. 2015, 6, 23–28. [Google Scholar] [CrossRef]
- Horgan, A.M.; Seruga, B.; Pond, G.R.; Alibhai, S.M.; Amir, E.; De Wit, R.; Eisenberger, M.A.; Tannock, I.F. Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J. Geriatr. Oncol. 2014, 5, 119–126. [Google Scholar] [CrossRef]
- Khalaf, D.J.; Sunderland, K.; Eigl, B.; Kollmannsberger, C.K.; Ivanov, N.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Gleave, M.; et al. Health-related Quality of Life for Abiraterone Plus Prednisone Versus Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer: Results from a Phase II Randomized Trial. Eur. Urol. 2019, 75, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, S.M.H.; Breunis, H.; Hansen, A.R.; Gregg, R.; Warde, P.; Timilshina, N.; Tomlinson, G.; Joshua, A.M.; Hotte, S.; Fleshner, N.; et al. Examining the ability of the Cancer and Aging Research Group tool to predict toxicity in older men receiving chemotherapy or androgen-receptor–targeted therapy for metastatic castration-resistant prostate cancer. Cancer 2021, 127, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, S.M.H.; Breunis, H.; Feng, G.; Timilshina, N.; Hansen, A.; Warde, P.; Gregg, R.; Joshua, A.; Fleshner, N.; Tomlinson, G.; et al. Association of Chemotherapy, Enzalutamide, Abiraterone, and Radium 223 With Cognitive Function in Older Men with Metastatic Castration-Resistant Prostate Cancer. JAMA Netw. Open 2021, 4, e2114694. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Oudard, S.; Özgüroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Tannock, I.F. Improving Treatment for Advanced Prostate Cancer. N. Engl. J. Med. 2019, 381, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Tran, B. Global Disparity in Access to Novel Therapeutics for Metastatic Prostate Cancer. JCO Oncol. Pract. 2022, 18, 57–59. [Google Scholar] [CrossRef]
- Bocci, G.; Kerbel, R.S. Pharmacokinetics of metronomic chemotherapy: A neglected but crucial aspect. Nat. Rev. Clin. Oncol. 2016, 13, 659–673. [Google Scholar] [CrossRef]
- Fontana, A.; Bocci, G.; Galli, L.; D’Arcangelo, M.; DeRosa, L.; Fioravanti, A.; Orlandi, P.; Barletta, M.T.; Landi, L.; Bursi, S.; et al. Metronomic cyclophosphamide in elderly patients with advanced, castration-resistant prostate cancer. J. Am. Geriatr. Soc. 2010, 58, 986–988. [Google Scholar] [CrossRef]
- Delos Santos, K.; Sivanathan, L.; Lien, K.; Emmenegger, U. Clinical Trials of Low-Dose Metronomic Chemotherapy in Castration-Resistant Prostate Cancer. In Metronomic Chemotherapy: Pharmacology and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 119–134. [Google Scholar] [CrossRef]
- Caffo, O.; Facchini, G.; Biasco, E.; Ferraù, F.; Morelli, F.; Donini, M.; Buttigliero, C.; Calvani, N.; Guida, A.; Chiuri, V.E.; et al. Activity and safety of metronomic cyclophosphamide in the modern era of metastatic castration-resistant prostate cancer. Futur. Oncol. 2019, 15, 1115–1123. [Google Scholar] [CrossRef]
- Calcagno, F.; Mouillet, G.; Adotevi, O.; Maurina, T.; Nguyen, T.; Montcuquet, P.; Curtit, E.; Kleinclauss, F.; Pivot, X.; Borg, C.; et al. Metronomic cyclophosphamide therapy in hormone-naive patients with non-metastatic biochemical recurrent prostate cancer: A phase II trial. Med. Oncol. 2016, 33, 89. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.; Morelli, F.; Naglieri, E.; Gnoni, A.; Chiuri, V.E.; Orlando, L.; Fedele, P.; Cinieri, S. Metronomic chemotherapy with cyclophosphamide plus low dose of corticosteroids in advanced castration-resistant prostate cancer across the era of taxanes and new hormonal drugs. Med. Oncol. 2019, 36, 80. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Dabkara, D.; Biswas, B.; Ghosh, J. Metronomic therapy in metastatic castrate-resistant prostate cancer: Experience from a tertiary cancer care center. Indian J. Cancer 2018, 55, 94. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, L.; Galli, L.; Orlandi, P.; Fioravanti, A.; Di Desidero, T.; Fontana, A.; Antonuzzo, A.; Biasco, E.; Farnesi, A.; Marconcini, R.; et al. Docetaxel plus oral metronomic cyclophosphamide: A phase II study with pharmacodynamic and pharmacogenetic analyses in castration-resistant prostate cancer patients. Cancer 2014, 120, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Di Desidero, T.; DeRosa, L.; Galli, L.; Orlandi, P.; Fontana, A.; Fioravanti, A.; Marconcini, R.; Giorgi, M.; Campi, B.; Saba, A.; et al. Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients. Investig. New Drugs 2016, 34, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Autorino, R.; De Laurentiis, M.; Forestieri, V.; Romano, C.; Prudente, A.; Giugliano, F.; Imbimbo, C.; Mirone, V.; De Placido, S. Thalidomide in combination with oral daily cyclophosphamide in patients with pretreated hormone refractory prostate cancer: A phase I clinical trial. Cancer Biol. Ther. 2007, 6, 313–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dickinson, P.D.; Peel, D.N.Y.; Sundar, S. Metronomic chemotherapy with cyclophosphamide and dexamethasone in patients with metastatic carcinoma of the prostate. Br. J. Cancer 2012, 106, 1464–1465. [Google Scholar] [CrossRef][Green Version]
- Fontana, A.; Galli, L.; Fioravanti, A.; Orlandi, P.; Galli, C.; Landi, L.; Bursi, S.; Allegrini, G.; Fontana, E.; Di Marsico, R.; et al. Clinical and Pharmacodynamic Evaluation of Metronomic Cyclophosphamide, Celecoxib, and Dexamethasone in Advanced Hormone-refractory Prostate Cancer. Clin. Cancer Res. 2009, 15, 4954–4962. [Google Scholar] [CrossRef]
- Gebbia, V.; Serretta, V.; Borsellino, N.; Valerio, M.R. Salvage Therapy with Oral Metronomic Cyclophosphamide and Methotrexate for Castration-refractory Metastatic Adenocarcinoma of the Prostate Resistant to Docetaxel. Urology 2011, 78, 1125–1130. [Google Scholar] [CrossRef]
- Glode, L.M.; Barqawi, A.; Crighton, F.; Crawford, E.D.; Kerbel, R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer 2003, 98, 1643–1648. [Google Scholar] [CrossRef]
- Hatano, K.; Nonomura, N.; Nishimura, K.; Kawashima, A.; Mukai, M.; Nagahara, A.; Nakai, Y.; Nakayama, M.; Takayama, H.; Tsujimura, A.; et al. Retrospective Analysis of an Oral Combination of Dexamethasone, Uracil plus Tegafur and Cyclophosphamide for Hormone-refractory Prostate Cancer. Jpn. J. Clin. Oncol. 2010, 41, 253–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jellvert, Å.; Lissbrant, I.F.; Edgren, M.; Övferholm, E.; Braide, K.; Olvenmark, A.-M.E.; Kindblom, J.; Albertsson, P.; Lennernäs, B. Effective oral combination metronomic chemotherapy with low toxicity for the management of castration-resistant prostate cancer. Exp. Ther. Med. 2011, 2, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, J.L. Efficacy of metronomic oral cyclophosphamide with low dose dexamethasone and celecoxib in metastatic castration-resistant prostate cancer. Asia-Pac. J. Clin. Oncol. 2016, 13, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Mandel, P.; Amsberg, G.; Strölin, P.; Graefen, M.; Steuber, T. Metronomic chemotherapy with oral cyclophosphamide: An individual option for the metastatic castration-resistant prostate cancer patient? Urol. A 2019, 58, 410–417. [Google Scholar] [CrossRef]
- Kubota, H.; Fukuta, K.; Yamada, K.; Hirose, M.; Naruyama, H.; Yanai, Y.; Yamada, Y.; Watase, H.; Kawai, N.; Tozawa, K.; et al. Feasibility of metronomic chemotherapy with tegafur-uracil, cisplatin, and dexamethasone for docetaxel-refractory prostate cancer. J. Rural Med. 2017, 12, 112–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ladoire, S.; Eymard, J.C.; Zanetta, S.; Mignot, G.; Martin, E.; Kermarrec, I.; Mourey, E.; Michel, F.; Cormier, L.; Ghiringhelli, F. Metronomic oral cyclophosphamide prednisolone chemotherapy is an effective treatment for metastatic hormone-refractory prostate cancer after docetaxel failure. Anticancer Res. 2010, 30, 4317–4323. [Google Scholar]
- Lord, R.; Nair, S.; Schache, A.; Spicer, J.; Somaihah, N.; Khoo, V.; Pandha, H. Low Dose Metronomic Oral Cyclophosphamide for Hormone Resistant Prostate Cancer: A Phase II Study. J. Urol. 2007, 177, 2136–2140. [Google Scholar] [CrossRef]
- Meng, L.-J.; Wang, J.; Fan, W.-F.; Pu, X.-L.; Liu, F.-Y.; Yang, M. Evaluation of oral chemotherapy with capecitabine and cyclophosphamide plus thalidomide and prednisone in prostate cancer patients. J. Cancer Res. Clin. Oncol. 2012, 138, 333–339. [Google Scholar] [CrossRef]
- Nelius, T.; Klatte, T.; De Riese, W.; Haynes, A.; Filleur, S. Clinical outcome of patients with docetaxel-resistant hormone-refractory prostate cancer treated with second-line cyclophosphamide-based metronomic chemotherapy. Med. Oncol. 2009, 27, 363–367. [Google Scholar] [CrossRef]
- Nicolini, A.; Mancini, P.; Ferrari, P.; Anselmi, L.; Tartarelli, G.; Bonazzi, V.; Carpi, A.; Giardino, R. Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC). Biomed. Pharmacother. 2004, 58, 447–450. [Google Scholar] [CrossRef]
- Nishimura, K.; Nonomura, N.; Ono, Y.; Nozawa, M.; Fukui, T.; Harada, Y.; Imazu, T.; Takaha, N.; Sugao, H.; Miki, T.; et al. Oral Combination of Cyclophosphamide, Uracil plus Tegafur and Estramustine for Hormone-Refractory Prostate Cancer. Oncology 2001, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Moriya, F.; Koga, N.; Matsueda, S.; Sasada, T.; Yamada, A.; Kakuma, T.; Itoh, K. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2016, 65, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, P.; Fontana, A.; Fioravanti, A.; Di Desidero, T.; Galli, L.; Derosa, L.; Canu, B.; Marconcini, R.; Biasco, E.; Solini, A.; et al. VEGF-A polymorphisms predict progression-free survival among advanced castration-resistant prostate cancer patients treated with metronomic cyclophosphamide. Br. J. Cancer 2013, 109, 957–964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tralongo, P.; Bordonaro, S.; Di Mari, A.; Cappuccio, F.; Giuliano, S.R. Chemotherapy in frail elderly patients with hormone-refractory prostate cancer: A “real world” experience. Prostate Int. 2016, 4, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Vorob’Ev, N.A.; Nosov, A.K.; Vorob’Ev, A.V.; Moiseenko, B.M. Efficacy of standard docetaxel and metronomic cyclophosphamide chemotherapy in patients with hormone-resistant prostate cancer: Comparative analysis. Probl. Oncol. 2011, 57, 753–758. [Google Scholar]
- Wang, J.; McGuire, T.R.; Britton, H.C.; Schwarz, J.K.; Loberiza, F.R.; Meza, J.L.; Talmadge, J.E. Lenalidomide and cyclophosphamide immunoregulation in patients with metastatic, castration-resistant prostate cancer. Clin. Exp. Metastasis 2015, 32, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.J.; Blumenstein, B.A.; Crawford, E.D.; Boileau, M.; Rivkin, S.E.; Fletcher, W.S. Cyclophosphamide, methotrexate, and 5-fluorouracil in the treatment of metastatic prostate cancer. A southwest oncology group study. Cancer 1993, 71, 3975–3978. [Google Scholar] [CrossRef]
- Yashi, M.; Nishihara, D.; Mizuno, T.; Yuki, H.; Masuda, A.; Kambara, T.; Betsunoh, H.; Abe, H.; Fukabori, Y.; Muraishi, O.; et al. Metronomic Oral Cyclophosphamide Chemotherapy Possibly Contributes to Stabilization of Disease in Patients with Metastatic Castration-Resistant Prostate Cancer: A Prospective Analysis of Consecutive Cases. Clin. Genitourin. Cancer 2014, 12, e197–e203. [Google Scholar] [CrossRef]
- Lien, K.; Georgsdottir, S.; Sivanathan, L.; Chan, K.; Emmenegger, U. Low-dose metronomic chemotherapy: A systematic literature analysis. Eur. J. Cancer 2013, 49, 3387–3395. [Google Scholar] [CrossRef]
- Crivellari, D.; Gray, K.P.; Dellapasqua, S.; Puglisi, F.; Ribi, K.; Price, K.N.; Láng, I.; Gianni, L.; Spazzapan, S.; Pinotti, G.; et al. Adjuvant pegylated liposomal doxorubicin for older women with endocrine nonresponsive breast cancer who are NOT suitable for a “standard chemotherapy regimen”: The CASA randomized trial. Breast 2013, 22, 130–137. [Google Scholar] [CrossRef]
- Colleoni, M.; Gray, K.P.; Gelber, S.; Láng, I.; Thürlimann, B.; Gianni, L.; Abdi, E.A.; Gomez, H.; Linderholm, B.K.; Puglisi, F.; et al. Low-Dose Oral Cyclophosphamide and Methotrexate Maintenance for Hormone Receptor–Negative Early Breast Cancer: International Breast Cancer Study Group Trial 22-00. J. Clin. Oncol. 2016, 34, 3400–3408. [Google Scholar] [CrossRef] [PubMed]
- Nasr, K.E.; Osman, M.A.M.; Elkady, M.S.; Ellithy, M.A. Metronomic methotrexate and cyclophosphamide after carboplatin included adjuvant chemotherapy in triple negative breast cancer: A phase III study. Ann. Transl. Med. 2015, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Rochlitz, C.; Bigler, M.; Von Moos, R.; Bernhard, J.; Matter-Walstra, K.; Wicki, A.; Zaman, K.; Anchisi, S.; Küng, M.; Na, K.-J.; et al. SAKK 24/09: Safety and tolerability of bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer—A multicenter, randomized phase III trial. BMC Cancer 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Liu, X.; Zhou, Q.; Yang, K.-Y.; Jin, F.; Zhu, X.-D.; Shi, M.; Hu, G.-Q.; Hu, W.-H.; Sun, Y.; et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: A multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet 2021, 398, 303–313. [Google Scholar] [CrossRef]
- Patil, V.; Noronha, V.; Dhumal, S.B.; Joshi, A.; Menon, N.; Bhattacharjee, A.; Kulkarni, S.; Ankathi, S.K.; Mahajan, A.; Sable, N.; et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: An open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob. Health 2020, 8, e1213–e1222. [Google Scholar] [CrossRef]
- Simkens, L.H.J.; van Tinteren, H.; May, A.; Tije, A.J.T.; Creemers, G.-J.M.; Loosveld, O.J.L.; de Jongh, F.E.; Erdkamp, F.L.G.; Erjavec, Z.; van der Torren, A.M.E.; et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- Hagman, H.; Frödin, J.-E.; Berglund, Å.; Sundberg, J.; Vestermark, L.W.; Albertsson, M.; Fernebro, E.; Johnsson, A. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: The Nordic ACT2 trial. Ann. Oncol. 2016, 27, 140–147. [Google Scholar] [CrossRef]
- Cramarossa, G.; Lee, E.K.; Sivanathan, L.; Georgsdottir, S.; Lien, K.; Santos, K.D.; Chan, K.; Emmenegger, U. A systematic literature analysis of correlative studies in low-dose metronomic chemotherapy trials. Biomarkers Med. 2014, 8, 893–911. [Google Scholar] [CrossRef]
- Ling, H.H.; Lin, Y.-C. Metronomic Oral Cyclophosphamide in 2 Heavily Pretreated Patients with Metastatic Castration-resistant Prostate Cancer With Homologous Recombination Deficiency (HRD): A Case Report. Clin. Genitourin. Cancer 2018, 17, 157–160. [Google Scholar] [CrossRef]
- Mittmann, N.; Liu, N.; Cheng, S.Y.; Seung, S.J.; Saxena, F.E.; Hong, N.J.L.; Earle, C.C.; Cheung, M.C.; Leighl, N.B.; Coburn, N.G.; et al. Health system costs for cancer medications and radiation treatment in Ontario for the 4 most common cancers: A retrospective cohort study. CMAJ Open 2020, 8, E191–E198. [Google Scholar] [CrossRef]
- Trogdon, J.G.; Falchook, A.D.; Basak, R.; Carpenter, W.R.; Chen, R.C. Total Medicare Costs Associated with Diagnosis and Treatment of Prostate Cancer in Elderly Men. JAMA Oncol. 2019, 5, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Timilshina, N.; Emmenegger, U.; Smoragiewicz, M.; Sander, B.; Alibhai, S.; Chan, K.K. A cost-utility analysis of apalutamide for metastatic castration-sensitive prostate cancer. Can. Urol. Assoc. J. 2021, 16, E126–E131. [Google Scholar] [CrossRef] [PubMed]
- Simsek, C.; Esin, E.; Yalcin, S. Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience. J. Oncol. 2019, 2019, 5483791. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Bocci, G.; Tuccori, M.; Emmenegger, U.; Liguori, V.; Falcone, A.; Kerbel, R.S.; Del Tacca, M. Cyclophosphamide-methotrexate ‘metronomic’ chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann. Oncol. 2005, 16, 1243–1252. [Google Scholar] [CrossRef]
- Mason, N.T.; Burkett, J.M.; Nelson, R.S.; Pow-Sang, J.M.; Gatenby, R.A.; Kubal, T.; Peabody, J.W.; Letson, G.D.; McLeod, H.L.; Zhang, J. Budget Impact of Adaptive Abiraterone Therapy for Castration-Resistant Prostate Cancer. Am. Health Drug Benefits 2021, 14, 15–20. [Google Scholar]
- West, J.B.; Dinh, M.N.; Brown, J.S.; Zhang, J.; Anderson, A.R.; Gatenby, R.A. Multidrug Cancer Therapy in Metastatic Castrate-Resistant Prostate Cancer: An Evolution-Based Strategy. Clin. Cancer Res. 2019, 25, 4413–4421. [Google Scholar] [CrossRef]
- Kummar, S.; Wade, J.L.; Oza, A.; O’Sullivan, C.G.; Chen, A.P.; Gandara, D.R.; Ji, J.; Kinders, R.J.; Wang, L.; Allen, D.; et al. Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Investig. New Drugs 2016, 34, 355–363. [Google Scholar] [CrossRef]
- Rivkin, S.E.; Moon, J.; Iriarte, D.S.; Bailey, E.; Sloan, H.L.; Goodman, G.E.; Bondurant, A.E.; Velijovich, D.; Wahl, T.; Jiang, P.; et al. Phase Ib with expansion study of olaparib plus weekly (Metronomic) carboplatin and paclitaxel in relapsed ovarian cancer patients. Int. J. Gynecol. Cancer 2019, 29, 325–333. [Google Scholar] [CrossRef]
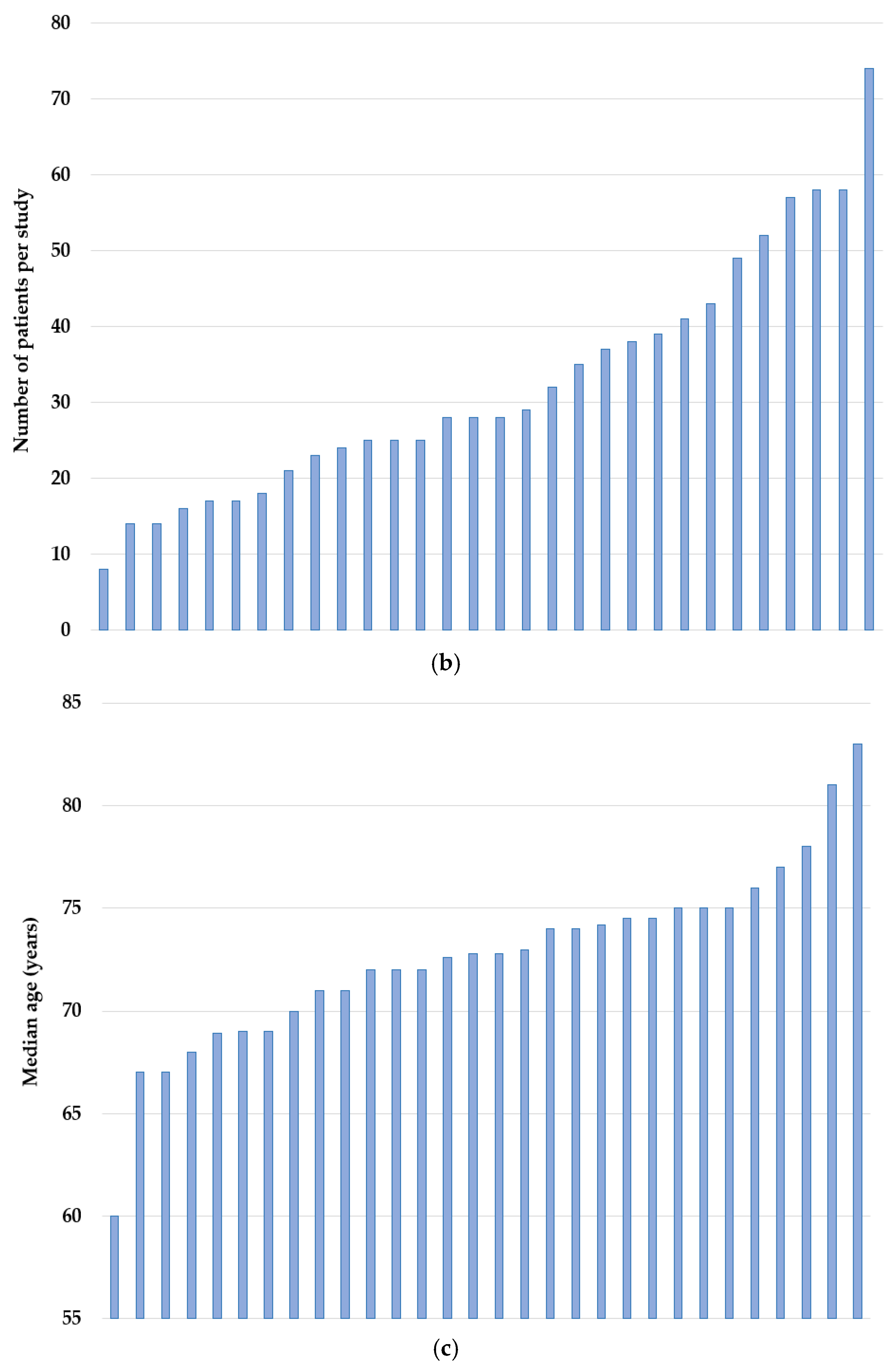

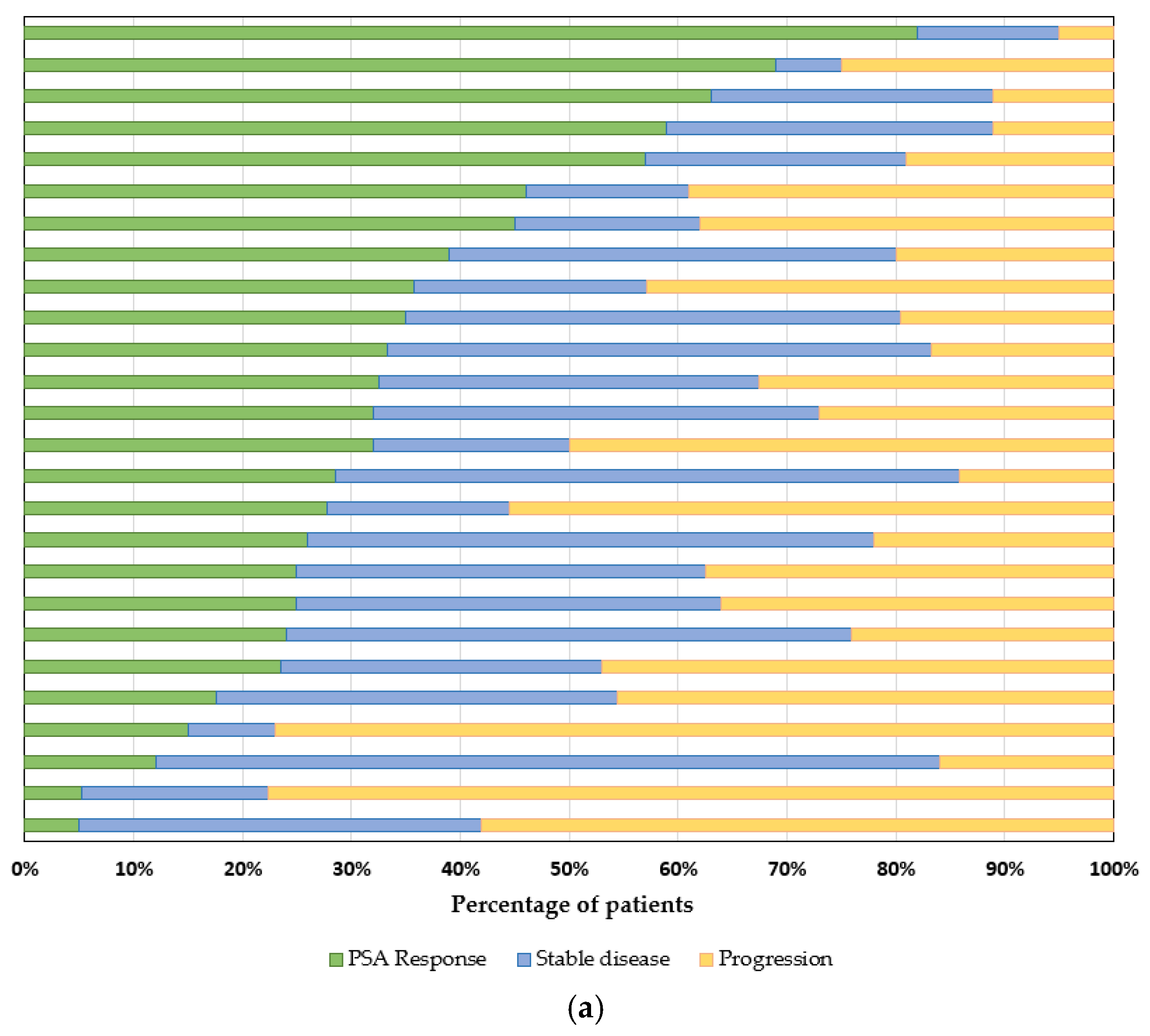
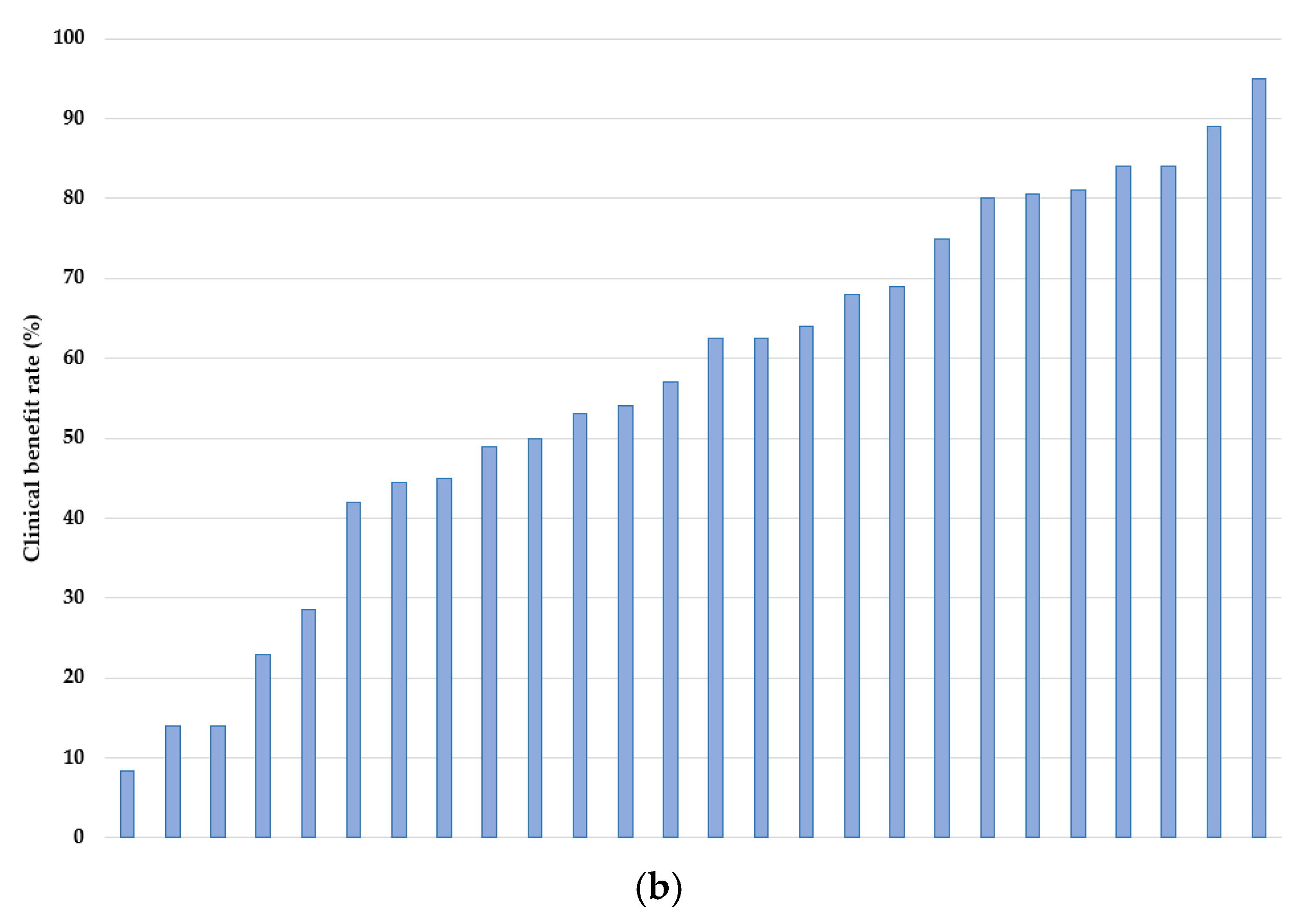
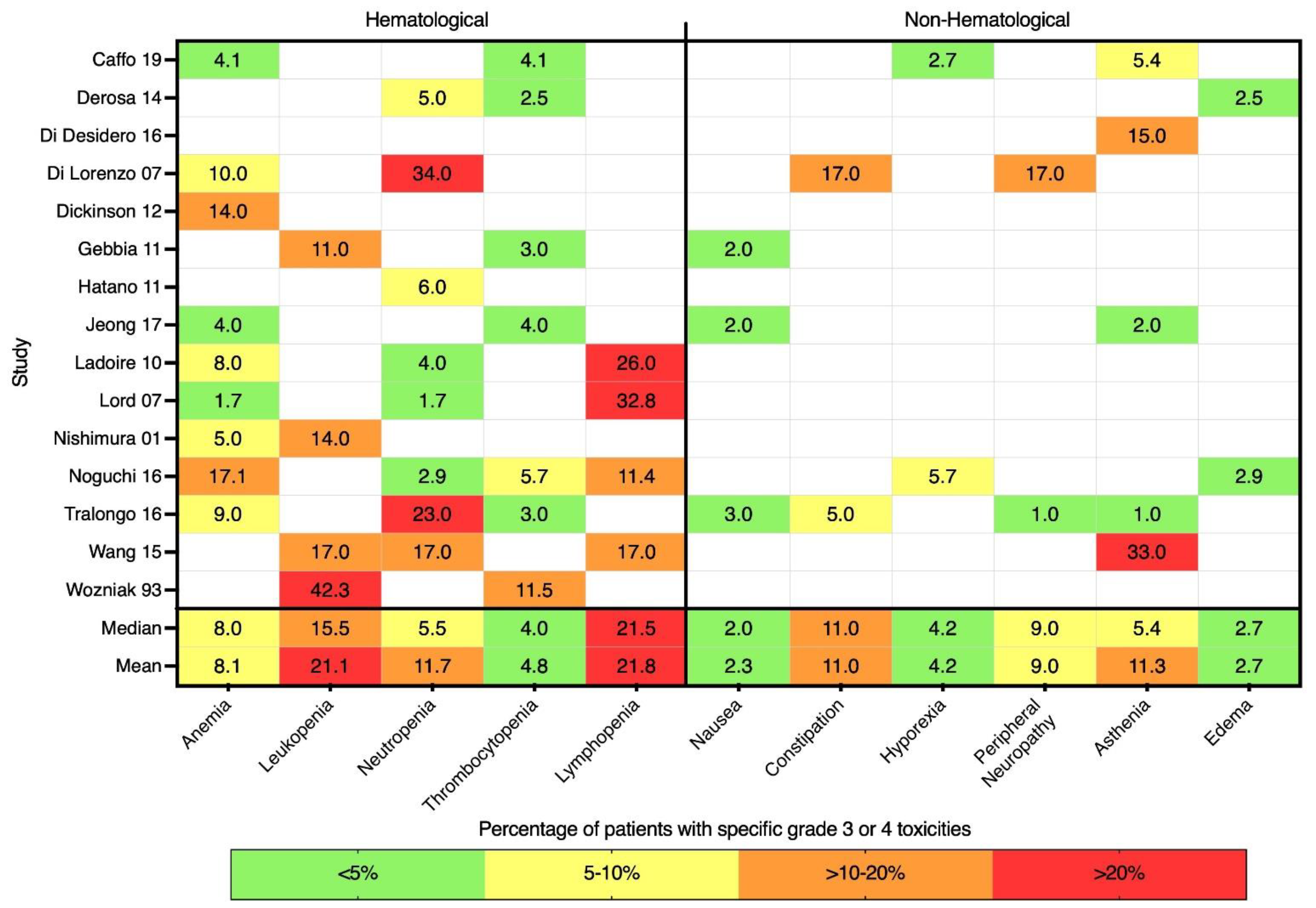

| First Author Name | Years | Study Type | Location | N | Age, Median (Range) | Reference |
|---|---|---|---|---|---|---|
| Caffo et al. | 2019 | retrospective | Italy | 8 | 74 (56–95) | [21] |
| Calcagno et al. | 2016 | prospective | France | 14 | 69 (57–82) | [22] |
| Calvani et al. | 2019 | retrospective | Italy | 14 | 75 (56–87) | [23] |
| Dabkara et al. | 2018 | retrospective | India | 16 | 74.5 (59–83) | [24] |
| Derosa et al. | 2014 | prospective | Italy | 17 | 72 (52–79) | [25] |
| Di Desidero et al. | 2016 | prospective | Italy | 17 | 73 (63–86) | [26] |
| Di Lorenzo et al. | 2007 | prospective | Italy | 18 | 67 (46–75) | [27] |
| Dickinson et al. | 2012 | retrospective | UK | 21 | 75 (N/A) | [28] |
| Fontana et al. | 2009 | prospective | Italy | 23 | 74.5 (54–91) | [29] |
| Fontana et al. | 2010 | retrospective | Italy | 24 | 83 (78–92) | [19] |
| Gebbia et al. | 2011 | prospective | Italy | 25 | 72 (56–83) | [30] |
| Glode et al. | 2003 | retrospective | USA | 25 | 72.6 (54–88) | [31] |
| Hatano et al. | 2011 | retrospective | Japan | 25 | 71 (49–90) | [32] |
| Jellvert et al. | 2011 | prospective | Sweden | 28 | 60 (45–75) | [33] |
| Jeong & Lee | 2017 | prospective | Korea | 28 | 71 (49–88) | [34] |
| Knipper et al. | 2019 | retrospective | Germany | 28 | 78 (N/A) | [35] |
| Kubota et al. | 2017 | prospective | Japan | 29 | 74.2 (66–88) | [36] |
| Ladoire et al. | 2010 | prospective | France | 32 | 74 (55–88) | [37] |
| Lord et al. | 2007 | prospective | UK | 35 | 69 (51–86) | [38] |
| Meng et al. | 2012 | retrospective | China | 38 | 72.8 (69–78) | [39] |
| Nelius et al. | 2010 | prospective | USA | 39 | 68 (42–85) | [40] |
| Nicolini et al. | 2004 | prospective | Italy | 41 | 72 (62–84) | [41] |
| Nishimura et al. | 2001 | prospective | Japan | 43 | 70 (50–82) | [42] |
| Noguchi et al. | 2016 | prospective | Japan | 49 | 68.6 (48–80) | [43] |
| Orlandi et al. | 2013 | retrospective | USA | 52 | 81 (52–92) | [44] |
| Tralongo et al. | 2016 | prospective | Italy | 57 | 77 (72–82) | [45] |
| Vorob’ev et al. | 2011 | retrospective | Russia | 58 | 72.8 * (56–85) | [46] |
| Wang et al. | 2015 | prospective | USA | 58 | 76 (50–86) | [47] |
| Wozniak et al. | 1993 | prospective | USA | 74 | 67 (55–78) | [48] |
| Yashi et al. | 2014 | prospective | Japan | 37 | 75 (67.8–79.3) | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshad, S.; Sidhu, A.K.; Khan, N.; Naoum, A.; Emmenegger, U. Metronomic Chemotherapy for Advanced Prostate Cancer: A Literature Review. J. Clin. Med. 2022, 11, 2783. https://doi.org/10.3390/jcm11102783
Parshad S, Sidhu AK, Khan N, Naoum A, Emmenegger U. Metronomic Chemotherapy for Advanced Prostate Cancer: A Literature Review. Journal of Clinical Medicine. 2022; 11(10):2783. https://doi.org/10.3390/jcm11102783
Chicago/Turabian StyleParshad, Shruti, Amanjot K. Sidhu, Nabeeha Khan, Andrew Naoum, and Urban Emmenegger. 2022. "Metronomic Chemotherapy for Advanced Prostate Cancer: A Literature Review" Journal of Clinical Medicine 11, no. 10: 2783. https://doi.org/10.3390/jcm11102783
APA StyleParshad, S., Sidhu, A. K., Khan, N., Naoum, A., & Emmenegger, U. (2022). Metronomic Chemotherapy for Advanced Prostate Cancer: A Literature Review. Journal of Clinical Medicine, 11(10), 2783. https://doi.org/10.3390/jcm11102783







