Influence of Advanced Organ Support (ADVOS) on Cytokine Levels in Patients with Acute-on-Chronic Liver Failure (ACLF)
Abstract
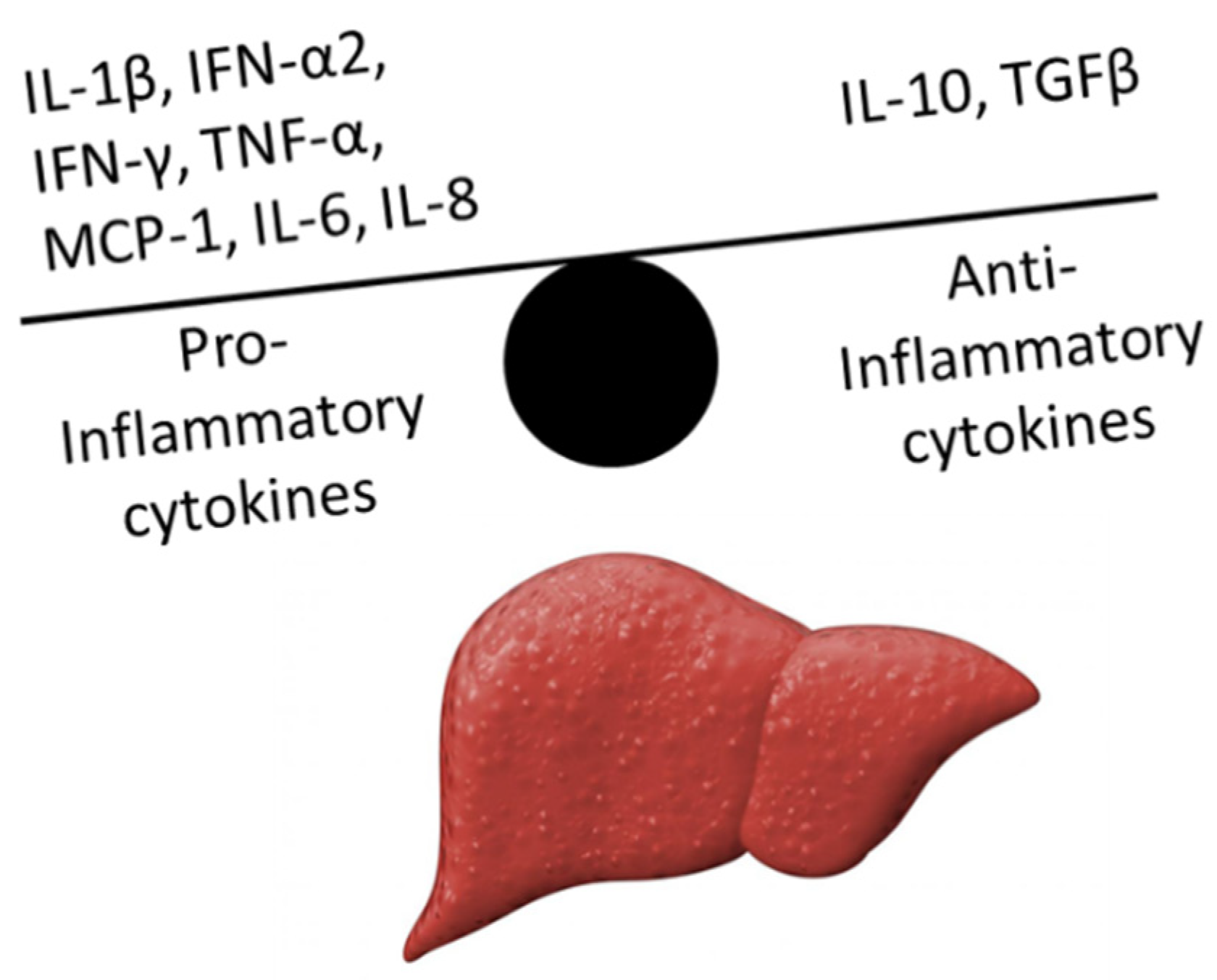
:1. Introduction
2. Materials and Methods
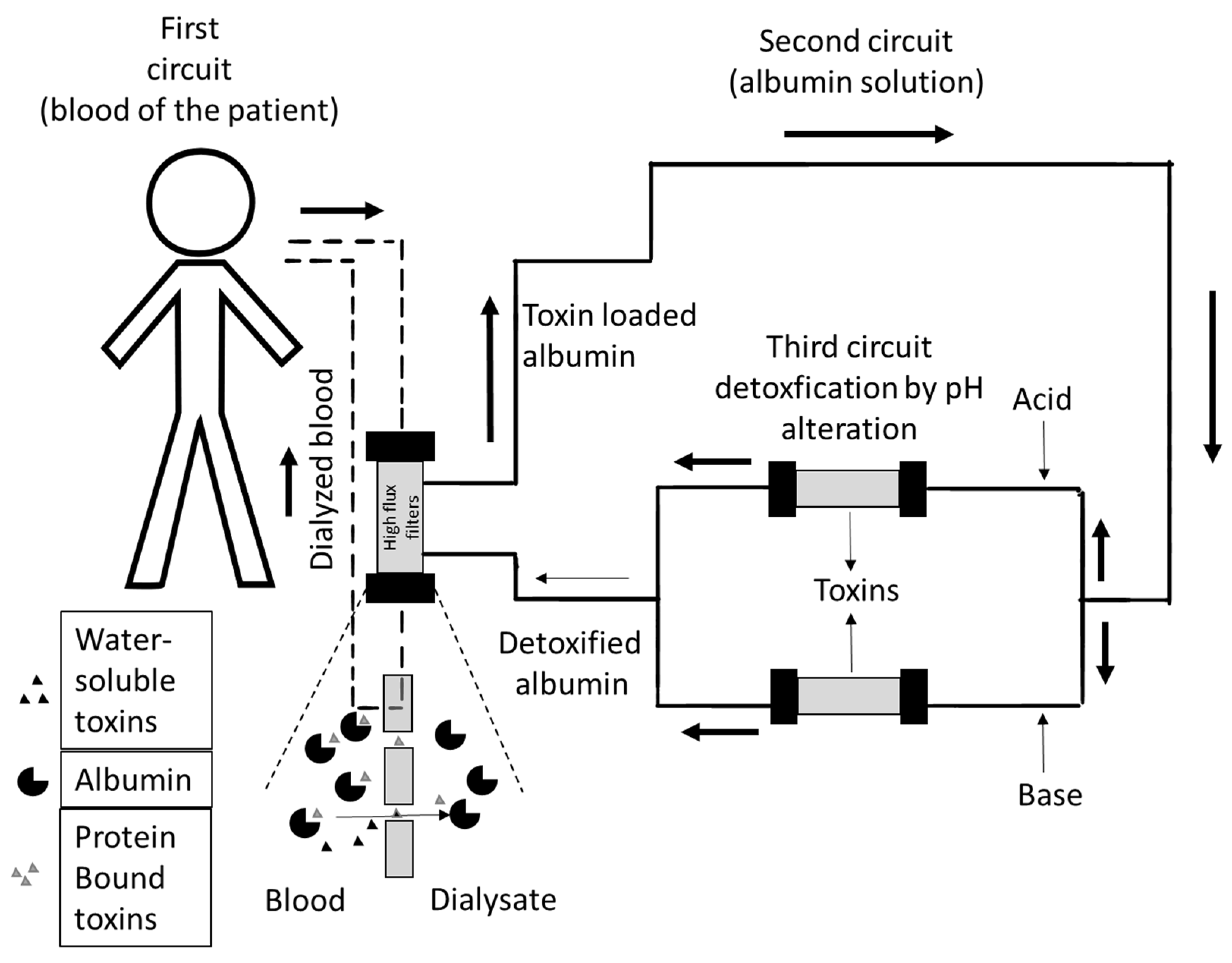
2.1. ADVanced Organ Support (ADVOS)
2.2. Study Design and Endpoint
2.3. Inclusion and Exclusion Criteria
2.4. ADVOS Treatment Parameters
2.5. Quantification of Cytokine Levels by Cytometric Bead Array
2.6. Ingenuity Pathway Analysis (IPA®)
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
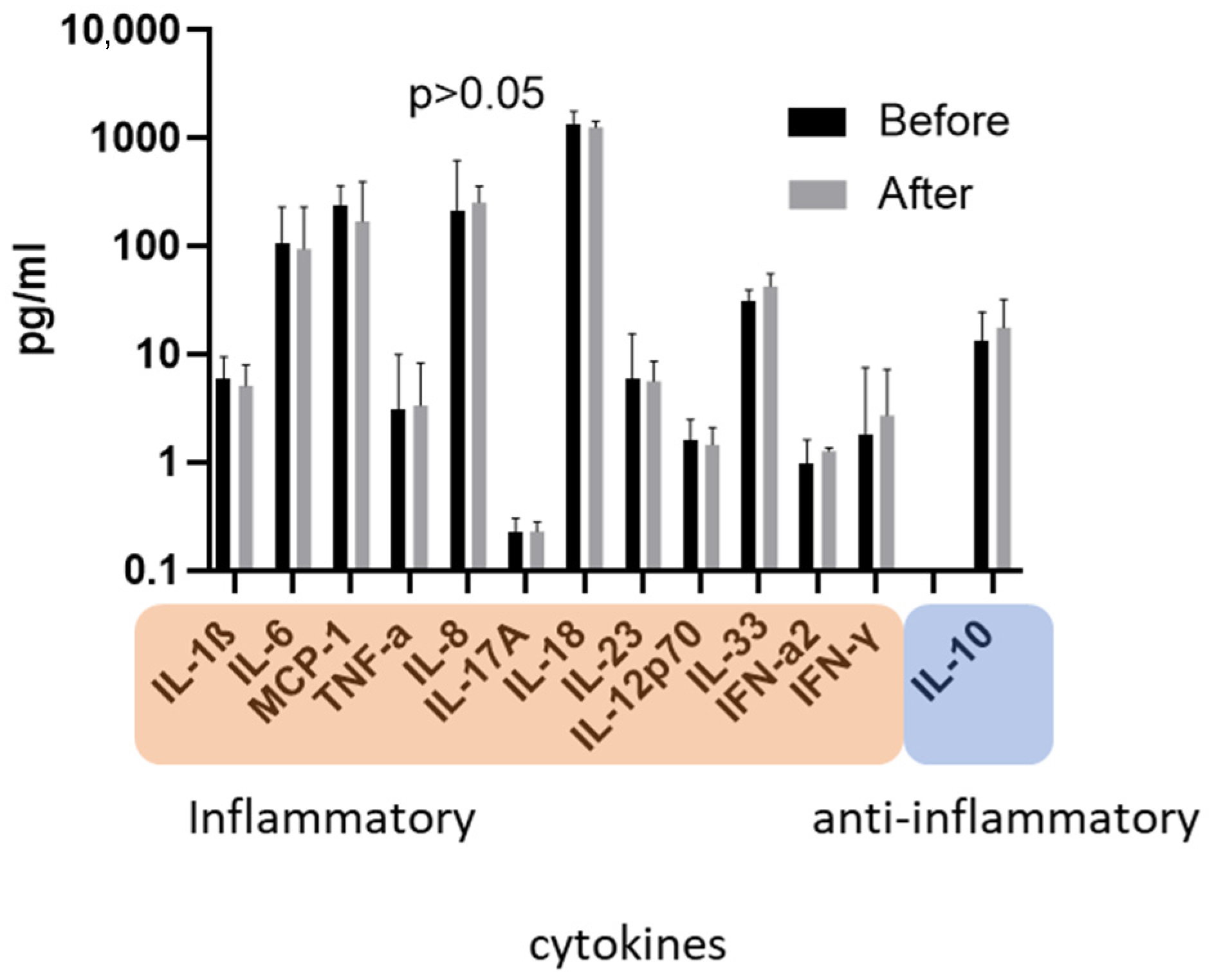
3.2. Concentration of Cytokines before versus after ADVOS
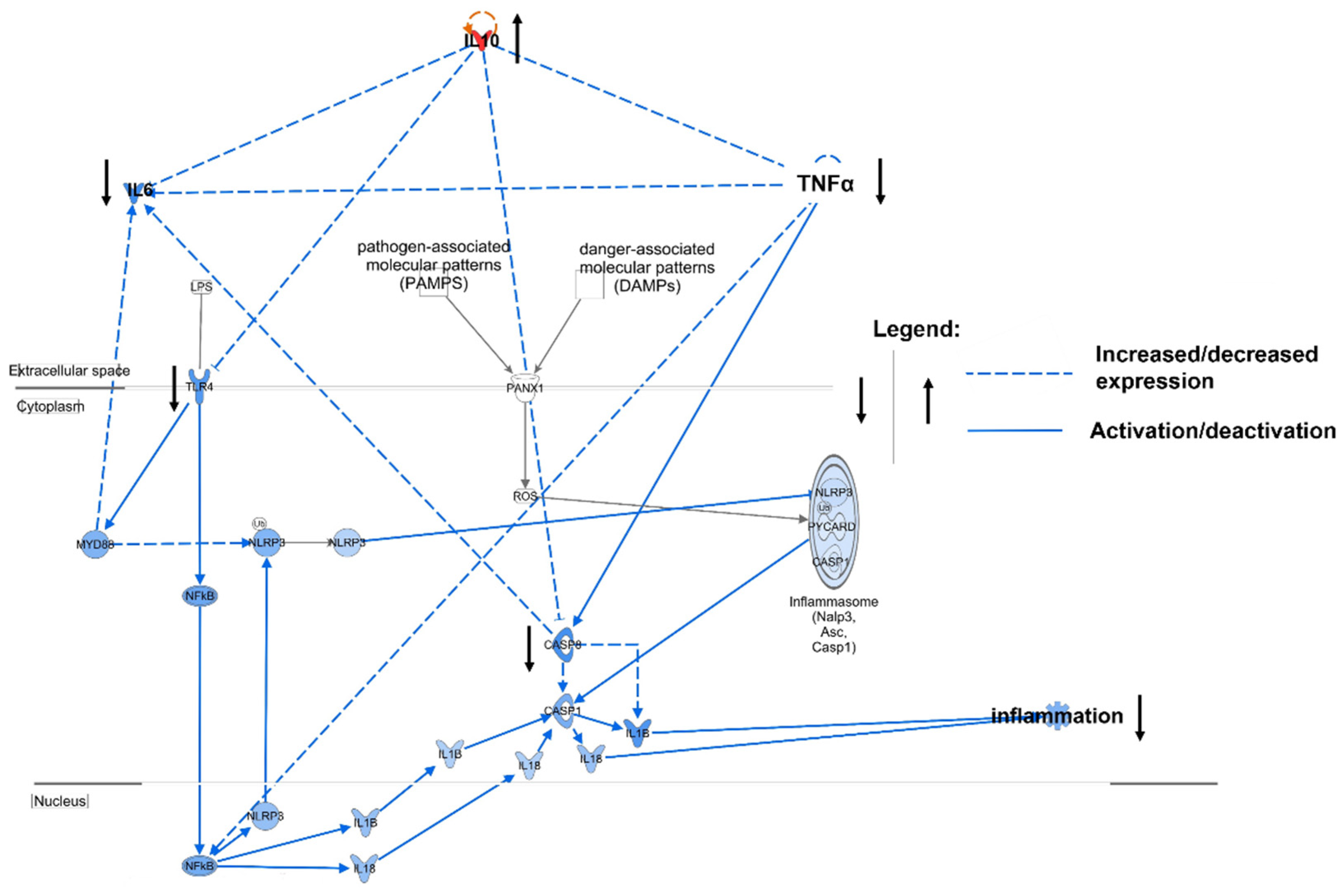
3.3. Relevance of Cytokines in ACLF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Gerbes, A.L.; Labenz, J.; Appenrodt, B.; Dollinger, M.; Gundling, F.; Gülberg, V.; Holstege, A.; Lynen-Jansen, P.; Steib, C.J.; Trebicka, J.; et al. [Updated S2k-Guideline “Complications of liver cirrhosis”. German Society of Gastroenterology (DGVS)]. Z. Gastroenterol. 2019, 57, E168. [Google Scholar] [PubMed] [Green Version]
- Mahmud, N.; Kaplan, D.E.; Taddei, T.H.; Goldberg, D.S. Incidence and Mortality of Acute-on-Chronic Liver Failure Using Two Definitions in Patients with Compensated Cirrhosis. Hepatology 2019, 69, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. Acute-on-chronic liver failure: A new syn-drome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 2020, 74, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75, S49–S66. [Google Scholar] [CrossRef]
- Tsipotis, E.; Shuja, A.; Jaber, B.L. Albumin Dialysis for Liver Failure: A Systematic Review. Adv. Chronic Kidney Dis. 2015, 22, 382–390. [Google Scholar] [CrossRef]
- Huber, W.; Henschel, B.; Schmid, R.; Al-Chalabi, A. First clinical experience in 14 patients treated with ADVOS: A study on feasibility, safety and efficacy of a new type of albumin dialysis. BMC Gastroenterol. 2017, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Kaps, L.; Ahlbrand, C.J.; Gadban, R.; Nagel, M.; Labenz, C.; Klimpke, P.; Holtz, S.; Boedecker, S.; Michel, M.; Kremer, W.M.; et al. Applicability and safety of discontinuous Advanced Organ Support (ADVOS) in the treatment of patients with acute-on-chronic liver failure (ACLF) outside of intensive care. PLoS ONE 2021, 16, e0249342. [Google Scholar] [CrossRef]
- Zaal, A.; Li, R.E.; Lubbers, J.; Bruijns, S.C.M.; Kalay, H.; van Kooyk, Y.; Van Vliet, S.J. Activation of the C-Type Lectin MGL by Terminal GalNAc Ligands Reduces the Glycolytic Activity of Human Dendritic Cells. Front. Immunol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Ruchakorn, N.; Ngamjanyaporn, P.; Suangtamai, T.; Kafaksom, T.; Polpanumas, C.; Petpisit, V.; Pisitkun, T.; Pisitkun, P. Performance of cytokine models in predicting SLE activity. Arthritis Res. Ther. 2019, 21, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; van den Hil, F.E.; Mummery, C.L.; Orlova, V.V. Generation and Functional Characterization of Monocytes and Macrophages Derived from Human Induced Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2020, 52, e108. [Google Scholar] [CrossRef] [PubMed]
- Koutsakos, M.; Rowntree, L.C.; Hensen, L.; Chua, B.Y.; van de Sandt, C.E.; Habel, J.R.; Zhang, W.; Jia, X.; Kedzierski, L.; Ashhurst, T.M.; et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021, 2, 100208. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Huppertsberg, A.; Choteschovsky, N.; Klefenz, A.; Durak, F.; Schrörs, B.; Diken, M.; Eichler, E.; Rosigkeit, S.; Schmitt, S.; et al. pH-degradable, bisphosphonate-loaded nanogels attenuate liver fibrosis by repolarization of M2-type macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2122310119. [Google Scholar] [CrossRef]
- Hashimoto, S.; Komuro, I.; Yamada, M.; Akagawa, K.S. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J. Immunol. 2001, 167, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.H.; Aggarwal, S.; Ho, W.H.; Foster, J.; Zhang, Z.; Stinson, J.; Wood, W.I.; Goddard, A.D.; Gurney, A.L. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000, 275, 31335–31339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Nagasawa, M.; Takada, H.; Hara, T.; Tsuchiya, S.; Agematsu, K.; Yamada, M.; Kawamura, N.; Ariga, T.; Tsuge, I.; et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J. Exp. Med. 2011, 208, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, R.; Mitra, D.; Chakrabarti, S. HIV-1 gp120 protein downregulates Nef induced IL-6 release in immature dentritic cells through interplay of DC-SIGN. PLoS ONE 2013, 8, e59073. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Gao, S.; Liang, S.; Jotwani, R.; Zhou, H.; Suttles, J.; Scott, D.A.; Lamont, R.J. The Role of JAK-3 in Regulating TLR-Mediated Inflammatory Cytokine Production in Innate Immune Cells. J. Immunol. 2013, 191, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Frasca, L.; Nasso, M.; Spensieri, F.; Fedele, G.; Palazzo, R.; Malavasi, F.; Ausiello, C.M. IFN-gamma arms human dendritic cells to perform multiple effector functions. J. Immunol. 2008, 180, 1471–1481. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Lügering, N.; Pauels, H.-G.; Schulze-Osthoff, K.; Domschke, W.; Kucharzik, T. IL-10 induces apoptosis in human monocytes involving the CD95 receptor/ligand pathway. Eur. J. Immunol. 2000, 30, 1769–1777. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, R.; Xu, X.; Liu, Y.; Zhang, H.; Zhai, X.; Hu, X. IL-10 Reduces Levels of Apoptosis in Toxoplasma gondii-Infected Trophoblasts. PLoS ONE 2013, 8, e56455. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, P.; Glorieux, G.; Vanholder, R. Interleukin/cytokine profiles in haemodialysis and in continuous peritoneal dialysis. Nephrol. Dial. Transplant. 2004, 19 (Suppl. 5), v41–v45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canaud, B.; Stephens, M.P.; Nikam, M.; Etter, M.; Collins, A. Multitargeted interventions to reduce dialysis-induced systemic stress. Clin. Kidney J. 2021, 14, i72–i84. [Google Scholar] [CrossRef]
- Bowry, S.K.; Kircelli, F.; Himmele, R.; Nigwekar, S.U. Blood-incompatibility in haemo-dialysis: Alleviating inflammation and effects of coagulation. Clin. Kidney J. 2021, 14, i59–i71. [Google Scholar] [CrossRef]
- Canivet, E.; Lavaud, S.; Wong, T.; Guenounou, M.; Willemin, J.C.; Potron, G.; Chanard, J. Cuprophane but not synthetic membrane induces increases in serum tumor necrosis fac-tor-alpha levels during hemodialysis. Am. J. Kidney Dis. 1994, 23, 41–46. [Google Scholar] [CrossRef]
- Lonnemann, G.; Haubitz, M.; Schindler, R. Hemodialysis- Associated Induction of Cytokines. Blood Purif. 1990, 8, 214–222. [Google Scholar] [CrossRef]
- Varela, M.P.; Kimmel, P.L.; Phillips, T.M.; Mishkin, G.J.; Lew, S.Q.; Bosch, J.P. Biocompatibility of Hemodialysis Membranes: Interrelations between Plasma Complement and Cytokine Levels. Blood Purif. 2001, 19, 370–379. [Google Scholar] [CrossRef]
- de Vriese, A.S.; Colardyn, F.A.; Philippé, J.J.; Vanholder, R.C.; de Sutter, J.H.; Lameire, N.H. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 1999, 10, 846–853. [Google Scholar] [CrossRef]
- Bouré, T.; Vanholder, R. Biochemical and Clinical Evidence for Uremic Toxicity. Artif. Organs 2004, 28, 248–253. [Google Scholar] [CrossRef]
- Pereira, B.J.; Snodgrass, B.R.; Hogan, P.J.; King, A.J. Diffusive and convective transfer of cytokine-inducing bacterial products across hemodialysis membranes. Kidney Int. 1995, 47, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouten, W.E.M.; Grooteman, M.P.C.; van Houte, A.-J.; Schoorl, M.; van Limbeek, J.; Nubé, M.J. Effects of dialyser and dialysate on the acute phase reaction in clinical bicarbonate dialysis. Nephrol. Dial. Transplant. 2000, 15, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trebicka, J.; Amoros, A.; Pitarch, C.; Titos, E.; Alcaraz-Quiles, J.; Schierwagen, R.; Deulofeu, C.; Fernandez-Gomez, J.; Piano, S.; Caraceni, P.; et al. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front. Immunol. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Inclusion | |
| 1. | Clinical or histological evidence of liver cirrhosis * |
| 2. | Acute decompensation indicated by ascites (II–III), deterioration of laboratory parameters or hepatic encephalopathy (West Haven criteria grade ≥ I) in line with CLIF consortium organ Failure Score ≥ 1 |
| 3. | Bilirubin ≥ 3 mg/dL and sudden prothrombin time-INR > 1.4 |
| 4. | HRS-AKI with anuria/oliguria diagnosed according to the EASL HRS/ACLF guidelines [4] |
| Exclusion | |
| 1. | Mean arterial pressure ≤ 50 mmHg despite volume expansion |
| 2. | Patients aged <18 years |
| Total Patients, n (%) | 15 (100%) | |
| Male, n (%) | 9 (60%) | |
| Age (years), median (IQR) | 50.5 (42.2; 56.5) | |
| Type of liver failure (%) | ACLF (100%) | |
| Etiology of Cirrhosis | ||
| Alcohol, n (%) | 11 (73%) | |
| Viral hepatitis, n (%) | 2 (13%) | |
| Cholestatic/Autoimmune, n (%) | 1 (7%) | |
| Other/mixed, n (%) | 1 (7%) | |
| ACLF Trigger | ||
| Unknown, n (%) | 5 (33%) | |
| Infections, n (%) | 6 (40%) | |
| Varical bleeding, n (%) | 4 (27%) | |
| Liver Function | ||
| Child-Pugh score, n (%) | 3 B (20%), 12 C (80%) | |
| MELD, median (IQR) | 38 (35; 40) | |
| CLIF-C ACLF Calculator, Median | ||
| CLIF-C ACLF score (IQR) | 52 (48; 56) | |
| ACLF grade (IQR) | 15 (2; 3) | |
| CLIF Organ Failure Score (IQR) | 11 (11; 12) | |
| Liver failure (IQR) | 3 (3; 3) | |
| Kidney failure (IQR) | 3 (3; 3) | |
| Cerebral failure (IQR) | 2 (1; 2) | |
| Coagulation failure (IQR) | 1 (1; 3) | |
| Circulatory failure, n (IQR) | 1 (1; 1) | |
| Lung failure, n (IQR) | 1 (1; 1) | |
| One-month probability of dying (IQR) | 33 (24; 44) | |
| Laboratory, Median | ||
| Sodium, mmol/L (IQR) | 136 (130; 143) | |
| Potassium, mmol/L (IQR) | 4 (3; 5.2) | |
| BUN, mg/dL (IQR) | 43 (35; 71.3) | |
| SCr, mmol/L (IQR) | 2.8 (2.3; 4.6) | |
| INR, median (IQR) | 1.9 (1.6; 4.6) | |
| Bilirubin, mg/dL (IQR) | 23.4 (17; 34.7) | |
| CRP, mg/L (IQR) | 43 (25; 65) | |
| Albumin, g/L (IQR) | 20 (17; 31.3) | |
| Thrombocytes, count/nL (IQR) | 87 (54; 111) | |
| WBC, count/nL (IQR) | 15 (7.3; 18.9) |
| Treatment, Median | ||
| Dialysis time at blood sampling, minutes (IQR) | 480 (360; 480) | |
| Total ADVOS cycles during hospitalization, (IQR) | 5 (3.3; 8) | |
| Vasopressor therapy | 0 | |
| Outcome, n (%) | ||
| Liver transplantation | 2 (13%) | |
| One-month mortality | 7 (46%) | |
| Overall mortality | 8 (53%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaps, L.; Schleicher, E.M.; Medina Montano, C.; Bros, M.; Gairing, S.J.; Ahlbrand, C.J.; Michel, M.; Klimpke, P.; Kremer, W.M.; Holtz, S.; et al. Influence of Advanced Organ Support (ADVOS) on Cytokine Levels in Patients with Acute-on-Chronic Liver Failure (ACLF). J. Clin. Med. 2022, 11, 2782. https://doi.org/10.3390/jcm11102782
Kaps L, Schleicher EM, Medina Montano C, Bros M, Gairing SJ, Ahlbrand CJ, Michel M, Klimpke P, Kremer WM, Holtz S, et al. Influence of Advanced Organ Support (ADVOS) on Cytokine Levels in Patients with Acute-on-Chronic Liver Failure (ACLF). Journal of Clinical Medicine. 2022; 11(10):2782. https://doi.org/10.3390/jcm11102782
Chicago/Turabian StyleKaps, Leonard, Eva Maria Schleicher, Carolina Medina Montano, Matthias Bros, Simon Johannes Gairing, Constantin Johannes Ahlbrand, Maurice Michel, Pascal Klimpke, Wolfgang Maximilian Kremer, Stefan Holtz, and et al. 2022. "Influence of Advanced Organ Support (ADVOS) on Cytokine Levels in Patients with Acute-on-Chronic Liver Failure (ACLF)" Journal of Clinical Medicine 11, no. 10: 2782. https://doi.org/10.3390/jcm11102782
APA StyleKaps, L., Schleicher, E. M., Medina Montano, C., Bros, M., Gairing, S. J., Ahlbrand, C. J., Michel, M., Klimpke, P., Kremer, W. M., Holtz, S., Boedecker-Lips, S. C., Galle, P. R., Kraus, D., Schattenberg, J. M., Labenz, C., & Weinmann-Menke, J. (2022). Influence of Advanced Organ Support (ADVOS) on Cytokine Levels in Patients with Acute-on-Chronic Liver Failure (ACLF). Journal of Clinical Medicine, 11(10), 2782. https://doi.org/10.3390/jcm11102782








