Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center
Abstract
:1. Introduction
2. Materials and Methods
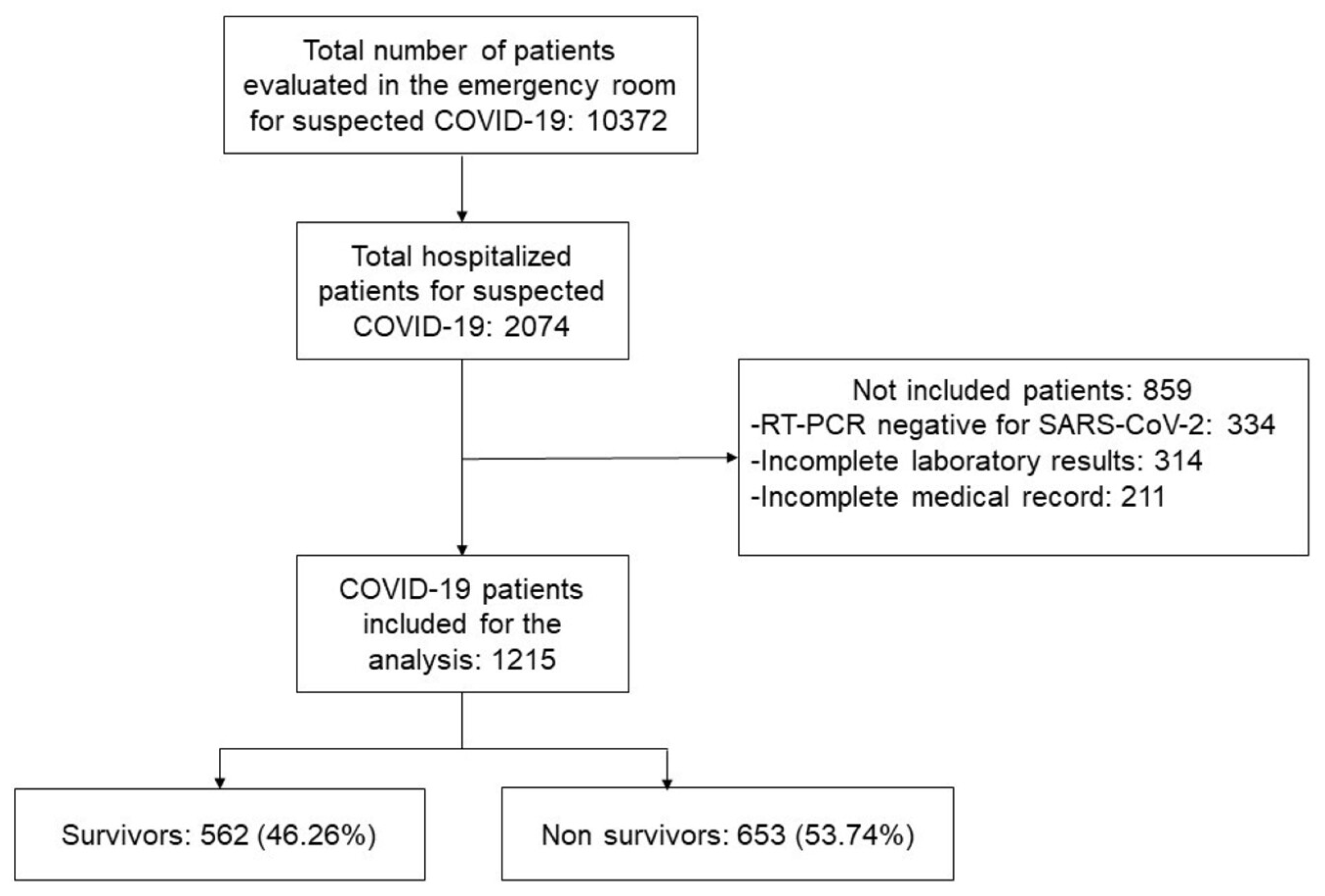
2.1. Study Design and Patient Population
2.2. Data Collection
2.3. COVID-19 Diagnosis
2.4. Definition of Variables
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. ROC Analysis to Determine Cut-Off Points
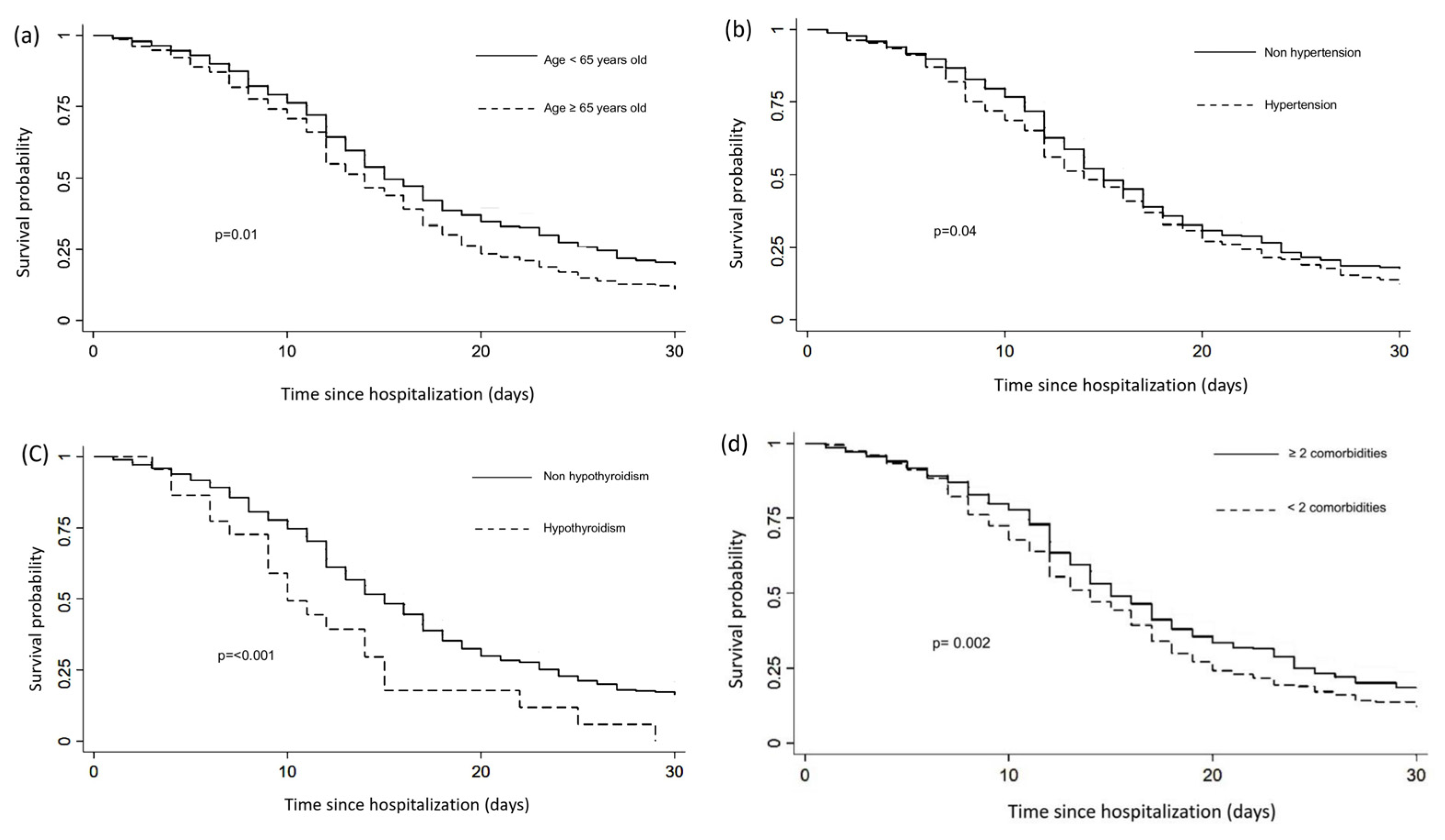
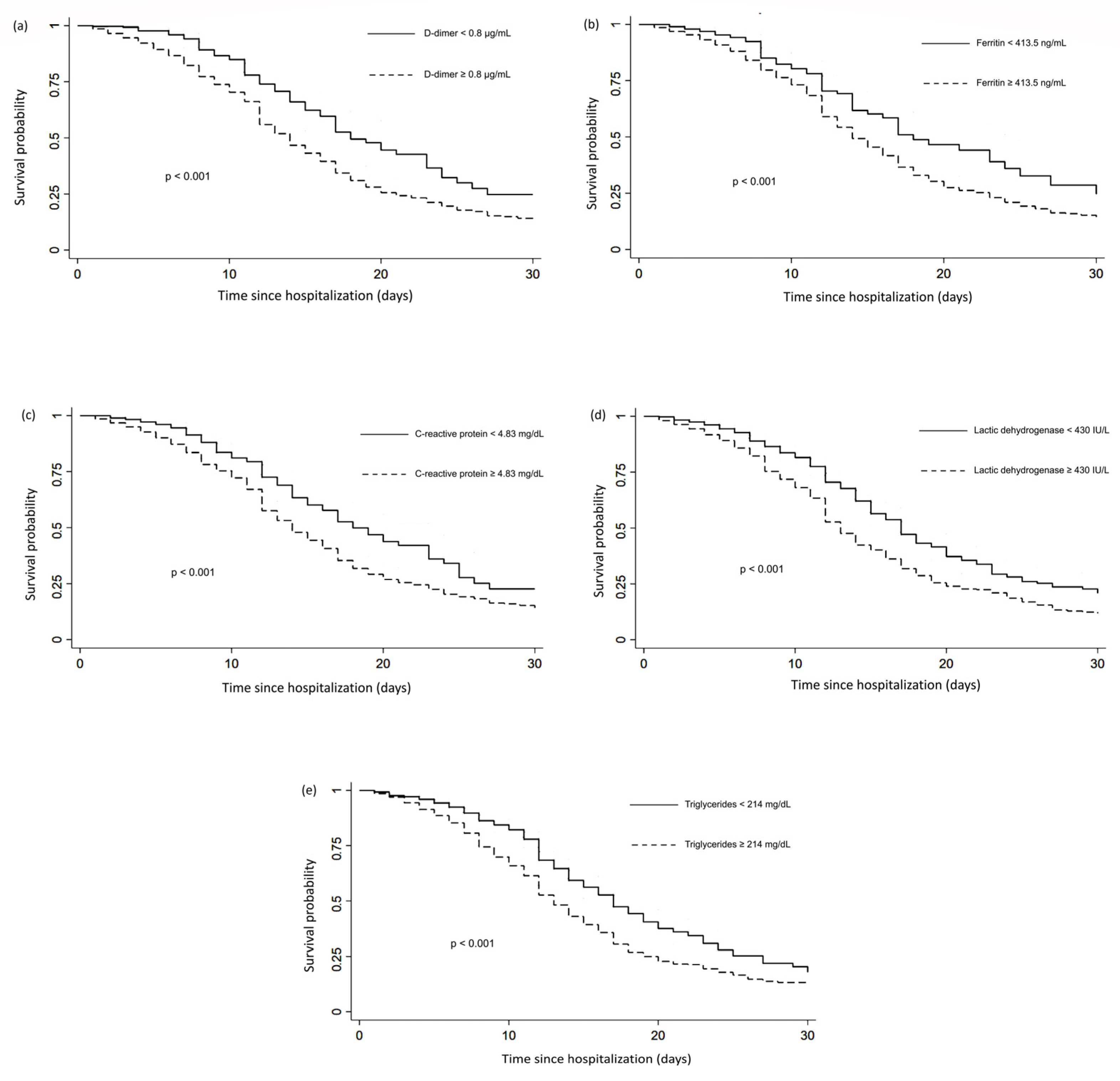
3.3. Cox Proportional Hazards and Kaplan–Meier Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albarrán-Sánchez, A.; Ramírez-Rentería, C.; Anda-Garay, J.C.; Noyola-García, M.E.; Alberti-Minutti, P.; Flores-Padilla, G.; Guizar-García, L.A.; Contreras-García, C.E.; Marrero-Rodríguez, D.; Taniguchi-Ponciano, K.; et al. Differences in mortality rate among patients hospitalized with severe COVID-19 according to their body mass index. Obes. Sci. Pract. 2021, 1–10. [Google Scholar] [CrossRef]
- Vidal-Cevallos, P.; Higuera-De-La-Tijera, F.; Chávez-Tapia, N.C.; Sanchez-Giron, F.; Cerda-Reyes, E.; Rosales-Salyano, V.H.; Servin-Caamaño, A.; Vázquez-Medina, M.U.; Méndez-Sánchez, N. Lactate-dehydrogenase associated with mortality in hospitalized patients with COVID-19 in Mexico: A multi-centre retrospective cohort study. Ann. Hepatol. 2021, 24, 100338. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Bahena-López, J.P.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; González-Díaz, A.; Márquez-Salinas, A.; Aguilar-Salinas, C.A. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J. Clin. Endocrinol. Metab. 2020, 105, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Parra-Bracamonte, G.M.; Lopez-Villalobos, N.; Parra-Bracamonte, F.E. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann. Epidemiol. 2020, 52, 93–98.e2. [Google Scholar] [CrossRef]
- Escobedo-de la Peña, J.; Rascón-Pacheco, R.A.; de Jesús Ascencio-Montiel, I.; González-Figueroa, E.; Fernández-Gárate, J.E.; Medina-Gómez, O.S.; Borja-Aburto, V.H. Hypertension, Diabetes and Obesity, Major Risk Factors for Death in Patients with COVID-19 in Mexico. Arch. Med. Res. 2021, 52, 443–449. [Google Scholar] [CrossRef]
- Johns Hopkins University. COVID-19 Map–Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 May 2022).
- Global Initiative for Chronic Obstructive Lung Disease. GOLD Report 2020. Glob. Initiat. Chronic. Obstr. Lung. Dis. 2020, 1–141. [Google Scholar]
- Global Initiative for Asthma. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention (Updated 2020). Rev. Fr. Allergol. Immunol. Clin. 2020, 36, 685–704. Available online: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf (accessed on 1 April 2022).
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Dos Santos, G.R.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Sunwoo, J.B. Natural Killer Cells and Thyroid Diseases. Endocrinol. Metab. 2019, 34, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Liu, F.; Liu, J.; Cao, G.; Yang, C.; Liu, W.; Tu, C.; Zhu, M.; Xiong, B. COVID-19 patients with hypertension have more severe disease: A multicenter retrospective observational study. Hypertens. Res. 2020, 43, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, B.; Grimm, D.; Krüger, M.; Kopp, S.; Infanger, M.; Wehland, M. SARS-CoV-2 and hypertension. Physiol. Rep. 2021, 9, e14800. [Google Scholar] [CrossRef]
- Chu, C.; Zeng, S.; Hasan, A.A.; Hocher, C.-F.; Krämer, B.K.; Hocher, B. Comparison of infection risks and clinical outcomes in patients with and without SARS-CoV-2 lung infection under renin-angiotensin-aldosterone system blockade: Systematic review and me-ta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 2475–2492. [Google Scholar] [CrossRef]
- AbdelGhaffar, M.M.; Omran, D.; Elgebaly, A.; Bahbah, E.I.; Afify, S.; AlSoda, M.; El-Shiekh, M.; ElSayed, E.S.; Shaaban, S.S.; AbdelHafez, S.; et al. Prediction of mortality in hospitalized Egyptian patients with Coronavirus disease-2019: A multicenter retrospective study. PLoS ONE 2022, 17, e0262348. [Google Scholar] [CrossRef]
- Pinto, L.C.; Bertoluci, M.C. Type 2 diabetes as a major risk factor for COVID-19 severity: A meta-analysis. Arch. Endocrinol. Metab. 2020, 64, 199–200. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Tang, Y.; Cheng, Q. Diabetes increases the mortality of patients with COVID-19: A meta-analysis. Acta Diabetol. 2021, 58, 139–144. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Dunn, D.; Turner, C. Hypothyroidism in Women. Nurs. Womens. Health 2016, 20, 93–98. [Google Scholar] [CrossRef]
- Van Gerwen, M.; Alsen, M.; Little, C.; Barlow, J.; Naymagon, L.; Tremblay, D.; Sinclair, C.F.; Genden, E. Outcomes of Patients with Hypothyroidism and COVID-19: A Retrospective Cohort Study. Front. Endocrinol. 2020, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Damara, F.A.; Muchamad, G.R.; Ikhsani, R.; Syafiyah, A.H.; Bashari, M.H. Thyroid disease and hypothyroidism are asso-ciated with poor COVID-19 outcomes: A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2021, 15, 102312. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Lund, H.; Chen, Y.; Zhang, J.; Osinski, K.; Jones, S.Z.; Kreuziger, L.B.; López, J.A.; Benjamin, I.J.; Silverstein, R.L.; et al. Hypertriglyceridemia during hospitalization independently associates with mortality in patients with COVID-19. J. Clin. Lipidol. 2021, 15, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, J.; He, W.; Chen, L.; Yuan, G.; Dong, F.; Chen, W.; Cao, Y.; Yang, J.; Cai, L.; et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia 2020, 34, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; O’Donnell, M.R. Epidemiology, clinical course, and out-comes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, X.; Wei, Y.; Li, X.; Gong, Q.; Dong, L.; Zhong, J. Laboratory Predictors of COVID-19 Mortality: A Retrospective Analysis from Tongji Hospital in Wuhan. Mediat. Inflamm. 2021, 2021, 6687412. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evidence-Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Chalmers, S.; Khawaja, A.; Wieruszewski, P.M.; Gajic, O.; Odeyemi, Y. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: The role of inflammatory biomarkers. World J. Crit. Care Med. 2019, 8, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Duman, A.; Akoz, A.; Kapci, M.; Ture, M.; Orun, S.; Karaman, K.; Turkdogan, K.A. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: Predictive role of lactate dehydrogenase. Am. J. Emerg. Med. 2016, 34, 2167–2171. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Mu, S.; Wei, W.; Jin, C.; Tong, C.; Song, Z.; Zha, Y.; Xue, Y.; Gu, G. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: A retrospective and observational study. Aging 2020, 12, 11245–11258. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin–from iron, through inflammation and auto-immunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. Available online: https://pubmed.ncbi.nlm.nih.gov/34883281 (accessed on 6 December 2021). [CrossRef] [PubMed]

| Variable | Non-Survivors (n = 653) | Survivors (n = 562) | Differences (95% CI) * | p |
|---|---|---|---|---|
| Age, median years, mean ± SD | 61.21 ± 14.23 | 54.13 ± 15.09 | 7.08 (8.73 to 5.42) | <0.001 a |
| Gender, n (%) | 0.15 b | |||
| Women | 230 (35.22) | 221 (39.32) | −4.1 (−9.5 to 1.3) | |
| Men | 423 (64.78) | 341 (60.68) | 4.1 (−1.3 to 9.5) | |
| Symptoms, n (%) | ||||
| Fever | 452 (69.33) | 348 (61.92) | 7.41 (2.05 to 12.76) | 0.007 b |
| Odynophagia | 219 (33.59) | 234 (41.64) | −8.05 (−13.50 to −2.59) | 0.004 b |
| Chest pain | 263 (40.34) | 266 (47.33) | −6.99 (−12.57 to −1.40) | 0.01 b |
| Asthenia | 425 (65.18) | 354 (62.99) | 2.19 (−3.22 to 7.60) | 0.42 b |
| Myalgia | 341 (52.3) | 313 (55.69) | −3.39 (−9.00 to 2.22) | 0.23 b |
| Headache | 273 (41.87) | 262 (46.62) | −4.75 (−10.34 to 0.84) | 0.09 b |
| Rhinorrhea | 130 (19.94) | 141 (25.09) | −5.15 (−9.86 to −0.43) | 0.03 b |
| Anosmia | 112 (17.18) | 131 (23.1) | −5.92 (−10.44 to −1.39) | 0.008 b |
| Shortness of breath | 520 (79.75) | 385 (68.51) | 11.24 (6.31 to 16.16) | <0.001 b |
| Comorbidities, n (%) | ||||
| COPD | 23 (3.53) | 12 (2.14) | 1.39 (−0.46 to 3.24) | 0.14 b |
| Asthma | 87 (13.32) | 73 (12.99) | 0.33 (−3.48 to 4.14) | 0.86 b |
| T2D | 183 (28.02) | 112 (19.93) | 8.09 (3.31 to 12.86) | <0.001 b |
| Hypertension | 225 (34.46) | 124 (22.06) | 12.4 (7.39 to 17.40) | <0.001 b |
| Heart disease | 35 (5.36) | 20 (3.56) | 1.8 (−0.50 to 4.10) | 0.13 b |
| Nephropathy | 48 (7.35) | 40 (7.12) | 0.23 (−2.68 to 3.14) | 0.87 b |
| Immunodeficiency | 23 (3.52) | 20 (3.56) | −0.04 (−2.12 to −2.04) | 0.79 b |
| Liver disease | 12 (1.84) | 3 (0.53) | 1.31 (0.11 to 2.50) | 0.04 b |
| Hypothyroidism | 20 (3.06) | 2 (0.36) | 2.7 (1.28 to 4.11) | <0.001 b |
| ≥2 comorbidities | 276 (42.27) | 129 (22.95) | 19.32 (14.17–24.4) | <0.001 b |
| Biochemical markers | ||||
| Fasting plasma glucose, mg/dL median (IQR) | 135 (106–206) | 113 (95–162) | 22 (13 to 30) | <0.001 c |
| D-dimer, μg/mL median (IQR) | 2.07 (1.05–5.97) | 1.01 (0.56–2.54) | 1.06 (0.75 to 1.36) | <0.001 c |
| Lactic dehydrogenase, median IU/L (IQR) | 512 (362–655) | 374.5 (278–503.5) | 137.5 (110 to 161) | <0.001 c |
| Ferritin, ng/mL median (IQR) | 1275 (709.1–1622) | 977.7 (448–1395.94) | 297.3 (174.64 to 419.55) | <0.001 c |
| C-reactive protein, mg/dL median (IQR) | 14.2 (7.58–21.82) | 9.49 (2.63–14.2) | 4.71(3.48 to 5.83) | <0.001 c |
| Triglycerides, mg/dL median (IQR) | 226 (163–277) | 190.5 (136–226) | 35.5 (27 to 43) | <0.001 c |
| Variable | Crude HR | 95% CI | p | Adjusted HR * | 95% CI | p |
|---|---|---|---|---|---|---|
| Age < 40 years | 0.65 | 0.48–0.88 | 0.007 | - | - | - |
| Age ≥ 65 years | 1.30 | 1.12–1.52 | 0.001 | 1.1 | 0.93–1.30 | 0.22 |
| Male gender | 1.02 | 0.87–1.20 | 0.74 | 1 | 0.84–1.19 | 0.97 |
| Shortness of breath | 1.25 | 1.03–1.51 | 0.021 | 1.07 | 0.87–1.31 | 0.4 |
| T2D | 1.04 | 0.88–1.24 | 0.58 | 0.92 | 0.76–1.11 | 0.42 |
| Hypertension | 1.17 | 0.99–1.37 | 0.52 | 1.14 | 0.95–1.36 | 0.14 |
| Hypothyroidism | 1.86 | 1.19–2.91 | 0.006 | 1.91 | 1.08–3.39 | 0.02 |
| COPD | 1.17 | 0.77–1.77 | 0.45 | 0.92 | 0.56–1.51 | 0.74 |
| Asthma | 1.08 | 0.86–1.35 | 0.48 | 0.93 | 0.72–1.18 | 0.56 |
| CKD | 0.88 | 0.66–1.18 | 0.41 | 0.73 | 0.52–1.03 | 0.7 |
| Immunodeficiency | 1.09 | 0.72–1.65 | 0.67 | 1.13 | 0.7–1.84 | 0.6 |
| Heart disease | 1.45 | 1.03–2.05 | 0.03 | 1.42 | 0.97–2.09 | 0.06 |
| Liver disease | 1.09 | 0.62–1.94 | 0.74 | 0.88 | 0.43–1.81 | 0.74 |
| D–dimer ≥ 0.8 μg/mL | 1.75 | 1.38–2.21 | <0.001 | 1.38 | 1.07–1.77 | 0.01 |
| Lactic dehydrogenase ≥ 430 IU/L | 1.56 | 1.33–1.83 | <0.001 | 1.33 | 1.12–1.57 | 0.001 |
| Ferritin ≥ 413.5 ng/mL | 1.51 | 1.17–1.94 | 0.001 | 1.23 | 0.94–1.62 | 0.12 |
| CRP ≥ 4.83 mg/dL | 1.57 | 1.26–1.95 | <0.001 | 1.40 | 1.11–1.76 | 0.004 |
| Triglycerides ≥ 214 mg/dL | 1.54 | 1.31–1.80 | <0.001 | 1.38 | 1.17–1.63 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Gameros, C.A.; Vargas-Ortega, G.; Rendón-Macias, M.E.; Cuevas-García, C.F.; Colín-Martínez, T.; Sánchez-Hurtado, L.A.; Balcázar-Hernández, L.J.; De la Cruz-Rodríguez, I.E.; Pérez-Dionisio, E.K.; Retana-Torres, P.M.; et al. Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center. J. Clin. Med. 2022, 11, 2780. https://doi.org/10.3390/jcm11102780
Romero-Gameros CA, Vargas-Ortega G, Rendón-Macias ME, Cuevas-García CF, Colín-Martínez T, Sánchez-Hurtado LA, Balcázar-Hernández LJ, De la Cruz-Rodríguez IE, Pérez-Dionisio EK, Retana-Torres PM, et al. Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center. Journal of Clinical Medicine. 2022; 11(10):2780. https://doi.org/10.3390/jcm11102780
Chicago/Turabian StyleRomero-Gameros, Carlos Alfonso, Guadalupe Vargas-Ortega, Mario Enrique Rendón-Macias, Carlos Fredy Cuevas-García, Tania Colín-Martínez, Luis Alejandro Sánchez-Hurtado, Lourdes Josefina Balcázar-Hernández, Iván Emilio De la Cruz-Rodríguez, Enid Karina Pérez-Dionisio, Perla Michelle Retana-Torres, and et al. 2022. "Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center" Journal of Clinical Medicine 11, no. 10: 2780. https://doi.org/10.3390/jcm11102780
APA StyleRomero-Gameros, C. A., Vargas-Ortega, G., Rendón-Macias, M. E., Cuevas-García, C. F., Colín-Martínez, T., Sánchez-Hurtado, L. A., Balcázar-Hernández, L. J., De la Cruz-Rodríguez, I. E., Pérez-Dionisio, E. K., Retana-Torres, P. M., García-Montesinos, E. S., López-Moreno, M. A., Intriago-Alor, M., Waizel-Haiat, S., & González-Virla, B. (2022). Risk Factors Associated with Mortality among Patients with COVID-19: Analysis of a Cohort of 1213 Patients in a Tertiary Healthcare Center. Journal of Clinical Medicine, 11(10), 2780. https://doi.org/10.3390/jcm11102780






