1. Introduction
Regular physical activity has many beneficial effects with regard to the cardiovascular system: it induces a physiological remodeling of the heart and results in molecular and cellular adaptive changes, which themselves have a cardioprotective effect. The long-term and moderate practice of sports, especially aerobic–isotonic ones, are even encouraged in those with chronic cardiac diseases [
1]. Even in the older population, regular physical activity has been shown to slow degenerative cardiac changes and significantly improve quality of life [
2].
Concerning young athletes, there are both morphological and functional adaptations of the heart in relation to sustained and intense physical activity. Morphologically, the myocardium suffers a process of hypertrophy and the chambers become dilated, as to adapt to the increased hemodynamic stress. The main difference between these physiological adaptative changes and the pathological ones, which are encountered in chronic cardiac diseases, lies in the fact that hemodynamic stress is intermittent in physical activity, whereas in chronic cardiac disease it is continuous. These structural modifications, especially the enlargement of the chambers, together with the increased vagal tonus, are the ones that, in turn, lead to electrophysiological modifications [
3].
Most of the electrophysiological changes, in the context of constant physical activity, which have an electrocardiographic (ECG) expression, can be interpreted as within the normal limits without warranting further investigation. However, they are not to be confused with pathological ECG patterns, which have an underlying cardiac pathology and which can act as triggers for adverse events, such as sudden cardiac death [
4,
5] (see
Table 1).
The 2020 European Society of Cardiology (ESC) guidelines on sports cardiology and exercise in patients with cardiovascular disease are a milestone in both the field of cardiology and sports medicine. As the first edition, they introduce numerous up-to-date information and recommendations for the practice of physical exercise in both healthy individuals and persons with known cardiovascular pathologies. Among the potential adverse cardiovascular events, the two most dangerous are sudden cardiac arrest (SCA) and sudden cardiac death (SCD). As tragic as they may be, especially if occurring in young healthy individuals, SCA and SCD are not reported in all countries; therefore, their incidences cannot be accurately estimated. However, according to most recent statistics, SCA is diagnosed in 1 out of 80,000 high-school-aged athletes and in 1 out of 50,000 college-aged athletes. Based on gender, ethnicity, and type of sports practiced, the most at-risk group is represented by male, black, basketball athletes in the United States, or football athletes in Europe [
6,
7,
8,
9,
10,
11] (see
Table 2).
The most important etiology of SCD in young athletes is congenital cardiac structural disorders; yet, a significant number of autopsies (44%) cannot identify a cause, these being the autopsy-negative sudden unexplained deaths (AN-SUD) [
6,
7,
12,
13,
14,
15].
The current guidelines recommend, for the routine screening for cardiovascular disease in young athletes, the patient’s history, a physical examination, and a 12-lead standard ECG, which actually outweighs the first two. Although the cardiac ultrasound could offer significantly more information, especially regarding the congenital anomalies, a lack of evidence restricts its implementation in the standard screening protocols [
6,
16,
17,
18,
19,
20,
21,
22].
There are a few methods that allow the assessment of cardiorespiratory fitness (CRF) in youths which are currently used, such as the 20-m shuttle run test (20mSRT). It is a feasible, reliable, and easy to put in practice method of evaluation, and is currently being discussed to be included in the standardized evaluation protocols [
23].
Given the wide spectrum of parameters evaluated (pulmonary, cardiovascular, muscular and oxidative) and the multitude of data and correlations it provides, the cardiopulmonary exercise testing (CPET) is an essential tool in current practice [
24]. It is also useful in healthy individuals and in the athletic performance assessment, as maximal oxygen uptake (VO
2 max) is directly linked to the exercise capacity [
25,
26]. Of course, normal parameters vary, as elite aerobic athletes can reach VO
2 max values over 80 mL/kg/min, compared to untrained individuals who are in the 30–45 mL/kg/min range [
27]. The test can be performed on a treadmill (mostly in the United States) or on the cycle ergometer (mostly in Europe). The cycle ergometer testing has a low associated cardiovascular risk in evaluating young healthy athletes [
28,
29] (see
Table 3).
A particular role of CPET in evaluating athletes is to differentiate between their physiological left-ventricular hypertrophy (LVH) and hypertrophic cardiomyopathy (HCM) [
30,
31].
Table 3.
CPET indications (adapted from Löllgen et al. [
31]).
Table 3.
CPET indications (adapted from Löllgen et al. [
31]).
| CPET Indications | References |
|---|
| Asymptomatic individuals | Patients | Follow-up assessment during training | [31] |
Latent disease diagnosis Risk factor identifications Physical performance ability assessment Guidance/monitorization of training
| | |
Cardiac troponins (cTn) are regulatory components of the cardiac muscle contraction. Cardiac troponin I (cTnI) is of interest due to its phosphorylation sites, which regulate different contractile responses [
32,
33]. It has been shown that physiological left ventricular hypertrophy, which is specific to trained persons, causes a reversible increase in Ca
2+-dependent force production and in Ca
2+-sensitivity in left ventricular (LV) cardiomyocytes. Together with a reduction in cTnI phosphorylation, it is suggestive for an adaptive measure and for the preserved or even increased contractile function, despite the morphology changes [
34]. In contrast, untrained persons who are subjected to a sudden physical effort do register increases in cTnI levels, although they are not pathological [
35]. Additionally, of interest is the fact that peak levels of hs-cTn are registered between 3 and 4 h after peak physical stress [
36].
Myoglobin is a biomarker produced by both the myocardial and skeletal muscle cells. Therefore, its levels rise in cardiac diseases (myocarditis, acute coronary syndromes) as well as muscular stress or strenuous physical effort. It can aid the differential diagnosis of cardiac or muscular damage, whether its levels rise independently or in conjunction with other cardiac biomarkers. Its use in establishing the diagnosis of acute coronary syndrome is low in the absence of other biomarkers’ elevation (CK-MB, cTn); however, it is the one whose levels rise earliest in the serum [
37,
38,
39].
The MB isoenzyme of creatine-kinase (CK-MB) is specific to the cardiac muscle. On its own it cannot predict the cardiovascular risk accurately, unless it is associated with the rise of cTn; thus, its use is still being debated. Its practical utility resides in situations where the estimated glomerular filtration rate (eGFR) is below 15 mL/min/m
2, or in recent-onset acute coronary syndromes [
40,
41].
The N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels do increase during intense physical exercise, especially in non-trained, amateur athletes. Yet, when not correlated with ECG changes, they are not significant for a cardiovascular pathology [
35]. Its release in the blood is more associated with aging and with the effort-generated hypertensive stress on the ventricular and atrial walls [
42,
43,
44]. Of course, it is essential in diagnosing potential ischemic changes in the myocardium, especially in conjunction with cTn, and with proven benefit in risk stratification in athletes [
45,
46,
47].
While not being specific markers of myocardial cytolysis, D-dimers levels reflect intravascular fibrinolysis and show higher concentrations in patients with severe atherosclerosis or other causes of coronary stenosis [
48]. They have more use in confirming or infirming venous thromboembolism, acute aortic dissection, cardioembolic stroke, or left atrium thrombosis in patients with atrial fibrillation [
49]. Additionally, an independent rise in their levels can be specific in persons with implantable cardiac devices [
50].
The aim of our study was to assess the relationship between effort-induced cardiac biomarker variations and CPET parameters, and their usefulness in the evaluation of young athletes.
2. Materials and Methods
2.1. Experimental Approach
We conducted a complete cardiovascular evaluation of professional football players, with an emphasis on cardiopulmonary exercise testing (CPET) and blood biomarkers (cTnI, myoglobin, CK-MB, NT-proBNP and D-dimers) in order to evaluate and assess the cardiovascular risk. The study was designed following the recommendations for clinical research contained in the Helsinki Declaration of the World Medical Association, and the protocol was approved by both the Ethics Committee of the Clinical Rehabilitation Hospital in Iași, Romania, approved on 24 March 2021, and the Ethics Research Committee of the “Grigore T. Popa” University of Medicine and Pharmacy in Iași, Romania, nr. 72, approved on 25 April 2021.
2.2. Participants and Protocol
We evaluated professional football players (n = 19). Football club trainers from the region of Moldova in the counties of Iași and Vaslui were informed of the ongoing study and were given our contact data. They themselves informed the registered football players aged between 18 and 20 years, and the first 30 to voluntarily contact us were automatically selected to be evaluated. In order for them to be included, they had to participate actively in regular training during the season of the last year, or at least the last months. Those who had missed part of the last months of training or playing due to injury, or who were in the recovery period after an injury of any sort, were excluded, as well as those with cardio-pulmonary pathological findings during the initial evaluation.
The participants were all male, aged between 18 and 20 years old (mean 18.47 ± 0.841), and fully informed about the procedures, research, and protocols used. They were asked to halt any strenuous training sessions or games a minimum of 24 h before the evaluation, and not to consume any foods or beverages other than water before the initial blood sampling, so as not to interfere with the blood sugar values. Afterwards, they could have a small snack or light meal at least an hour before the CPET. They were admitted through day hospitalization in August and September 2021. Upon admission, they filled out the informed consent forms regarding all the procedures and their inclusion in the study, and underwent rapid COVID-19 antigen testing, which, if negative, would allow them to further proceed with the investigations. They underwent a clinical and paraclinical evaluation with an emphasis on the cardiovascular system, followed by a 12-lead resting ECG, TTE, and CPET. Blood samples for the measurements of cardiac biomarkers values were taken at rest before the procedures and 3 h after finishing the CPET.
2.3. Initial Evaluation
After the initial blood samples were taken, a complete clinical evaluation was conducted with an emphasis on the cardiovascular system with its four major components (inspection, palpation, percussion and auscultation). The resting blood pressure (BP) and heart rate (HR) values were measured comparatively on the both upper limbs and in the orthostatism using a Rossmax X3 BT automatic blood pressure monitor with a brachial cuff. Height and weight were measured on a SECA digital measuring station.
2.4. Resting ECG
A standard 12-lead resting ECG was performed on a BTL-08 LC device, both in post-expiratory apnea and in deep post-inspiratory apnea, and every morphological change was analyzed to be considered as either normal for an athlete or pathological (see
Table 1), and possibly contraindicate further procedures.
2.5. Cardiac Ultrasound
The standard transthoracic echocardiographic evaluation was performed on a Toshiba Aplio device, prior to the CPET, to assess the cardiac function and to exclude any possible contraindications, using the M-mode, pulse wave Doppler (PWD), continuous wave Doppler (CWD), and color Doppler methods, according to the European Society of Cardiology (ESC) and European Association of Cardiovascular Imaging (EACVI) protocols.
2.6. Cardiopulmonary Exercise Testing
Functional capacity was assessed by cardiopulmonary exercise testing (CPET) on the BTL CardioPoint software (version 2.32 manufactured by BTL Industries Ltd., Herfordshire, UK) and the BTL-compatible device. We used a progressive maximal symptom-limited CPET protocol on the cycle ergometer, specifically tailored for the athletes: they started on a workload of 15 Watts which was set to increase every 30 s with 12.5 Watts. The duration of the testing was between 10 and 12 min and the recovery period was 10 min.
The most important CPET parameters were: maximal work rate (absolute value, WR (Watt) and percentage of the predicted value, WR% (%)); oxygen uptake with maximal aerobic capacity (absolute value, VO2 max (mL per min) and percentage of the predicted value, VO2 max%); carbon dioxide output (VCO2 (mL per min)); oxygen uptake at the anaerobic threshold (AT) (mL per min); peak value of the respiratory exchange ratio (RER) defined as the ratio between VCO2 and VO2; maximal heart rate (HR (bpm)); O2 pulse as the ratio of VO2 to heart rate, reflecting the amount of O2 extracted per heartbeat; ventilatory efficiency expressing the rise in minute ventilation (VE) relative to VCO2 (VE/VCO2 slope); and heart rate reserve (difference between maximal HR and resting HR, HRR (bpm)). VO2, VCO2, and AT were also expressed as values normalized by body weight (mL per min per km).
Metabolic efficiency was assessed by measuring the increase in VO2 over the rate of increase in work rate (ΔVO2/ΔWR). The slope of this relationship expresses the ability of the muscle to extract O2 during exercise.
Blood pressure was monitored every 2 min using the auscultatory method, while a real-time 12-lead ECG was recorded.
To clinically determine the intensity of the exercise, a subjective rating of the intensity of perceived fatigue was determined by a 6 to 20 Borg scale of perceived exertion.
The test was halted, according to current recommendations, when the subject requested it, upon symptoms or fatigue occurrence, when the blood pressure (BP) measurement exceeded 220 mmHg for the systolic value, or 120 mmHg for the diastolic value, or when suggestive ischemic ECG patterns appeared.
2.7. Cardiac Biomarker Determination
Peripheral venous blood was collected at rest for the basic laboratory parameters and also to determine the mentioned biomarker values at rest. Three hours after finalizing the CPET, another sample was taken to measure the variations of the biomarker values after the stress test, so as to allow them time to appear and reach certain levels in the blood. All blood sampling was taken from the antecubital veins and in kept dedicated vacutainers (with sodium citrate for D-dimers and lithium heparin for CK-MB, Myo, cTnI, and NT-proBNP). Their measurements were performed immediately after, using whole blood on the FIA 8000 device from Getein Biotechnology Co. Ltd. on its dedicated panels: triple tests for CK-MB, myoglobin, and cTnI (CK-MB: measuring range 2.5–80 ng/mL, lower detection limit ≤ 2.5 ng/mL, recovery 96%; myoglobin: measuring range 30–1000 ng/mL, lower detection limit ≤ 30 ng/mL, recovery 95%; cTnI: measuring range 0.5–50 ng/mL, lower detection limit ≤ 0.5 ng/mL, recovery rate 95%, measuring time 15 min), double tests for cTnI and NT-proBNP (cTnI: measuring range 0.5-50 ng/mL, lower detection limit ≤ 0.5 ng/mL, recovery rate 95%; NT-proBNP: measuring range 10–12,000 pg/mL, lower detection limit ≤ 100 ng/mL, recovery 99%, measuring time 18 min) and a single test for D-dimers (D-dimers: measuring range 0.1–10 mg/L, lower detection limit ≤ 0.1 mg/L, recovery rate 99%, measuring time 7 min) were completed. All the assays were stored, according to manufacturer specifications, at a temperature between 4 and 30 degrees Celsius and used before the expiration date was reached and within 1 h since each foil was opened.
2.8. Data analysis and Statistics
Data analysis was performed using SPSS 20.0 (Statistical Package for the Social Sciences, Chicago, IL, USA). Data were presented as mean ± standard deviation (SD), or as median with an interquartile range for continuous variables. These variables were compared by the non-parametric Mann–Whitney U test, considering that their distribution did not satisfy the assumption of normality, due to the small number of subjects included in the study. For the same reason, correlations between continuous variables were assessed by calculating the Spearman correlation coefficients. A two-sided p-value < 0.05 was considered significant for all analyses.
4. Discussion
Laboratory explorations are gaining more and more ground and sports cardiology represents the new trend in medicine. Therefore, as confirmed by previous studies, their combination is an opportunity for future research [
51].
The initial evaluation showed findings consistent with athletes with a normal BMI. In the few cases where BMI was over 25 kg/m2, this was due to the higher muscle mass, as the abdominal circumference was normal. ECG interpretation also showed heart rate and morphology patterns which are considered normal in an athletic population as part of the effort adaptation process (sinus bradycardia, right bundle branch block). The cardiac ultrasound highlighted parameters of increased cardiovascular performance, such as a higher ejection fraction and a mild left ventricular hypertrophy, which, once again, is part of the adaptation to effort process in athletes.
In addition, cardiorespiratory functional evaluations are becoming more used in current practice and screening. As obesity and the sedentary lifestyle are becoming more prevalent, especially among the young population, cardiorespiratory functional evaluations are useful tools for assessing the fitness level. In recent studies, CRF has been proven to correlate negatively with parameters such as BMI and the sedentary lifestyle at younger ages [
52].
A study published by Olekšák et al. involved the evaluation of CPET on young Slovenian footballers. They compared the CPET parameters on children and adolescents and concluded that some of them were physiologically higher in athletes and with growing age (VO
2 max, Watt max) [
53].
The CPET parameters evaluated on the 19 subjects were comparable with those in other studied athlete cohorts. It is interesting to note that some of the registered values, such as a lower VO2 max than predicted, were consistent with the tests which were halted for high BP values. This once again proves the utility of this particular evaluation and shows how physical performance CRF can be limited by an abrupt rise in BP.
Other investigations whose utility has been confirmed in recent studies include biomarker measurements. In a paper published by Mahanty et al., cTn, BNP and hypoxanthine were proven as means of assessing the cardiovascular impact of intense physical activity. Therefore, they are being considered to be implemented in the future as part of screening protocols [
54].
A metanalysis published in 2015 concluded that cTnT, hs-cTnT, BNP, NT-proBNP, and D-dimers do suffer serum level changes when a person is performing a high-intensity physical effort, which may interfere with their interpretation in an emergency unit when an acute coronary syndrome, heart failure or pulmonary embolism can be suspected [
55].
When investigating young football players, after a full-time football match, cTnI and NT-proBNP levels rose above the baseline and remained elevated even 24 h after the game, yet they never reached pathologically significant values. This rise, compared to our study can be attributed to the higher intensity and duration of the football match (90 min, on average) compared to a CPET [
56]. Similarly, blood samples taken from participants in the 2016 Barcelona marathon showed an increase in NT-proBNP levels; however, the intensity of the physical stress and its duration were considerably higher than during a CPET [
44].
Another study published in 2021 included individuals that participated in 2018 in the North Sea Race, a 91 km leisure sport mountain bike race. Prior to the race, they performed a CPET. Their cTnI levels were measured before, 3 h, and 24 h after both the race and CPET. The peak values were reached at the 3-h mark, though it should be once again mentioned that, in both cases, the duration and intensity of the physical exercise were higher than in the CPET which we conducted. However, a noticeable inter-individual variation was also observed [
57].
There are several mechanisms incriminated for this pattern of variation in biomarker blood levels, such as microvascular ischemia, deficiencies in cardiac metabolism, a systemic inflammatory surge, or even an impaired renal function during intense exercise [
58]. This particular dynamic of cTnI concentrations, with an early peak followed by a rapid normalization within hours (maximum 48–72 h), renders an active myocyte necrosis highly improbable, and rather suggests the above-mentioned secondary mechanisms. In our study, including highly trained athletes, we did not observe any variation of cTnI or NT-proBNP, compared to baseline. These somehow atypical kinetics can be explained by the rather short duration of the CPET, performed by apparently healthy, well-trained individuals, without the deleterious effects of prolonged and exhausting sports that can presumably represent important triggers for the release of cardiac biomarkers. This finding is consistent with the results of Marshall et al., who recently highlighted a similar pattern of troponin fluctuation, with significant variations compared to baseline occurring only in subjects who performed a moderate or intense training regimen. Very interestingly, the same authors noted that a shorter duration of high-intensity exercise induced a more important increase in troponin compared to prolonged, but less intensive, training [
59]. Basically, these heterogeneous patterns outline the importance of the duration, intensity, and type of training when assessing cardiac biomarkers. A promising future scenario also assumes the use of novel cardiac biomarkers, such as the soluble suppression of tumorigenesis-2 (sST2) for the early detection of subclinical myocardial injury during sports. Being a marker of increased myocardial strain, fibrosis, and neurohormonal activation, sST2 exhibits a superior prognosis value compared to NT-proBNP or cTnI in patients presenting acute myocardial injury [
60,
61].
Recent studies have shown that cardiac biomarkers have an important negative predictive value. Thus, low or undetectable hs-cTnI levels can help exclude an inducible myocardial ischemia, in both patients with known coronary artery disease (CAD) and patients without [
62,
63,
64].
Comparing male and female football players who had CK-MB values measured before, immediately after, and 15 min after a running training session, researchers described a slight increase in CK-MB values immediately post-exercise, most notably in the women’s group, yet these values returned to baseline at the 15 min timepoint. The groups comprised both genders and the measurements were performed at different timepoints (immediately after and 15 min after). This study was comparable to ours with regard to the type of sports practiced and the number of participants [
65].
Another study measured myoglobin and CK levels immediately after, at the 24 h, 48 h and 72 h timepoints after a high-intensity intermittent running protocol. The results showed a higher increase in myoglobin levels and a more modest one in CK levels, with a return to the baseline values within 24 h for both parameters [
66].
On a longer time span, CK and myoglobin levels were measured during a 12-day training period, with blood samples taken prior, at the 6-day mark, and on the 12th day at the end of the training period. CK values peaked on the 6th day, with a drop afterward, while myoglobin peaked on the 12th day [
67].
By combining the results of both the cardiac biomarker measurements and of the CPET we can observe how biomarkers are also useful in the assessment of CRF, as they are released into the bloodstream when the cardiovascular stress is at higher values.
The main limitation of our study is represented by the small number of participants, which was not sufficient for more complex statistical analysis tests and could not offer sufficient data for further correlations (see
Appendix A).
However, given the low number of publications and existing studies of this design, which combines the complete cardiovascular evaluation of athletes starting from the history, physical examination, 12-lead ECG and cardiac ultrasound and focusing on the combination of the CPET and cardiac biomarker measurements, it is definitely a starting point for further research and future studies. This is also supported by the significant statistical results which were obtained.
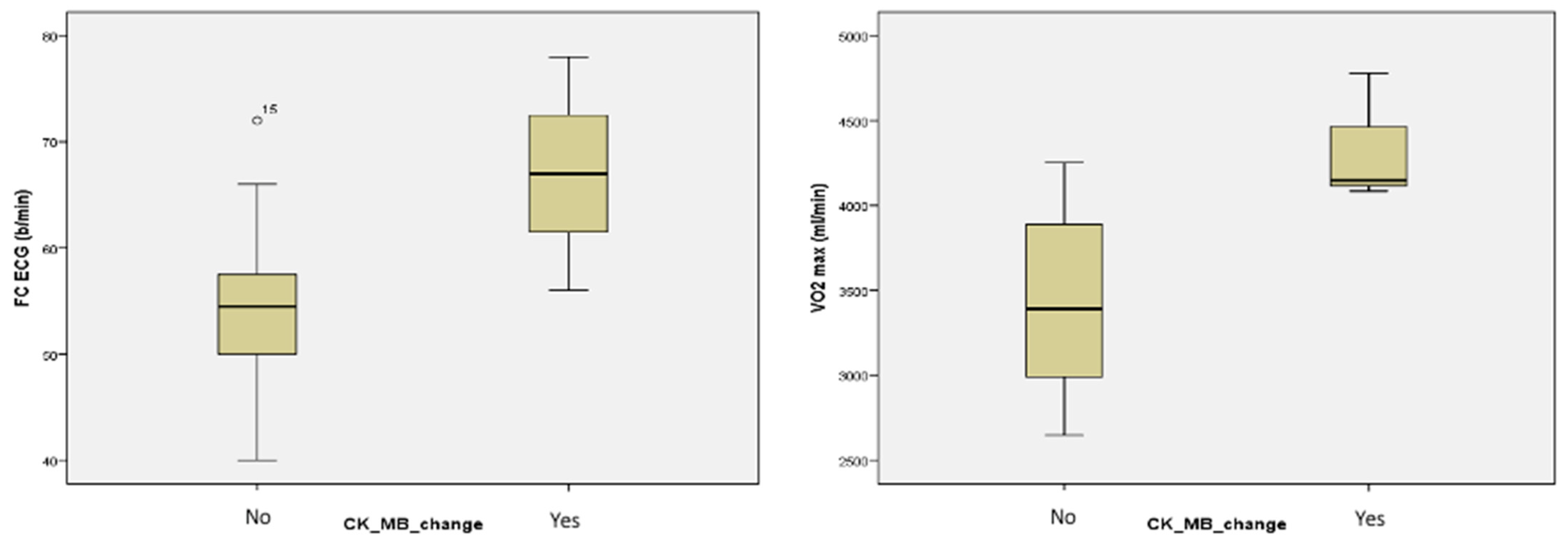
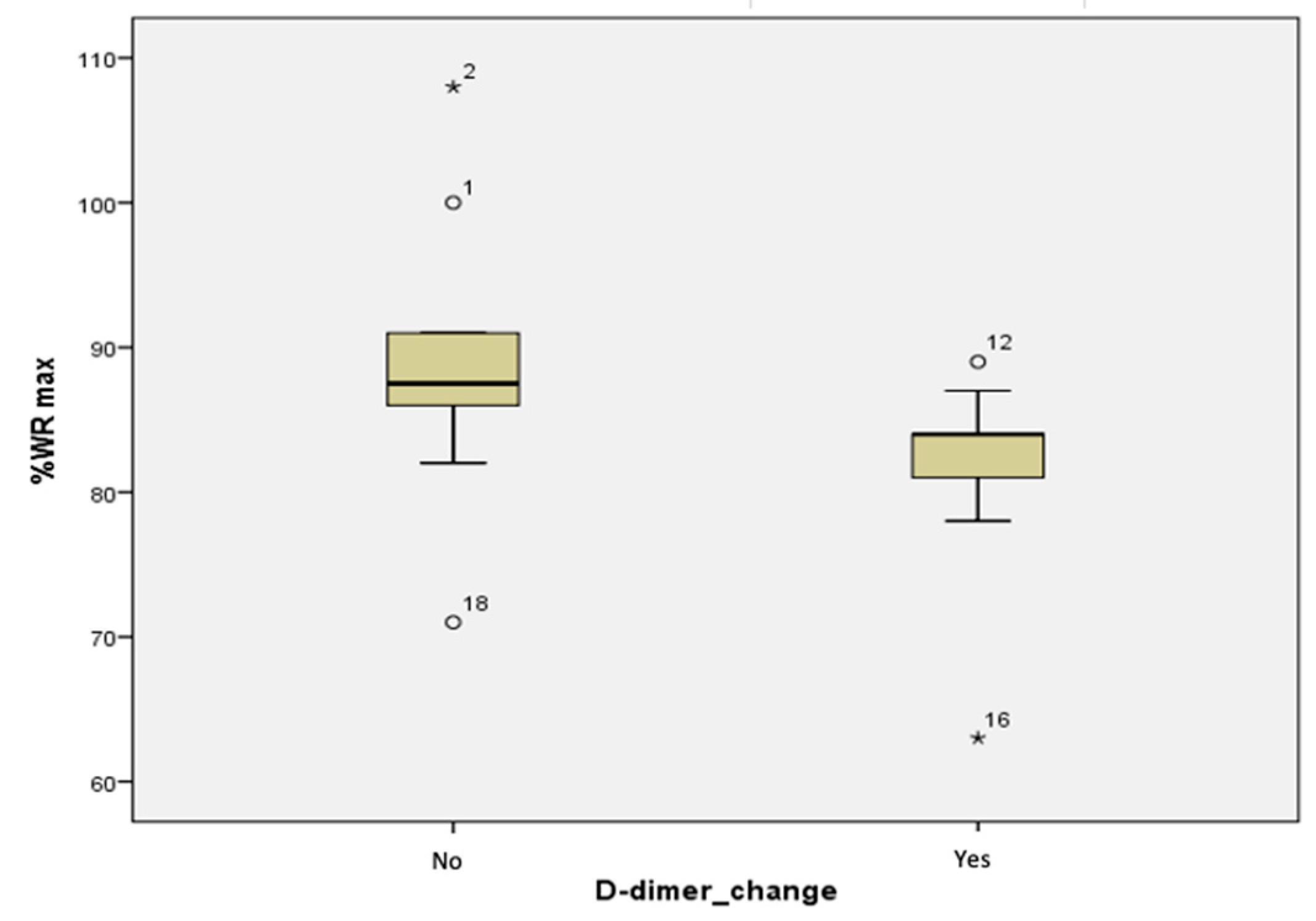
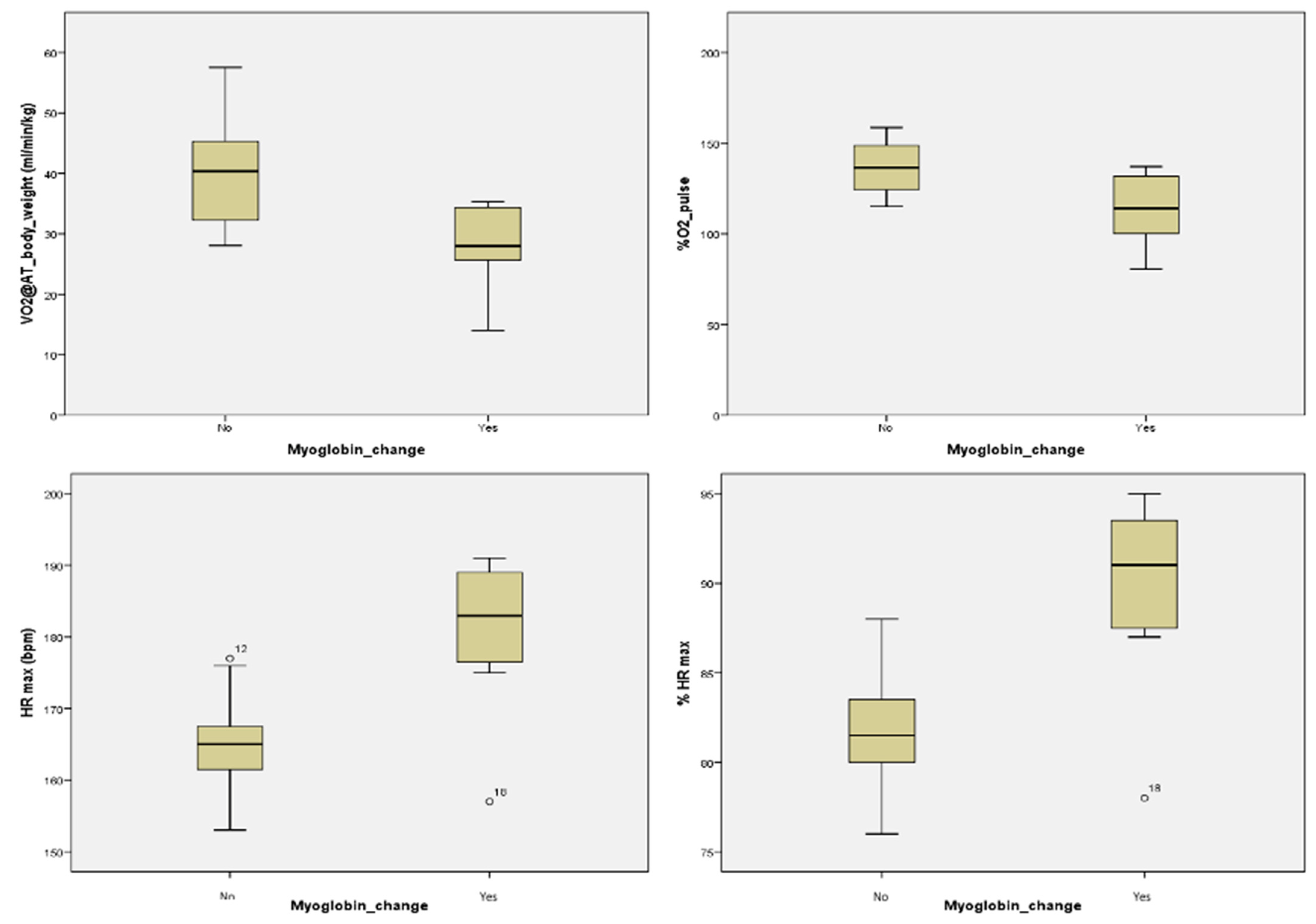
Our study offers a more complete approach than other studies, with the combination of CPET and biomarker measurements, and the established correlations so far encourage future research on larger groups, even though the biomarkers which suffered blood level changes (CK-MB, myoglobin, D-dimers) were not specific on their own for coronary diseases [
37,
38,
39,
40,
41,
48]. This association of CPET and biomarkers is also useful to be implemented in cardiac and respiratory rehabilitation evaluations, as shown by a 2021 study conducted by Wang et al., where the improvement of CHF patients’ parameters was monitored using these dynamics [
68]. Apart from a higher number of subjects, serial measurements of cardiac biomarkers at more timepoints, especially at 12 and 24 h, would offer more indication of their full dynamics in relation to induced physical stress.