The Impact of Different Ventilatory Strategies on Clinical Outcomes in Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Groups
2.2. Outcomes
2.3. Data Collection
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Overall COVID-19 Population
3.2. Characteristics of the COVID-19 Population According to the Type of Ventilatory Support
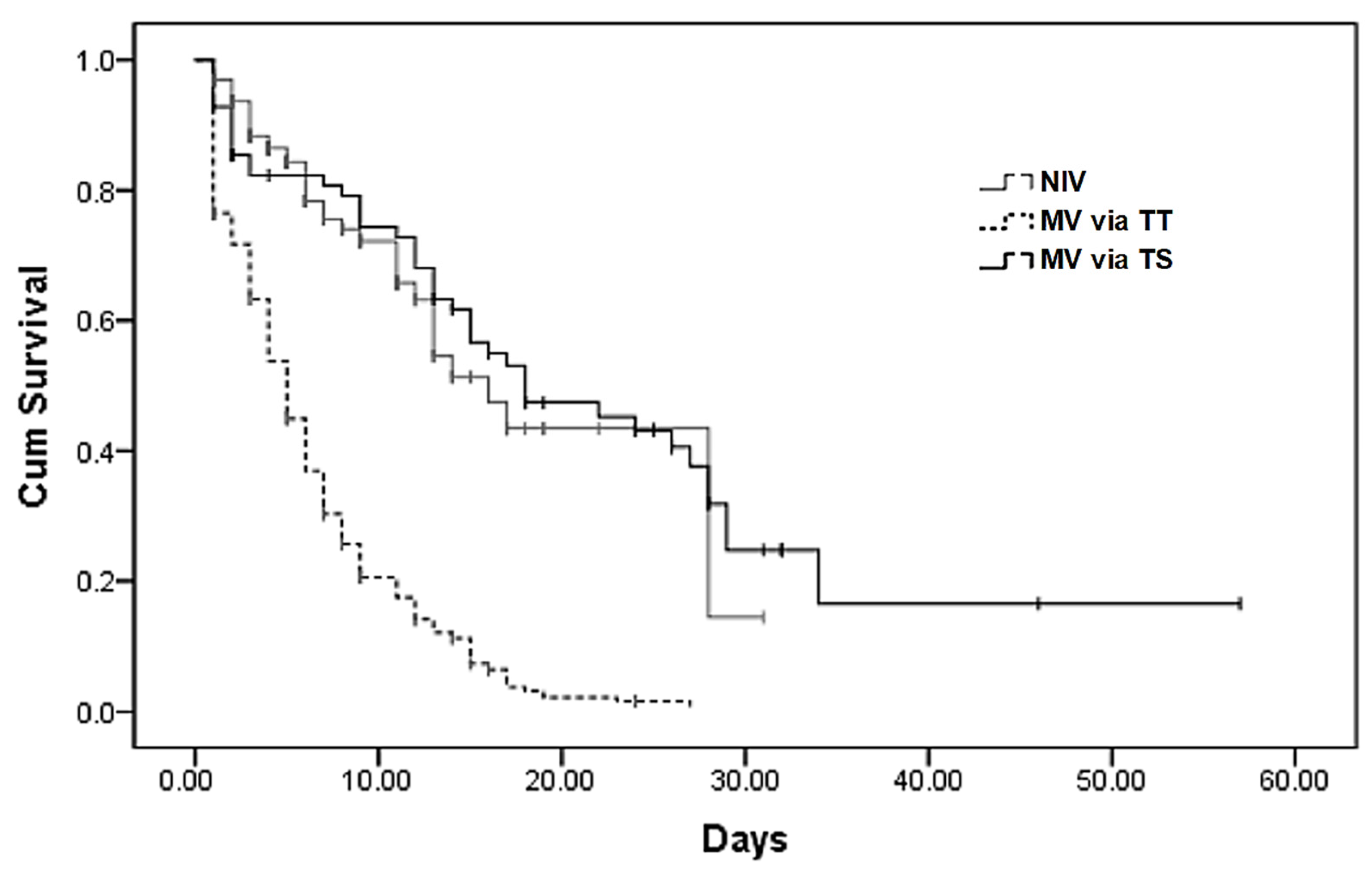
3.3. Impact of Different Ventilator Strategies on Mortality
3.4. Impact of Different Ventilator Strategies on ICU Length of Stay
3.5. Impact of the Timing of Airway Management on Mortality
3.6. Risk Factors Associated with Mortality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| ARDS | Acute respiratory distress syndrome |
| BiPAP | Bilevel positive airway pressure |
| BMI | Body mass index |
| CI95 | 95% confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| ICU | Intensive care unit |
| IQR | Interquartile ratio |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| MODS | Multiple organ dysfunction syndrome |
| MV | Mechanical ventilation |
| NIV | Non-invasive ventilation |
| OR | Odds ratio |
| P-SILI | Patient self-inflicted lung injury |
| PE | Pulmonary embolism |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SOFA | Sequential organ failure assessment |
| TS | Tracheostomy |
| TT | Tracheal tube |
References
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Wkly 2020, 2, 113–122. Available online: https://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2020.032 (accessed on 16 January 2022). [CrossRef]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortegiani, A.; Russotto, V.; Antonelli, M.; Azoulay, E.; Carlucci, A.; Conti, G.; Demoule, A.; Ferrer, M.; Hill, N.S.; Jaber, S.; et al. Ten important articles on noninvasive ventilation in critically ill patients and insights for the future: A report of expert opinions. BMC Anesthesiol. 2017, 17, 122. [Google Scholar] [CrossRef] [Green Version]
- Benito, D.A.; Bestourous, D.E.; Tong, J.Y.; Pasick, L.J.; Sataloff, R.T. Tracheotomy in COVID-19 Patients: A Systematic Review and Meta-analysis of Weaning, Decannulation, and Survival. Otolaryngol. Head Neck Surg. 2021, 165, 398–405. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Ball, L.; Silva, P.L.; Cruz, F.F.; Pelosi, P.; Rocco, P.R. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: A narrative review. Br. J. Anaesth. 2021, 127, 353–364. [Google Scholar] [CrossRef]
- Ball, L.; Robba, C.; Herrmann, J.; Gerard, S.E.; Me, Y.X.; Pigati, M.; Berardino, A.; Iannuzzi, F.; Battaglini, D.; Brunetti, I.; et al. Early versus late intubation in COVID-19 patients failing helmet CPAP: A quantitative computed tomography study. Respir. Physiol. Neurobiol. 2022, 301, 103889. [Google Scholar] [CrossRef]
- Menga, L.S.; Cese, L.D.; Bongiovanni, F.; Lombardi, G.; Michi, T.; Luciani, F.; Cicetti, M.; Timpano, J.; Ferrante, M.C.; Cesarano, M.; et al. High Failure Rate of Noninvasive Oxygenation Strategies in Critically Ill Subjects with Acute Hypoxemic Respiratory Failure Due to COVID-19. Respir. Care 2021, 66, 705–714. [Google Scholar] [CrossRef]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosà, T.; Spadaro, S.; Bitondo, M.M.; Montomoli, J.; Falò, G.; Tonetti, T.; Cutuli, S.L.; et al. Effect of Helmet Noninvasive Ventilation vs. High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA 2021, 325, 1731–1743. [Google Scholar] [CrossRef]
- Parish, A.J.; West, J.R.; Caputo, N.D.; Janus, T.M.; Yuan, D.; Zhang, J.; Singer, D.J. Early Intubation and Increased Coronavirus Disease 2019 Mortality: A Propensity Score–Matched Retrospective Cohort Study. Crit. Care Explor. 2021, 3, e0452. [Google Scholar] [CrossRef]
- Rojas-Marte, G.; Hashmi, A.T.; Khalid, M.; Chukwuka, N.; Fogel, J.; Munoz-Martinez, A.; Ehrlich, S.; Waheed, M.A.; Sharma, D.; Sharma, S.; et al. Outcomes in Patients With COVID-19 Disease and High Oxygen Requirements. J. Clin. Med. Res. 2021, 13, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.; Sarff, L.; Banerjee, J.; Coffey, C.; Holtom, P.; Meurer, S.; Wald-Dickler, N.; Spellberg, B. Effect of mortality from COVID-19 on inpatient outcomes. J. Med. Virol. 2021, 94, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, K.-J.; Choi, S.H.; Lee, S.Y.; Kim, K.C.; Kim, E.J.; Lee, J. Clinical Significance of Timing of Intubation in Critically Ill Patients with COVID-19: A Multi-Center Retrospective Study. J. Clin. Med. 2020, 9, 2847. [Google Scholar] [CrossRef] [PubMed]
- Fayed, M.; Patel, N.; Yeldo, N.; Nowak, K.; Penning, D.H.; Torres, F.V.; Natour, A.K.; Chhina, A. Effect of Intubation Timing on the Outcome of Patients with Severe Respiratory Distress Secondary to COVID-19 Pneumonia. Cureus 2021, 13, e19620. [Google Scholar] [CrossRef]
- Hyman, J.B.; Leibner, E.S.; Tandon, P.; Egorova, N.N.; Bassily-Marcus, A.; Kohli-Seth, R.; Arvind, V.; Chang, H.L.; Lin, H.-M.; Levin, M.A. Timing of Intubation and In-Hospital Mortality in Patients with Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0254. [Google Scholar] [CrossRef]
- Pandya, A.; Kaur, N.A.; Sacher, D.; O’Corragain, O.; Salerno, D.; Desai, P.; Sehgal, S.; Gordon, M.; Gupta, R.; Marchetti, N.; et al. Ventilatory Mechanics in Early vs. Late Intubation in a Cohort of Coronavirus Disease 2019 Patients With ARDS: A Single Center’s Experience. Chest 2020, 159, 653–656. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; de Gonzalo-Calvo, D.; Torres, G.; de Batlle, J.; Gómez, S.; Moncusí-Moix, A.; Carmona, P.; Santisteve, S.; Monge, A.; et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: A prospective cohort study. Crit. Care 2022, 26, 18. [Google Scholar] [CrossRef]
- Pierson, D.J. Tracheostomy and weaning. Respir Care 2005, 50, 526–533. [Google Scholar]
- Ferro, A.; Kotecha, S.; Auzinger, G.; Yeung, E.; Fan, K. Systematic review and meta-analysis of tracheostomy outcomes in COVID-19 patients. Br. J. Oral Maxillofac. Surg. 2021, 59, 1013–1023. [Google Scholar] [CrossRef]
- Ji, Y.; Fang, Y.; Cheng, B.; Li, L.; Fang, X. Tracheostomy timing and clinical outcomes in ventilated COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2022, 26, 40. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; de Gonzalo-Calvo, D.; Torres, G.; de Batlle, J.; Gómez, S.; Moncusí-Moix, A.; Carmona, P.; Santisteve, S.; Monge, A.; et al. Tracheostomy Timing and Outcome in Severe COVID-19: The WeanTrach Multicenter Study. J. Clin. Med. 2021, 10, 2651. [Google Scholar] [CrossRef]
- Liu, C.C.; Livingstone, D.; Dixon, E.; Dort, J.C. Faculty Opinions recommendation of Early versus late tracheostomy: A systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2015, 152, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. ARDS Definition of Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Summary of Recommendation Statements. Kidney Int. Suppl. 2012, 2, 8–12. Available online: https://www.sciencedirect.com/science/article/pii/S2157171615310443 (accessed on 28 April 2022). [CrossRef] [Green Version]
- WHO. COVID-19 Clinical Management: Living Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 28 April 2022).
- Hazard, D.; Kaier, K.; Von Cube, M.; Grodd, M.; Bugiera, L.; Lambert, J.; Wolkewitz, M. Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: A multistate approach. BMC Med. Res. Methodol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.L.; Suen, J.Y.; White, N.; Dalton, H.J.; Fanning, J.; Corley, A.; Shrapnel, S.; Hinton, S.; Forsyth, S.; Parsons, R.; et al. Assessment of 28-Day In-Hospital Mortality in Mechanically Ventilated Patients with Coronavirus Disease 2019: An International Cohort Study. Crit. Care Explor. 2021, 3, e0567. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Ball, L.; Patroniti, N.; Loconte, M.; Brunetti, I.; Vena, A.; Giacobbe, D.R.; Bassetti, M.; Rocco, P.R.M.; et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020, 279, 103455. [Google Scholar] [CrossRef]
- Weaver, L.; Das, A.; Saffaran, S.; Yehya, N.; Scott, T.E.; Chikhani, M.; Laffey, J.G.; Hardman, J.G.; Camporota, L.; Bates, D.G. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: A computational modelling study. Ann. Intensive Care 2021, 11, 109. [Google Scholar] [CrossRef]
- Kallet, R.H. 2020 Year in Review: Mechanical Ventilation During the First Year of the COVID-19 Pandemic. Respir. Care 2021, 66, 1341–1362. [Google Scholar] [CrossRef]
- Gray, A.; Goodacre, S.; Newby, D.E.; Masson, M.; Sampson, F.; Nicholl, J. 3CPO Trialists. Noninvasive Ventilation in Acute Cardiogenic Pulmonary Edema. N. Engl. J. Med. 2008, 359, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Andriolo, B.N.G.; Andriolo, R.B.; Saconato, H.; Atallah, N.; Valente, O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst. Rev. 2015, 1, CD007271. [Google Scholar] [CrossRef] [PubMed]
- Livneh, N.; Mansour, J.; Lerner, R.K.; Feinmesser, G.; Alon, E. Early vs. late tracheostomy in ventilated COVID-19 patients—A retrospective study. Am. J. Otolaryngol. 2021, 42, 103102. [Google Scholar] [CrossRef] [PubMed]
- Polok, K.; Fronczek, J.; van Heerden, P.V.; Flaatten, H.; Guidet, B.; De Lange, D.W.; Fjølner, J.; Leaver, S.; Beil, M.; Sviri, S.; et al. Association between tracheostomy timing and outcomes for older critically ill COVID-19 patients: Prospective observational study in European intensive care units. Br. J. Anaesth. 2021, 128, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; De Vries, H.J.; Vlaar, A.P.J.; Van Der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C. Dutch COVID-19 Diaphragm Investigators Diaphragm Pathology in Critically III Patients With COVID-19 and Postmortem Findings From 3 Medical Centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Menon, T.; Gandhi, S.A.Q.; Tariq, W.; Sharma, R.; Sardar, S.; Arshad, A.M.; Adhikari, R.; Ata, F.; Kataria, S.; Singh, R. Impact of Chronic Kidney Disease on Severity and Mortality in COVID-19 Patients: A Systematic Review and Meta-analysis. Cureus 2021, 13, e14279. [Google Scholar] [CrossRef]
- Shi, C.; Wang, L.; Ye, J.; Gu, Z.; Wang, S.; Xia, J.; Xie, Y.; Li, Q.; Xu, R.; Lin, N. Predictors of mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 663. [Google Scholar] [CrossRef]
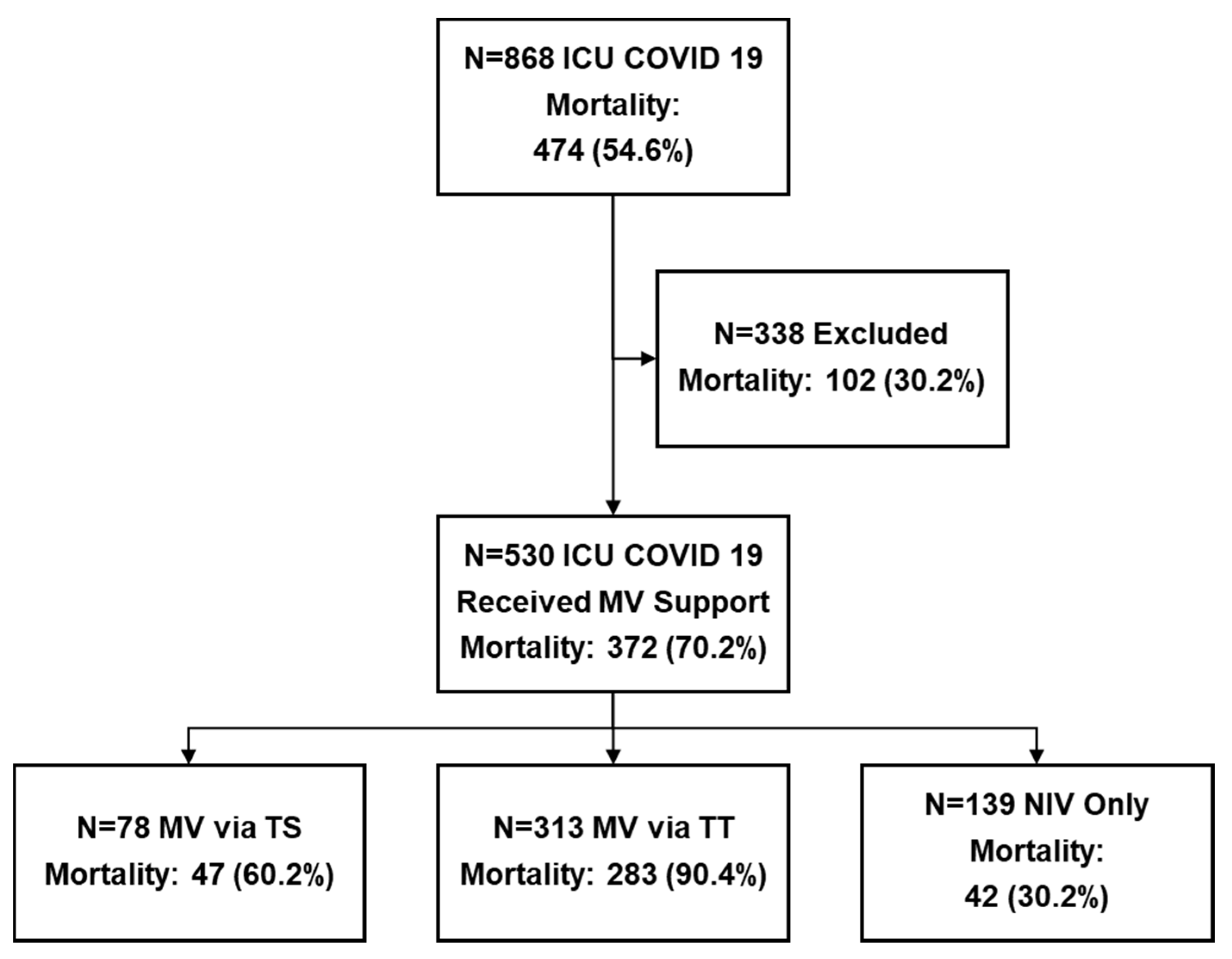
| Ventilation Approach | Overall n = 530 | NIV n = 139 | TT n = 313 | TS n = 78 | p-Value |
|---|---|---|---|---|---|
| Demographical data | |||||
| Age, years | 63.0 (62.1–63.1) | 63.0 (61.0–65.4) | 65.0 (63.5–66.5) | 60.1 (57.5–62.8) | 0.124 |
| Women, n (%) | 268 (50.6%) | 79 (51.8%) | 151 (48.2%) | 38 (48.7%) | 0.314 |
| Comorbidities | |||||
| Coronary artery disease, n (%) | 290 (54.7%) | 84 (60.4%) | 169 (54.0%) | 37 (47.4%) | 0.059 |
| Diabetes mellitus, n (%) | 150 (28.3%) | 40 (28.8%) | 91 (29.1%) | 19 (24.4%) | 0.691 |
| COPD, n (%) | 32 (6.0%) | 6 (4.3%) | 21 (6.7%) | 5 (6.4%) | 0.437 |
| Malignancy, n (%) | 151 (28.5%) | 46 (33.1%) | 82 (26.2%) | 23 (29.5%) | 0.391 |
| Chronic renal disease, n (%) | 71 (13.4%) | 19 (13.7%) | 48 (15.3%) | 4 (5.1%) | 0.164 |
| Obesity (BMI > 30 kg/m2), n (%) | 90 (17.0%) | 25 (18.0%) | 52 (16.6%) | 13 (16.7%) | 0.561 |
| COVID-19 severity | |||||
| Mild, n (%) | 17 (3.2%) | 2 (1.4%) | 12 (3.9%) | 3 (4%) | 0.803 |
| Moderate, n (%) | 86 (16.2%) | 24 (17.3%) | 53 (17.3) | 9 (12%) | |
| Severe and Critical, n (%) | 417 (78.7%) | 113 (81.3%) | 241 (78.8%) | 63 (84.0%) | |
| Complications | |||||
| Sepsis, n (%) | 188 (35.5%) | 19(13.7%) | 129 (41.2%) *,** | 40 (51.3%) * | <0.001 |
| Acute kidney injury, n (%) | 94 (17.7%) | 8 (5.8%) | 68 (21.7%) * | 18 (23.1%) * | <0.001 |
| MODS, n (%) | 158 (29.8%) | 8 (5.8%) | 121 (38.7%) * | 29 (37.2%) * | <0.001 |
| PE, n (%) | 47 (8.8%) | 10 (7.2%) | 33 (10.5%) | 4 (5.1%) | 0.804 |
| Stroke, n (%) | 7 (1.3%) | 0 (0) | 4 (1.3%) | 3 (3.86) | 0.058 |
| Secondary bacterial pneumonia, n (%) | 224 (42.3%) | 59 (42.4%) | 122 (39.0%) | 43 (55.1%) | 0.107 |
| ARDS, n (%) | 149 (28.1%) | 25 (18.0%) | 92 (29.4%) * | 32 (41.0%) * | <0.001 |
| Ventilation Approach | Overall n = 530 | NIV n = 139 | TT n = 313 | TS n = 78 | p-Value |
|---|---|---|---|---|---|
| Mean days since first symptoms of COVID-19 at hospital admission | 8.2 (7.1–9.3) | 8.2 (7.3–9.1) | 8.1 (7.4–8.7) | 9.0 (6.6–11.3) | 0.489 |
| Mean duration of NIV, days | 5.6 (4.2–7.1) | 8.58 (7.4–9.61) | 4.8 (4.1–5.4) * | 4.4 (3.1–5.7) * | <0.001 |
| Use of NIV before relying on invasive MV, n (%) | – | 194 (62%) | 41 (52.6%) | 0.155 | |
| Time from admission in ICU to intubation, days | 4.2 (3.6–4.9) | – | 4.3 (3.9–4.8) | 4.0 (3.0–5.0) | 0.365 |
| Duration of invasive mechanical ventilation, days | 9.7 (6.7–12.8) | – | 7.16 (6.4–7.9) ** | 19.9 (16.1–23.7) | <0.001 |
| Mean length of stay in ICU, days | 12.3 (8.4–16.2) | 10.5 (8.0–12.9) ** | 10.7 (8.3–13.3) ** | 22.2 (16.0–28.4) | <0.001 |
| Mean ventilator-free days | 6.9 (5.7–8.1) | 6.1 (3.4–8.9) | 6.9 (5.8–8.0) | 7.1 (5.0–9.2) | 0.240 |
| Discharged from the ICU, n (%) | 158 (29.8%) | 97 (69.8%) ** | 30 (9.6%) *,** | 31 (39.7%) | <0.001 |
| Mortality rates in the ICU, n (%) | 372 (70.2%) | 42 (30.2%) ** | 283 (90.4%) *,** | 47 (60.2%) | <0.001 |
| Mortality rates in Ward after discharge from ICU, n (%) | 18 (11.4%) | 6 (6.2%) ** | 7 (23.3%) *,** | 5 (16.12%) | 0.022 |
| Duration of hospitalization, days | 25.4 (18.6–32.2) | 25.3 (21.7–26.7) ** | 19.2 (12.5–24.9) *,** | 49.7 (37.5–62.0) | <0.001 |
| Survivors, n = 158 | Non-Survivors, n = 372 | p-Value | Univariate Analysis OR (p-Value; 95% CI) | Multivariate Analysis OR (p-Value; 95% CI) | |
|---|---|---|---|---|---|
| Age, years | 59.7 (58.4–61.2) | 65.7 (64.5–66.9) | <0.001 | 1.03 (<0.001; 1.02–1.04) | 1.03 (<0.001; 1.02–1.04) |
| Females, n (%) | 83 (52.5%) | 185 (49.7%) | 0.223 | - | - |
| Comorbidities | |||||
| Coronary artery disease, n (%) | |||||
| No | 72 (45.6%) | 168 (45.2%) | |||
| Yes | 86 (54.4%) | 204 (54.8%) | 0.503 | - | - |
| Diabetes mellitus, n (%) | |||||
| No | 115 (72.8%) | 265 (71.2%) | 0.437 | - | - |
| Yes | 43 (27.2%) | 107 (28.8%) | |||
| COPD, n (%) | |||||
| No | 149 (94.3%) | 349 (93.8%) | 0.291 | - | - |
| Yes | 9 (5.7%) | 23 (6.2%) | |||
| Malignancy, n (%) | |||||
| No | 108 (68.4%) | 271 (72.8%) | 0.295 | - | - |
| Yes | 50 (31.6%) | 101 (27.2%) | |||
| Chronic renal disease, n (%) | |||||
| No | 145 (91.8%) | 314 (84.4%) | 0.025 | 2.06 (0.024; 1.09–3.88) | 1.57 (0.04; 1.02–2.4) |
| Yes | 13 (8.2%) | 58 (15.6%) | |||
| Obesity (BMI > 30 kg/m2), n (%) | |||||
| No | 138 (84.8%) | 292 (82.3%) | 0.082 | 1.9 (0.01; 1.1–3.2) | 1.58 (0.02; 1.08–2.3) |
| Yes | 20 (15.2%) | 80 (17.7%) | |||
| Complications | |||||
| Sepsis, n (%) | |||||
| No | 126 (79.7%) | 216 (58.1%) | <0.001 | 2.8 (<0.001; 1.8–4.4) | 2.8 (<0.001; 1.8–4.3) |
| Yes | 32 (20.3%) | 156 (41.9%) | |||
| Acute kidney injury, n (%) | |||||
| No | 145 (91.8%) | 291 (78.2%) | <0.001 | 3.1 (<0.001; 1.7–5.8) | 1.7 (0.049; 1.05–2.9) |
| Yes | 13 (8.2%) | 81 (21.8%) | |||
| MODS, n (%) | |||||
| No | 138 (87.3%) | 234 (62.9%) | <0.001 | 4.1 (<0.001; 2.4–6.8) | 3.15 (<0.001; 1.9–5.1) |
| Yes | 20 (12.7%) | 138 (37.1%) | |||
| PE, n (%) | |||||
| No | 147 (93.0%) | 336 (90.3%) | 0.404 | - | - |
| Yes | 11 (7.0%) | 36 (9.7%) | |||
| Stroke, n (%) | |||||
| No | 155 (98.1%) | 368 (98.9%) | 0.431 | - | - |
| Yes | 3 (1.9%) | 4 (1.1%) | |||
| Secondary bacterial pneumonia, n (%) | |||||
| No | 92 (58.2%) | 214 (57.5%) | 0.924 | - | - |
| Yes | 66 (41.8%) | 158 (42.5%) | |||
| ARDS, n (%) | |||||
| No | 129 (81.6%) | 252 (67.7%) | <0.001 | 2.1 (0.001; 1.3–3.3) | 3.3 (<0.001; 2.1–5.1) |
| Yes | 29 (18.4%) | 120 (32.3%) | |||
| Ventilation approach: | |||||
| NIV, n (%) | 97 (69.8%) | 42 (30.2%) | <0.001 | 12.5 (<0.001; 7.9–19.7) | - |
| Invasive MV with TT, n (%) | 30 (9.6%) | 283 (90.4%) | <0.001 | 0.07 (<0.001; 0.05–0.11) | |
| Invasive MV with TS, n (%) | 31 (39.7%) | 47 (60.2%) | <0.001 | 6.2 (<0.001; 3.5–11.2) | 6.3 (<0.001; 3.4–11.6) |
| Early, n (%) (n = 36) | 16 (44.4%) | 20 (56.6%) | 0.542 | ||
| Late, n (%) (n = 42) | 16 (38.1%) | 26 (61.9%) | |||
| Mean days since first symptoms of COVID-19 at hospital admission | 8.7 (7.4–9.3) | 8.1 (7.6–8.7) | 0.091 | - | - |
| Mean duration of NIV, days | 6.4 (5.1–8.8) | 5.3 (4.7–7.0) | 0.072 | - | - |
| Use of NIV prior to invasive MV, n (%) | 22 (49.2%) | 213 (62.3%) | 0.028 | 2.0 (0.02; 1.1–3.7) | 2.7 (0.001; 1.5–4.1) |
| Time from admission in ICU to intubation, days | 4.1 (3.6–4.6) | 4.3 (3.8–4.9) | 0.563 | - | - |
| Duration of invasive MV days | 16.2 (12.8–19.6) | 8.5 (7.5–9.5) | <0.001 | 1.1 (<0.001; 1.03–1.1) | - |
| Mean length of stay in ICU, days | 13.4 (11.8–15.1) | 11.9 (8.7–13.3) | 0.072 | - | - |
| Mean ventilator-free days | 7.6 (2.1–14.9) | 1.7 (0.54–3.11) | 0.064 | - | - |
| Duration of hospitalization, days | 20.9 (19.1–22.8) | 14.8 (8.4–21.2) | 0.276 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocans, R.P.; Ozolina, A.; Battaglini, D.; Bine, E.; Birnbaums, J.V.; Tsarevskaya, A.; Udre, S.; Aleksejeva, M.; Mamaja, B.; Pelosi, P. The Impact of Different Ventilatory Strategies on Clinical Outcomes in Patients with COVID-19 Pneumonia. J. Clin. Med. 2022, 11, 2710. https://doi.org/10.3390/jcm11102710
Rocans RP, Ozolina A, Battaglini D, Bine E, Birnbaums JV, Tsarevskaya A, Udre S, Aleksejeva M, Mamaja B, Pelosi P. The Impact of Different Ventilatory Strategies on Clinical Outcomes in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine. 2022; 11(10):2710. https://doi.org/10.3390/jcm11102710
Chicago/Turabian StyleRocans, Rihards P., Agnese Ozolina, Denise Battaglini, Evita Bine, Janis V. Birnbaums, Anastasija Tsarevskaya, Sintija Udre, Marija Aleksejeva, Biruta Mamaja, and Paolo Pelosi. 2022. "The Impact of Different Ventilatory Strategies on Clinical Outcomes in Patients with COVID-19 Pneumonia" Journal of Clinical Medicine 11, no. 10: 2710. https://doi.org/10.3390/jcm11102710
APA StyleRocans, R. P., Ozolina, A., Battaglini, D., Bine, E., Birnbaums, J. V., Tsarevskaya, A., Udre, S., Aleksejeva, M., Mamaja, B., & Pelosi, P. (2022). The Impact of Different Ventilatory Strategies on Clinical Outcomes in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine, 11(10), 2710. https://doi.org/10.3390/jcm11102710







