Abstract
(1) Background: Given the high prevalence of non-alcoholic fatty liver disease (NAFLD) and the limitations of liver biopsies, multiple non-invasive tests (NITs) have been developed to identify non-alcoholic fatty liver disease (NAFLD) patients at-risk of progression. The availability of these new NITs varies from country to country, and little is known about their implementation and adoption in routine clinical practice. This study aims to explore barriers and facilitators that influence the adoption of NAFLD NITs, from healthcare professionals’ perspectives. (2) Methods: A cross-sectional study was performed using an exploratory mixed-methods approach. Twenty-seven clinicians from eight different countries with different specialties filled in our questionnaire. Of those, 16 participated in semi-structured interviews. Qualitative and quantitative data were collected and summarized using the recently published Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework for new medical technologies in healthcare organizations. (3) Results: Several factors were reported as influencing the uptake of NITs for NAFLD in clinical practice. Among those: insufficient awareness of tests; lack of practical guidelines and evidence for the performance of tests in appropriate patient populations and care settings; and absence of sufficient reimbursement systems were reported as the most important barriers. Other factors, most notably ‘local champions’, proper functional payment systems, and sufficient resources in academic hospitals, were indicated as important facilitating factors. (4) Conclusions: Clinicians see the adoption of NITs for NAFLD as a complex process that is modulated by several factors, such as robust evidence, practical guidelines, a proper payment system, and local champions. Future research could explore perspectives from other stakeholders on the adoption of NITs.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is an increasingly prevalent, complex, and progressive liver condition, which is presenting a growing challenge to healthcare systems internationally [1,2,3,4]. NAFLD patients are at risk of progression to more severe stages, such as non-alcoholic steatohepatitis (NASH) and/or advanced liver fibrosis and cirrhosis. The progressive nature of this disease and its high global prevalence highlight the importance of timely identification of patients at risk of progression and of assessing the severity of the disease.
Currently, liver biopsy is the reference standard for definitive diagnosis of NAFLD, detecting NASH, and accurately staging liver fibrosis in NAFLD patients. However, it is invasive, has risks of complications, and is subject to sampling variability and inter-observer variation in interpretation [5,6]. These limitations have fuelled interest in developing non-invasive tests (NITs) to evaluate NAFLD progression [3,7,8,9,10]. Multiple biomarker-based NITs have been developed in recent years. Some, like the Enhanced Liver Fibrosis (ELF) test and FibroScan, are advocated by clinical guidelines for assessing fibrosis in NAFLD patients [11]. However, recent surveys detail that the availability of these tests in clinics varies from country to country, influenced by factors such as different regulatory requirements, national policies, and insurance coverage [12,13].
The global need for and interest in new and accurate medical tests is not limited to the hepatology field. The discovery and development of biomarkers have been a highly exciting field of research, as novel markers may have substantial potential to improve health outcomes for a range of medical conditions. Large investments, by both academia and industry, have been made in this area, but the path from the discovery of biomarkers to implementation in innovative medical tests is long and winding. So far, very few regulatory-approved biomarkers have entered clinics [14,15,16].
The introduction of biomarker measurement into clinical practice is challenging. Several scientific, economic, and regulatory barriers need to be overcome before biomarkers can reach clinical practice [14,17,18,19]. Moreover, targeted adopters will respond heterogeneously to a new test [20]. As of yet, research focusing on identifying challenges at the implementation stage of the biomarker development pipeline has been scarce [21,22].
Different theoretical models have been developed to better understand users’ intention to accept a new technology [23]. One of these frameworks is the Non-adoption, Abandonment, and Challenges to the Scale-UP, Spread, and Sustainability of Health and Care Technologies (NASSS) framework, developed to theorize and evaluate challenges to the scale-up of health and care technologies [24,25]. This NASSS framework aims to detect the determinants of the implementation processes of complex technologies in healthcare in seven different domains. So far, it has been helpful in a range of applications [26,27,28].
In this study, we used the NASSS framework to investigate clinician-perceived barriers and facilitators to the adoption of NAFLD NITs. The tests were selected from a list of NITs that are evaluated in the LITMUS (Liver Investigation: Testing Marker Utility in Steatohepatitis) study, a multicenter project that aims to develop, validate, and qualify a defined set of biomarkers that can enable the detection of high-risk NAFLD patients [29].
2. Materials and Methods
2.1. Study Design and Registration
This exploratory study employed a cross-sectional design. Using a mixed-methods approach, we combined qualitative and quantitative methods to create a comprehensive picture of the adoption of influencing factors. The study protocol was made available through the Open Science Framework (https://osf.io/vhzkm, accessed on 8 May 2022) and is reported using the SRQR checklist (Supplementary Table S1) [30].
2.2. Theoretical Framework
We selected the NASSS framework to evaluate the adoption of the selected NITs because of its solid theoretical foundation and its focus on detecting determinants of the implementation processes of complex technologies in healthcare [24,25]. The NASSS framework lists potential determinants in seven domains: (1) the condition, (2) the technology, (3) the value proposition, (4) the adopters, (5) the organization, (6) the wider system, and (7) embedding and adaptation over time (see Figure 1).
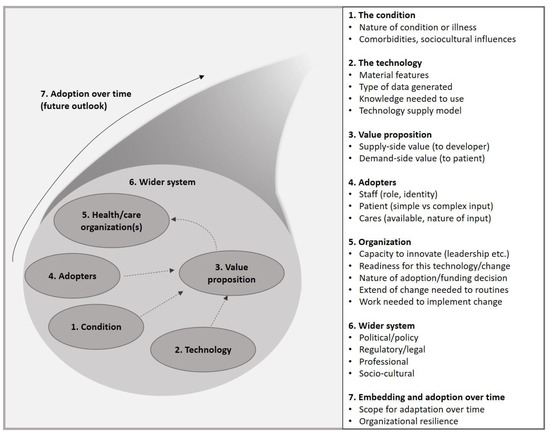
Figure 1.
NASSS (Non-adoption, Abandonments, Scale-Up, Spread, and Sustainability) framework.
2.3. Sampling and Consent to Participate
This study is focused on clinicians, as these important stakeholders in the healthcare system play a significant role in the implementation and dissemination of medical tests. Clinicians routinely request tests in clinical practice, interpret the results, and make clinical decisions based on them. We selected clinicians from multiple European countries, different specialties, and variable levels of experience in working with NAFLD patients through both purposeful and snowball sampling.
Initially, an invitation was sent to (1) a list, provided by the LITMUS consortium, of clinicians experienced with NAFLD care from different countries in Europe, (2) the Dutch NAFLD clinicians working group, (3) the French NAFLD clinicians working group, and (4) clinicians from Belgium, Scotland, Germany and England, identified through the researchers’ networks. All clinicians who participated in this study provided explicit consent before contributing and all answers were processed anonymously.
2.4. Data Collection
2.4.1. Scoping Phase
The study consisted of two phases: a scoping phase and a data collection phase (Figure 2). In the scoping phase, four interviews with NAFLD care experts were conducted, to create an initial list of tests and to discuss the most relevant concepts of the NASSS framework. Thereafter, a screening survey with the initial list of seven tests was sent to 129 clinicians, to gain knowledge about the current clinical use of each of the tests. The final list was assembled based on the answers to the screening surveys (Supplementary Table S2). The results from the screening survey informed the development of the final questionnaire and interview guide in the main data collection phase.
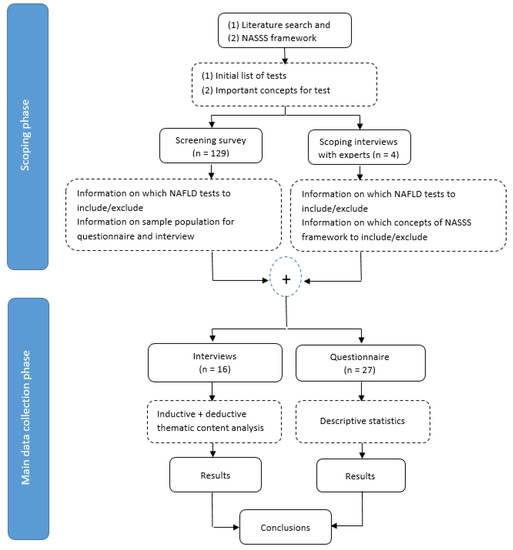
Figure 2.
Study design flow diagram.
Selected Tests
The final tests were selected based on the clinicians’ responses to the screening survey. See Supplementary Table S3 for the number of tests that the respondents use for their NAFLD patients in their current clinical practice. To capture both facilitating and hindering factors, we selected three tests with different dissemination levels: from a well-disseminated test, which was used by all the respondents (FibroScan), to a relatively new marker that is not yet well integrated into clinical care and was reported by only two clinicians (PRO-C3).
- FibroScan: FibroScan vibration-controlled transient elastography (VCTE) is a widely available ultrasound-based fibrosis test, which measures liver stiffness by estimating the velocity of propagation of a shear wave through liver tissue [31,32];
- Enhanced Liver Fibrosis (ELF) test: ELF is a moderately available serum biomarker panel, which consists of three components: type III procollagen peptide (PIIINP), hyaluronic acid (HA), and tissue inhibitor of metalloproteinase-1 (TIMP1) [33,34];
- PRO-C3: This procollagen-based marker is a relatively new serum biomarker with limited availability outside clinical trials. The procollagen type III N-terminal peptide (P3NP) is a by-product of the cleavage of procollagen III to produce collagen III [35].
Results of FibroScan and ELF are indicative of the amount of liver fibrosis while those of PRO-C3 are also indicative of fibrogenesis, the process of active fibrosis synthesis. All three have been studied across multiple liver diseases, including NAFLD [36,37,38].
2.4.2. Data Collection Phase
Qualitative Data Collection
Participants who responded to our screening survey were invited to participate in a semi-structured interview of approximately 40 min, conducted online, with a pre-defined topic guide (Supplementary Table S4). The topic guide was piloted on one respondent. Revisions were made to the final version after the piloting interview and the first couple of interviews.
Quantitative Data Collection
To supplement the findings from the semi-structured interviews we also disseminated a quantitative questionnaire, designed based on the questions from the topic guide to the respondents. We offered a five-point Likert scale for responses, ranging from “strongly disagree” to “strongly agree”. We also invited clinicians to rate their level of knowledge for each test and to indicate their opinion about the most important barrier and facilitator for adopting each test using two open-ended questions (Supplementary Table S5).
2.5. Data Analysis
2.5.1. Qualitative Data Analysis
All interviews were transcribed ad verbatim using transcription software Otter.ai version 2.1.41.612 (Lost Altos, CA, USA). Transcripts were analysed with the qualitative data analysis software ATLAS.ti version 9.1.2 (Berlin, Germany). Two authors (RE and YV) independently coded the first two interviews and discussed the codes. After reaching a consensus on the coding strategy, coding was completed using a combination of inductive and deductive thematic analysis and the NASSS domains as an analytical framework. RE led the analysis, and YV verified all codes, to achieve content conformity of the categories and themes. The quotations reported in the text were slightly edited to improve the readability while the meaning of the original texts was retained [39].
2.5.2. Quantitative Data Analysis
Collected data were evaluated using descriptive statistics in R software version 3.5.2 (Vienna, Austria). Responses to each question are reported as: Disagree (scores 1 and 2), Neutral (3), and Agree (4 and 5).
3. Results
Thirty-nine of 129 invited clinicians responded to our screening survey (Supplementary Table S6). Twenty-seven filled in our questionnaire (Table 1); 16 of those also participated in the interviews (Figure 3). Participants were from eight different countries and spanned a wide range of experience (3–36 years), and specialties (Table 1). All clinicians were using FibroScan in their clinical practice, three also used ELF, while none had clinical experience working with PRO-C3.

Table 1.
Baseline characteristics of questionnaire respondents and interview respondents.
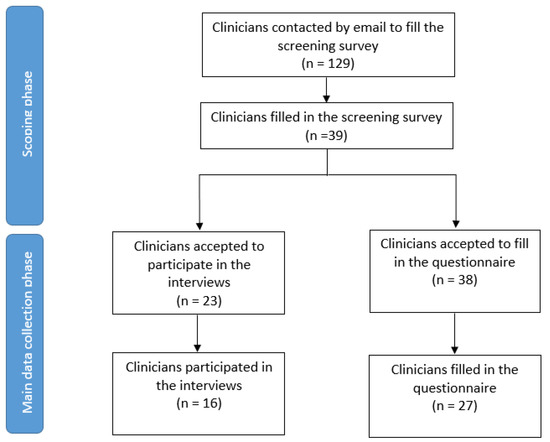
Figure 3.
Flow diagram of the respondents.
Clinicians reported several factors that could influence the adoption of these three tests. These factors were categorized based on the respective NASSS domains. The complete list of identified barriers and facilitators is reported in Table 2, while the main factors are captured below.

Table 2.
Summary of the main facilitators and barriers sorted under the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework’s domains.
3.1. Identified Factors Affecting Tests’ Adoption
3.1.1. The Condition
Clinicians defined NAFLD as a multi-system disease highly associated with obesity, metabolic syndrome, or type 2 diabetes mellitus. They described that its complex pathogenesis and natural course are not fully understood and considered it essential to investigate disease progression in patients with different degrees of NAFLD activity.
According to most clinicians, the complexity of NAFLD would not hamper the adoption of the three selected tests (see Table 3). However, they believed that accurate diagnosis and management of such a complex disease would be challenging if using a single test.
“In our more complex diseases, where the decision and treatment depends a little bit on the assessment of [the] fibrosis, FibroScan alone will not be sufficient and have to be complemented with something else.”(Hepatologist)

Table 3.
Results per items of “The Condition” and “The Technology” domains of the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework as reported by responders.
3.1.2. The Technology
Clinicians generally perceived the selected tests as easy to use in clinical practice. However, they mostly agreed that the test results are not always sufficient for decision making (Table 3). They specified that, practically, it is unlikely that a single test would be able to accurately rule in or rule out the disease. For this reason, they almost always use a combination of tests.
Concerns were also raised regarding the utility of the test. Almost all clinicians referred to the existence of a large grey zone, which impacts the discriminatory performance of these tests: they would only trust low and high values.
“So, there is this greyish area where the accuracy is not good enough. And the real low values give you a fair accuracy, there’s absence of significant fibrosis and certainly cirrhosis, and this higher limit where you’re sure that there is significant fibrosis and possibly even close to cirrhosis. And then there’s this greyish area where you’re not certain.”(Gastroenterologist)
The three selected tests were perceived to need different levels of knowledge for accurate performance in clinical practice. For FibroScan, for example, adequate training is required for proper implementation, while automated measurement of blood samples in laboratories can play a role as a facilitator for the adoption of blood-based markers. The human element in measurement with ultrasound-based tests, such as FibroScan, and the resulting intra-operator variability were believed to be factors that could challenge the current clinical practice and the test’s further adoption.
Furthermore, the results generated by the tests are not always easily interpretable. The complicated interpretation was mentioned as another critical factor influencing the usage of the tests, while the existence of a clear cut-off was believed to facilitate the interpretation and, consequently, test adoption.
“More important is how you interpret the results. And interpreting the result is a bit more complicated, and it requires knowledge of the tool and its pitfalls, particularly where you are likely to get false-positive results, and what to do when you suspect and therefore what to do as a result.”(Hepatologist)
Clinicians indicated an essential need for robust evidence of the adequate performance of the tests across different clinical settings. They stated that FibroScan and ELF have been substantially validated, while the available research is too limited to convincingly demonstrate PRO-C3 accuracy in detecting NAFLD progression. As these tests are used in different levels of care and clinical settings, which differ in the prevalence of at-risk NAFLD patients, a lack of studies that carefully consider these differences when estimating the performance of the test can considerably hamper adoption.
“At this stage, I don’t know how FIB-4, PRO-C3, and ELF perform in relation to each other and in different patient groups. I can imagine that advanced stages of NASH in certain patients such as morbidly obese patients or patients with diabetes will be better identified with the ELF test than with a FIB-4 test. This is something that we should investigate. We have very good data from the UK and other countries. But for example, we have no data in the Netherlands, our population could be different than the UK population. So we need to do more research and to establish the sensitivity and specificity in our population.”(Internist)
3.1.3. The Value Proposition
Apart from substantial concerns regarding the benefits of new tests for specific populations or health settings, clinicians also referred to the high costs of the tests as one of the main barriers to the adoption of new NITs (Table 2).
Although high-quality cost-effectiveness analyses of these three tests are scarce and approved therapies for treating NAFLD are not yet available, all clinicians agreed that a NIT could be beneficial, for both clinicians and patients, by reducing the number of invasive and costly biopsy procedures. They also stated that all new NITs should be compared with less costly, already available tests, such as FIB-4, which has shown acceptable performance for ruling out advanced fibrosis (Table 4).
“So they could be cost-effective, but I don’t think they will beat using only FIB-4. The problem with all these cost-effectiveness analyses is that they are heavily influenced by what you put into them. And it depends on who you talk to how you really should count these costs. So even though cost-effectiveness analysis are very, very important, they are sometimes skewed, depending on which researcher that does them. But compared to the more normal or ordinary tests, both ELF and PRO-C3 are quite expensive. So that’s a barrier.”(Hepatologist)

Table 4.
Results per items of “The Value Proposition” and “The Adopters” domains of the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework as reported by responders.
3.1.4. The Adopters
The participating clinicians reported different levels of knowledge about the three selected NITs (Supplementary Table S7). They stated that, while FibroScan is well implemented and widely used in most European countries, there is more limited understanding of the use of ELF and PRO-C3 in the NAFLD care pathway, which can significantly hinder the adoption of these tests (Table 2 and Table 4). The higher number of “neutral” answers for PRO-C3, compared to the other two tests, reflects the limited knowledge about this test that prevented the respondents from making strong statements about the test.
Differences in the level of knowledge among our respondents were not only observed between countries. Clinicians referred to this difference as a potential hampering factor that can affect test adoption across regions within the same country. They observed a considerable range in awareness in countries and settings where NAFLD is more prevalent. For instance, adopting a new NIT might be simpler in a tertiary care setting or an academic hepatology clinic in the UK than in a primary care setting in the Netherlands, with lower NAFLD prevalence.
The involvement of multiple stakeholders, from laboratory technicians to practitioners and hospital managers, makes implementation particularly challenging. Here, clinicians highlighted the important role of the so-called “local champions”. They stated that an enthusiastic clinician, who would initiate and closely monitor the adoption process, or a lab professional, willing to handle all practicalities in the lab, could significantly facilitate the adoption process of NITs.
“[as a clinician] if you come up with evidence, and as a group, or maybe even two clinics, in our case, vascular medicine, and hepatology, if you say this is important, then we’re going to have to be there in order to follow this development, or maybe even be leading in the development in the Netherlands, in order to convince boards of directors and insurance companies to provide financial support.”(Endocrinologist)
“[as a clinician] if you just come up with evidence, and as a group… in our case, vascular medicine, and hepatology, if you say this is important, then we’re going to have to be there in order to follow this development, or maybe even be leading in the development….[as] you’d have to convince [the] boards of directors to provide this funding.”(Endocrinologist)
3.1.5. The Organization
Clinicians indicated that academic hospitals typically have more resources available to start using new tests. In almost all clinical settings, the initiative for adopting a new NIT would start with a clinician who can convincingly demonstrate the clinical need (local champion). Engagement with the management team and allocation of budgets were described as other important factors for successful implementation (Table 2 and Table 5).
“It was initiated by clinicians, who really wanted something to identify patients with these disorders, NAFLD and NASH, and we realized that the echography [Conventional ultrasound] was not sufficient, and that the usual algorithms are not sensitive enough. So we wanted a more precise measurement [such as] the FibroScan to take better care of our patients. So it was initiated by clinicians. And then we started talking to management. Together, we organized the money to buy the machine and it was very easily implemented in our center…”(Internist)

Table 5.
Results per items of “The Organization”, “The Wider System”, and “The Future Outlook” domains of the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework as reported by responders.
Most clinicians believed that extensive work is needed to properly adopt ELF and PRO-C3 in clinical practice, with less effort required for FibroScan, which is already implemented in many countries (Table 5). Changes might be needed in both intra- and extra-organizational routines, such as training sessions and changes in laboratory routines. Clinicians involved in implementing clinical care pathways with ELF and FibroScan mentioned other necessary changes at the extra-organizational level. They explained the need for a referral management system that would enable general practitioners to simply order a new test and guide them to interpret results properly, referring patients to secondary care when needed.
“That will be mainly at the level of the clinical lab where all the technical things needs to happen. So it should be integrated in the machinery of the lab and in the protocols of the lab. And there should be knowledge of the technical staff at the lab, if there is any specific manipulation needed, which is not done automatically by the machine. So it’s mainly a question of looking into the technical aspects of the lab and the training of staff at the clinical lab.”(Hepatologist)
“And what we have put out there in primary care is some very clear [guidance], there’s a big box that says ELF [score] below this; fine reassure [the patient], ELF at this level; fine refer [the patient onwards]. So it’s quite prescriptive in the sense, they don’t have to think too much about what the ELF components are and what it’s telling them. They’re just being guided by the result.”(Endocrinologist)
3.1.6. The Wider System
Adoption of new NITs was perceived as a complex process, influenced by various national and international bodies. National expert groups, which include different specialists who actively collaborate to improve the care path for NAFLD patients, were named as one of the most important facilitators (Table 2). In addition, international consortiums and groups, such as the European Association for the Study of the Liver (EASL) were considered influential. Most of the clinicians agreed that current practice guidelines on NITs in NAFLD are sub-par (Table 5). FibroScan has appeared in many guidelines, but ELF and PRO-C3 are mostly absent.
“So cost-effectiveness are performed by people who aren’t even in that specialty area. So it’s a group of people who just look at the data: statisticians. And then there might be one advisor on the group who is from that area. It’s hard for them to argue sometimes against all these numbers.”(Hepatologist)
3.1.7. The Future Outlook
Whilst most clinicians were optimistic about the future adoption of the three selected NITs on a larger scale, there were differing views on where to implement them (Table 5). The blood-based tests were perceived appropriate for primary care level screening. Some clinicians thought that the future of non-invasive testing for NAFLD lies in shear-wave elastography incorporated in ultrasound machines since every hospital has ultrasound machines already. Others believe that fibrosis assessment in NAFLD needs to be a dedicated procedure, not an ancillary measurement, that would best benefit in its overall interpretation from the knowledge and experience of a hepatologist.
“Ultrasound elastography is an elastography technique incorporated into regular ultrasound machines, which are available in all hospitals. I think this technique has the best chance to become the first line [test for] measuring fibrosis in NAFLD.”(Gastroenterologist)
4. Discussion
This explorative mixed-methods study highlights the complexities of NAFLD NITs’ adoption process and may contribute to a deeper understanding of the challenges and influencing factors involved. Different barriers and facilitators from the clinicians’ perspectives were reported for the adoption of FibroScan, ELF, and PRO-C3 in Europe (Table 2). Insufficient knowledge and awareness of NAFLD and NITs, a lack of practical guidelines built upon robust evidence for specific patient populations and care settings, and the absence of reimbursement were perceived as some of the most important barriers. Other factors, such as the presence of local champions, a proper functional payment system, and resources in academic hospitals were seen to play a facilitating role in the process.
4.1. Strengths and Limitations
The use of a structured and validated framework and integration of qualitative and quantitative methods contributed to a systematic data collection and synthesis and increased the credibility and reliability of the findings [40,41]. Semi-structured nature of the interviews allowed participants the freedom to express their views on their own terms. The questionnaire and the semi-structured interviews constituted data triangulation and consequently increased the study’s internal validity and confidence in the findings [42].
Nevertheless, it is important to consider the potential limitations of this study. Despite a large number of invited clinicians, a small number of clinicians participated in the interviews. In addition, our group of participants was selected using a purposeful sampling strategy and mostly from western Europe, which can limit the generalizability of the findings [43]. This study was not designed to represent all clinicians who work with NAFLD patients. We included clinicians from different specialties and various countries with varying levels of experience to improve the generalizability. However, the list of barriers and facilitators might differ in other countries, which were not reported in this study, due to different reasons such as differences in their health care systems. This study was focused on clinicians, as one of the most important stakeholders in the adoption process of NITs, to gain their in-depth perspectives. Future studies should be performed on the larger and more heterogeneous samples to get other stakeholders’ insights and experiences.
4.2. Implications for Practice and Research
Over the past decades, health care systems have experienced waves of rapid development, with new insurance models, regulatory changes, and novel technologies. In these evolving health systems, the development and implementation of NITs as new technologies for detecting specific health conditions received a great deal of attention. A number of other studies have evaluated the introduction and dissemination of NITs in different medical fields to enhance understanding of the diseases and extend the monitoring and treatment options [28,44,45,46]. Some also evaluated various factors that can influence the adoption of these tests [46].
Multiple NITs have been developed and evaluated in the hepatology field. In parallel with a steady increase in the incidence of NAFLD at the global level, interest in these NAFLD NITs has grown. Several regional guidelines were published for the clinical management of NAFLD, but many health care settings still suffer from a lack of written practical pathways to identify patients and link them to care pathways [47,48], which makes diagnosing NAFLD an enduring challenge.
There have been many discussions about the preferred NAFLD care pathway, without a clear consensus on the NITs to be used [47]. This debate could influence practitioners’ decisions about including new tests in their routine clinical practice, as well as decisions at the hospital management level about adoption.
Our findings show that clinicians’ acceptance and adoption of new NAFLD NITs highly depend on a test’s ability to improve patient care in clinical practice. Robust evidence is required, demonstrating that the performance of new tests is substantively superior to that of already existing approaches, while the strong influence of differences in the pre-test probability and comorbidities on the performance of the test is sufficiently considered [36,47,49].
There is a lack of adequate head-to-head comparisons of new tests in clinical settings that differ in disease prevalence and patient characteristics, including primary care settings. This may contribute to the limited awareness and knowledge about these tests, consequently affecting their usage. In addition, this absence may also hamper the development of practical guidelines that define optimal disease diagnosis, patient management strategies, and a successful regulatory approval process.
Besides the need for robust evidence and clinical guidance, the high costs associated with many new diagnostic tests in different medical fields also create significant barriers to their widespread adoption [50,51]. The costs of the NAFLD NITs and their coverage by health insurance vary considerably between countries. When a new test is not advocated sufficiently by clinical guidelines, it is more likely not to be reimbursed. Reimbursement will often depend on convincing evidence in guidelines. This evidence-based healthcare paradigm [52,53] also affects local budgeting for NALFD NITs. Academic hospitals may be at an advantage, due to their ability to attract research grants, which bring more opportunities for adopting new tests for research purposes.
As in any other field of technology, the crucial role of local champions in the adoption of NAFLD tests should not be underestimated [54]. These clinicians with a special interest in and knowledge about a new test are vital for initiating the adoption process, increasing awareness and improving knowledge of NAFLD and NITs. As such, they can significantly facilitate the adoption process and influence functional reimbursement systems that serve the interests of both the health care system and patients.
5. Conclusions
Clinicians consider the adoption of new diagnostic NAFLD tests a complex process, one that can be promoted or restricted by several factors, such as robust evidence, practical guidelines, an adequate payment system, and local champions. Identifying these influencing factors helps clinicians and health decision-makers to identify areas for improvement in the test’s adoption process and more effectively implement new tests in clinical settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11102707/s1, Table S1: Standards for Reporting Qualitative Research (SRQR) checklist; Table S2: Screening survey; Table S3: Clinicians’ responses to the screening survey and the question of whether they use the selected tests for their NAFLD patients in their current clinical practice; Table S4: Topic guide; Table S5: Questionnaire; Table S6: Characteristics of the respondents to the screening survey; Table S7: Self-reported level of knowledge of questionnaire respondents from 0 to 5; Table S8: The LITMUS Investigators.
Author Contributions
Conceptualization, Y.V., R.E., T.H., W.S.J., J.S., A.G.H., V.R., M.W.L., Q.M.A. and P.M.M.B.; methodology, Y.V., R.E., T.H., W.S.J., J.S. and P.M.M.B.; software, Y.V. and R.E.; validation, Y.V., R.E. and P.M.M.B.; formal analysis, Y.V. and R.E.; investigation, Y.V., R.E. and P.M.M.B.; resources, Y.V., R.E. and P.M.M.B.; data curation, Y.V., R.E.; writing—original draft preparation, Y.V.; writing—review and editing, Y.V., R.E., T.H., W.S.J., J.S., A.G.H., V.R., M.W.L., Q.M.A. and P.M.M.B.; visualization, Y.V., R.E.; supervision, M.W.L. and P.M.M.B.; project administration, Y.V. and R.E.; funding acquisition, Q.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been performed as part of the Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) project, which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking, under grant agreement No. 777377. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors sincerely thank all the participants for giving their time and sharing their views, clinicians and researchers who brain-stormed with us and helped us at the protocol development phase (Jerome Boursier, Michelle M.A. Kip, Mohammad Taghi Ramezan Zadeh), and the LITMUS investigators (Supplementary Table S8).
Conflicts of Interest
A.G.H. is supported by the Dutch Gastroenterology Foundation, the Amsterdam UMC Fellowship and Health~Holland TKI PPP grants. Q.M.A. is a Newcastle NIHR Biomedical Research Centre investigator. The other authors have no conflict of interest.
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease–A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef]
- Brunt, E.M.; Clouston, A.D.; Goodman, Z.; Guy, C.; Kleiner, D.E.; Lackner, C.; Tiniakos, D.G.; Wee, A.; Yeh, M.; Leow, W.Q. Complexity of ballooned hepatocyte feature recognition: Defining a training atlas for artificial intelligence-based imaging in NAFLD. J. Hepatol. 2022, 76, 1030–1041. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; Group, L.S. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef]
- Sewell, C. Tissue Pathways for Liver Biopsies for the Investigation of Medical Disease and for Focal Lesions; The Royal College of Pathologists: London, UK, 2008. [Google Scholar]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Palayew, A.; Carrieri, P.; Ekstedt, M.; Marchesini, G.; Novak, K.; Ratziu, V.; Romero-Gómez, M.; Tacke, F.; Zelber-Sagi, S. European ‘NAFLD Preparedness Index’—Is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021, 3, 100234. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Palayew, A.; Tacke, F.; Ekstedt, M.; Marchesini, G.; Romero-Gómez, M.; Ratziu, V.; Novak, K.; Cortez-Pinto, H.; Anstee10, Q.M. The European NAFLD policy index–how well are countries prepared? Hepatology 2020, 72, 413A. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Development, Policy Issues for the Development and Use of Biomarkers in Health. 2011. Available online: www.oecd.org/health/biotech/49023036.pdf (accessed on 28 February 2022).
- Diamandis, E.P. The failure of protein cancer biomarkers to reach the clinic: Why, and what can be done to address the problem? BMC Med. 2012, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Biomarker failures. Clin. Chem. 2013, 59, 202–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frangogiannis, N.G. Biomarkers: Hopes and challenges in the path from discovery to clinical practice. Transl. Res. 2012, 159, 197–204. [Google Scholar] [CrossRef]
- Freue, G.V.C.; Meredith, A.; Smith, D.; Bergman, A.; Sasaki, M.; Lam, K.K.; Hollander, Z.; Opushneva, N.; Takhar, M.; Lin, D. Computational biomarker pipeline from discovery to clinical implementation: Plasma proteomic biomarkers for cardiac transplantation. PLoS Comput. Biol. 2013, 9, e1002963. [Google Scholar]
- Ioannidis, J.P.; Bossuyt, P.M. Waste, leaks, and failures in the biomarker pipeline. Clin. Chem. 2017, 63, 963–972. [Google Scholar] [CrossRef]
- Oldenburg, B.; Glanz, K. Diffusion of innovations. In Health Behavior and Health Education: Theory, Research, and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 313–330. [Google Scholar]
- Mischak, H.; Ioannidis, J.P.; Argiles, A.; Attwood, T.K.; Bongcam-Rudloff, E.; Broenstrup, M.; Charonis, A.; Chrousos, G.P.; Delles, C.; Dominiczak, A. Implementation of proteomic biomarkers: Making it work. Eur. J. Clin. Investig. 2012, 42, 1027–1036. [Google Scholar] [CrossRef]
- Pirmohamed, M. Acceptance of biomarker-based tests for application in clinical practice: Criteria and obstacles. Clin. Pharmacol. Ther. 2010, 88, 862–866. [Google Scholar] [CrossRef]
- Nilsen, P. Making sense of implementation theories, models, and frameworks. In Implementation Science 3.0; Springer: Cham, Switzerland, 2020; pp. 53–79. [Google Scholar]
- Greenhalgh, T.; Abimbola, S. The NASSS framework-a synthesis of multiple theories of technology implementation. Stud. Health Technol. Inf. 2019, 263, 193–204. [Google Scholar]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond adoption: A new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, M.G.U.; Baillie, A.J.; Louie, E.; Giannopoulos, V.; Wood, K.; Riordan, B.; Haber, P.; Morley, K. Application of the Non-adoption, Abandonment, Scale-up, Spread and, Sustainability (NASSS) Framework to evaluate the role of technology in the Pathways to Comorbidity Care (PCC) implementation project to improve management of comorbid substance use and mental disorders. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Banck, J.K.; Bernhardsson, S. Experiences from implementation of internet-delivered cognitive behaviour therapy for insomnia in psychiatric health care: A qualitative study applying the NASSS framework. BMC Health Serv. Res. 2020, 20, 729. [Google Scholar]
- Strohm, L.; Hehakaya, C.; Ranschaert, E.R.; Boon, W.P.; Moors, E.H. Implementation of artificial intelligence (AI) applications in radiology: Hindering and facilitating factors. Eur. Radiol. 2020, 30, 5525–5532. [Google Scholar] [CrossRef]
- About LITMUS. Available online: https://litmus-project.eu/about/ (accessed on 8 February 2022).
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Gurusamy, K.; Ntaoula, S.; Cholongitas, E.; Davidson, B.; Burroughs, A. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Rosenberg, W.M.; Voelker, M.; Thiel, R.; Becka, M.; Burt, A.; Schuppan, D.; Hubscher, S.; Roskams, T.; Pinzani, M.; Arthur, M.J. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004, 127, 1704–1713. [Google Scholar] [CrossRef]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008, 47, 455–460. [Google Scholar] [CrossRef]
- Nielsen, M.; Karsdal, M.A. Type III collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 21–30. [Google Scholar]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Löffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; Bossuyt, P.M. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef]
- Mak, A.L.; Lee, J.; van Dijk, A.-M.; Vali, Y.; Aithal, G.P.; Schattenberg, J.M.; Anstee, Q.M.; Brosnan, M.J.; Zafarmand, M.H.; Ramsoekh, D. Systematic Review with Meta-Analysis: Diagnostic Accuracy of Pro-C3 for Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Aishima, S.; Kage, M. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: A multicenter prospective study. J. Gastroenterol. 2020, 55, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Eldh, A.C.; Årestedt, L.; Berterö, C. Quotations in qualitative studies: Reflections on constituents, custom, and purpose. Int. J. Qual. Methods 2020, 19, 1609406920969268. [Google Scholar] [CrossRef]
- Lincoln, Y.S.; Guba, E.G. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Dir. Program Eval. 1986, 1986, 73–84. [Google Scholar] [CrossRef]
- Bryman, A. Integrating quantitative and qualitative research: How is it done? Qual. Res. 2006, 6, 97–113. [Google Scholar] [CrossRef]
- Heale, R.; Forbes, D. Understanding triangulation in research. Evid. Based Nurs. 2013, 16, 98. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015, 42, 533–544. [Google Scholar] [CrossRef]
- Roback, K.; Gäddlin, P.-O.; Nelson, N.; Persson, J. Adoption of medical devices: Perspectives of professionals in Swedish neonatal intensive care. Technol. Health Care 2007, 15, 157–179. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Di Carli, M.F. Methods and limitations of assessing new noninvasive tests: Part II: Outcomes-based validation and reliability assessment of noninvasive testing. Circulation 2008, 117, 2793–2801. [Google Scholar] [CrossRef]
- Hillman, B.J.; Winkler, J.D.; Phelps, C.E.; Aroesty, J.; Williams, A.P. Adoption and diffusion of a new imaging technology: A magnetic resonance imaging prospective. Am. J. Roentgenol. 1984, 143, 913–917. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Anstee, Q.M.; Hagström, H.; Cusi, K.; Cortez-Pinto, H.; Mark, H.E.; Roden, M.; Tsochatzis, E.A.; Wong, V.W.-S.; Younossi, Z.M. Defining comprehensive models of care for NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ekstedt, M.; Marchesini, G.; Mullen, J.; Novak, K.; Pericàs, J.M.; Roel, E.; Romero-Gómez, M.; Ratziu, V.; Tacke, F. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J. Hepatol. 2020, 72, 14–24. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.-F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 2017, 112, 740. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A. Barriers to the introduction of new medical diagnostic tests. Lab. Med. 2013, 44, e132–e136. [Google Scholar] [CrossRef]
- Lam, Y. Scientific challenges and implementation barriers to translation of pharmacogenomics in clinical practice. ISRN Pharmacol. 2013, 2013, 641089. [Google Scholar] [CrossRef] [PubMed]
- Fineout-Overholt, E.; Melnyk, B.M.; Schultz, A. Transforming health care from the inside out: Advancing evidence-based practice in the 21st century. J. Prof. Nurs. 2005, 21, 335–344. [Google Scholar] [CrossRef]
- Shelton, R.C.; Cooper, B.R.; Stirman, S.W. The sustainability of evidence-based interventions and practices in public health and health care. Annu. Rev. Public Health 2018, 39, 55–76. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Robert, G.; Macfarlane, F.; Bate, P.; Kyriakidou, O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004, 82, 581–629. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).