The Long-Term Effects of Budesonide Nasal Irrigation in Chronic Rhinosinusitis with Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Budenoside Nasal Irrigation
2.3. Clinical Parameters and Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Clinical Parameters of Subjects
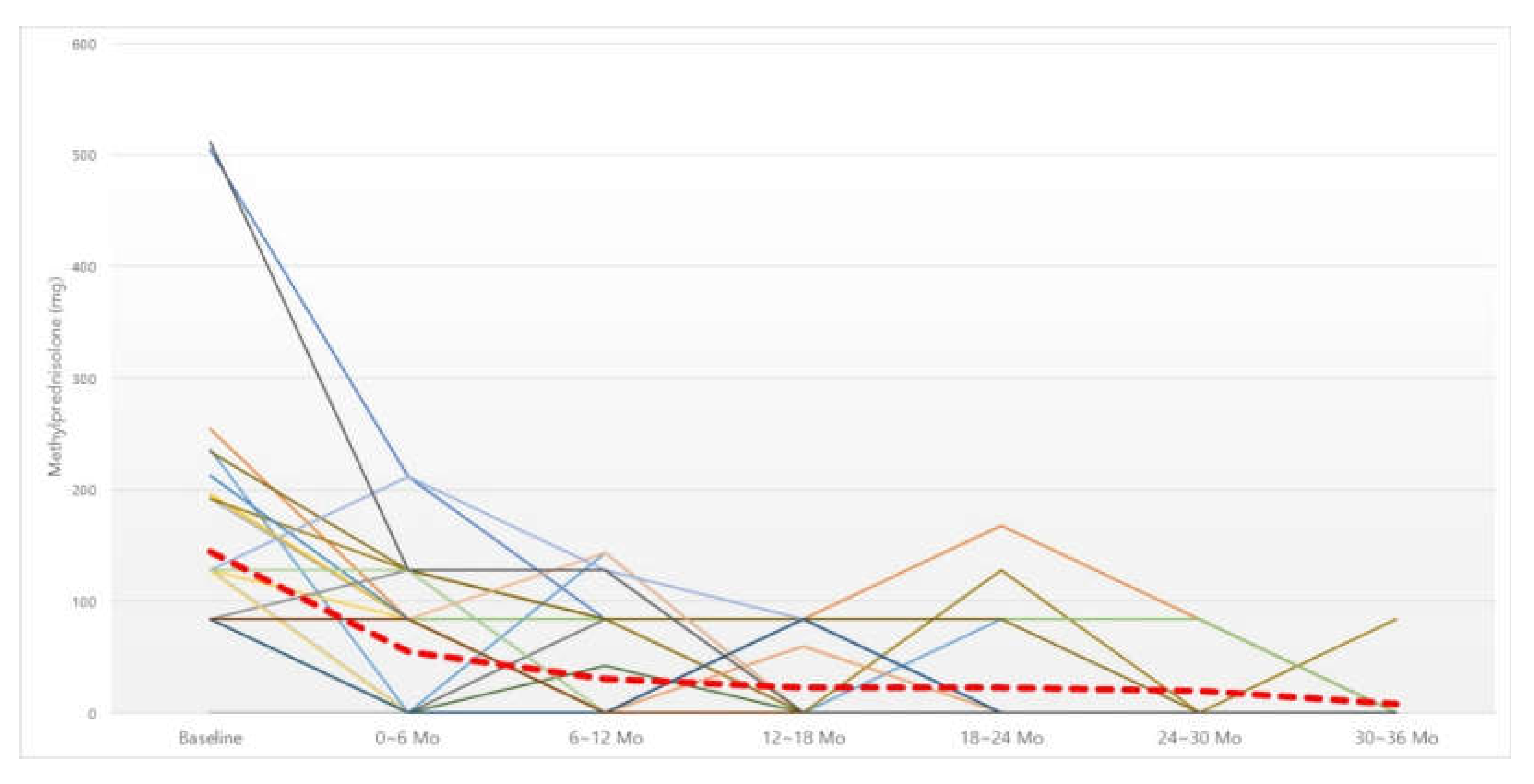
3.2. Six-Month Dosage of Oral Steroid
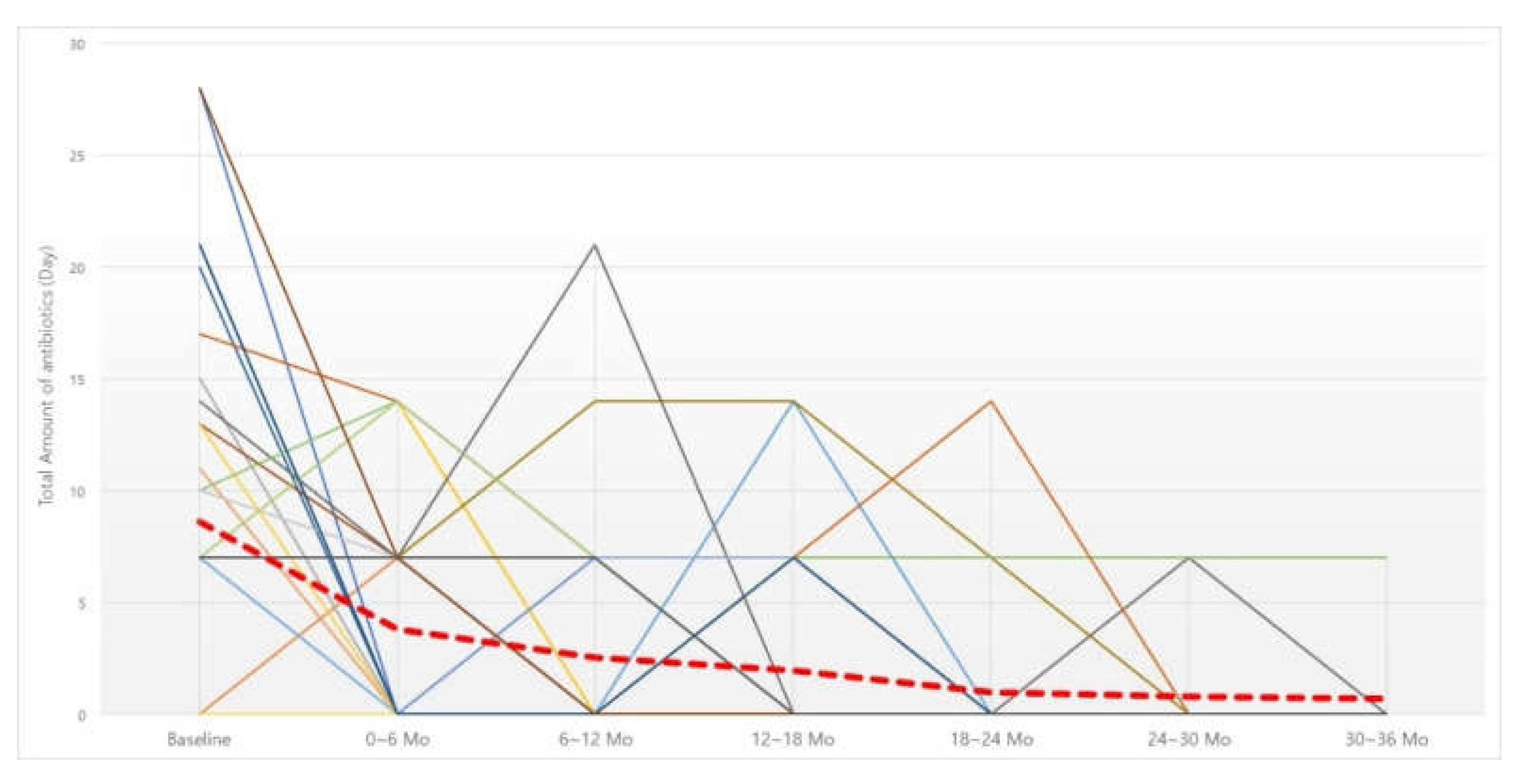
3.3. Six-Month Dosage of Antibiotics
3.4. LK Endoscopy Score
3.5. Nasal Symptoms Questionnaire (SNOT-22)
3.6. Clinical Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.H.; Kim, S.W. Considerations for the use of biologic agents in patients with chronic rhinosinusitis with nasal polyposis. Clin. Exp. Otorhinolaryngol. 2021, 14, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Z.; Chen, X.Z.; Huang, J.C.; Wang, Z.Y.; Xia, L.; Chen, X.H.; Lai, X.P.; Chang, L.H.; Zhang, G.H. Budesonide nasal irrigation improved Lund-Kennedy endoscopic score of chronic rhinosinusitis patients after endoscopic sinus surgery. Eur. Arch. Otorhinolaryngol. 2019, 276, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Kim, D.W. Th2 inflammatory responses in the development of nasal polyps and chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.; Mullol, J. Nasal polyps in patients with asthma: Prevalence, impact, and management challenges. J. Asthma Allergy 2016, 14, 45–53. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, D.K.; Dhong, H.J.; Eun, K.M.; Lee, K.E.; Kong, I.G.; Kim, H.Y.; Chung, S.K.; Kim, D.Y.; Rhee, C.S.; et al. Immunological Characteristics in Refractory Chronic Rhinosinusitis with Nasal Polyps Undergoing Revision Surgeries. Allergy Asthma Immunol. Res. 2019, 11, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Rudmik, L.; Hoy, M.; Schlosser, R.J.; Harvey, R.J.; Welch, K.C.; Lund, V.; Smith, T.L. Topical therapies in the management of chronic rhinosinusitis: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2013, 3, 281–298. [Google Scholar] [CrossRef]
- Periasamy, N.; Pujary, K.; Bhandarkar, A.M.; Bhandarkar, N.D.; Ramaswamy, B. Budesonide vs Saline Nasal Irrigation in Allergic Rhinitis: A Randomized Placebo-Controlled Trial. Otolaryngol. Head Neck Surg. 2020, 162, 979–984. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Seth, R.; Abelson, A.; Batra, P.S. Prevalence of metabolic bone disease among chronic rhinosinusitis patients treated with oral glucocorticoids. Am. J. Rhinol. Allergy 2010, 24, 215–219. [Google Scholar] [CrossRef]
- Winblad, L.; Larsen, C.G.; Hakansson, K.; Abrahamsen, B.; von Buchwald, C. The risk of osteoporosis in oral steroid treatment for nasal polyposis: A systematic review. Rhinology 2017, 55, 195–201. [Google Scholar] [CrossRef]
- Mullol, J.; Obando, A.; Pujols, L.; Alobid, I. Corticosteroid treatment in chronic rhinosinusitis: The possibilities and the limits. Immunol. Allergy Clin. N. Am. 2009, 29, 657–668. [Google Scholar] [CrossRef]
- Kang, T.W.; Chung, J.H.; Cho, S.H.; Lee, S.H.; Kim, K.R.; Jeong, J.H. The effectiveness of budesonide nasal irrigation after endoscopic sinus surgery in chronic rhinosinusitis with asthma. Clin. Exp. Otorhinolaryngol. 2017, 10, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, A.N.; Chandra, R.K.; Chang, D.; Conley, D.B.; Anju, T.P.; Grammer, L.C.; Schleimer, R.T.; Kern, R.C. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am. J. Rhinol. Allergy 2009, 23, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.; Scadding, G.K.; Lund, V.J.; Saleh, H. Treatment of chronic rhinosinusitis and its effects on asthma. Eur. Respir. J. 2006, 28, 68–74. [Google Scholar] [CrossRef]
- Lehrer, E.; Mullol, J.; Agredo, F.; Alobid, I. Management of chronic rhinosinusitis in asthma patients: Is there still a debate? Curr. Allergy Asthma Rep. 2014, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Gao, Y.; Wang, K.; Lou, H.; Meng, Y.; Wang, C. Long-term outcomes of different endoscopic sinus surgery in recurrent chronic rhinosinusitis with nasal polyps and asthma. Rhinology 2020, 58, 126–135. [Google Scholar] [CrossRef]
- Gill, A.S.; Smith, K.A.; Meeks, H.; Oakley, G.M.; Curtin, K.; LeClair, L.; Howe, H.; Orlandi, R.R.; Alt, J.A. Asthma increases long-term revision rates of endoscopic sinus surgery in chronic rhinosinusitis with and without nasal polyposis. Int. Forum Allergy Rhinol. 2021, 11, 1197–1206. [Google Scholar] [CrossRef]
- Thomas, W.W.; Harvey, R.J.; Rudmik, L.; Hwang, P.H.; Schlosser, R.J. Distribution of topical agents to the paranasal sinuses: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2013, 3, 691–703. [Google Scholar] [CrossRef]
- Tait, S.; Kallogjeri, D.; Suko, J.; Kukuljan, S.; Schneider, J.; Piccirillo, J.F. Effect of Budesonide Added to Large-Volume, Low-pressure Saline Sinus Irrigation for Chronic Rhinosinusitis: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 605–612. [Google Scholar] [CrossRef]
- Harvey, R.J.; Snidvongs, K.; Kalish, L.H.; Oakley, G.M.; Sacks, R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 461–470. [Google Scholar] [CrossRef]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int. Forum Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef]
- Song, Y.J.; Cho, S.W. Biomarkers in Chronic Rhinosinusitis with Nasal Polyp: Personalized Medicine Based on Endotype. Korean J. Otorhinolaryngol. Head Neck Surg. 2019, 62, 427–434. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, D.-K. New Discoveries Regarding Endotypes of Chronic Rhinosinusitis with Nasal Polyp. Korean J. Otorhinolaryngol. Head Neck Surg. 2017, 60, 431–436. [Google Scholar] [CrossRef][Green Version]
- Snidvongs, K.; Pratt, E.; Chin, D.; Sacks, R.; Earls, P.; Harvey, R.J. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2012, 2, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Seiberling, K.A.; Chang, D.F.; Nyirady, J.; Park, F.; Church, C.A. Effect of intranasal budesonide irrigations on intraocular pressure. Int. Forum Allergy Rhinol. 2013, 3, 704–707. [Google Scholar] [CrossRef]
- Smith, K.A.; French, G.; Mechor, B.; Rudmik, L. Safety of long-term high-volume sinonasal budesonide irrigations for chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 228–232. [Google Scholar] [CrossRef]
- Soudry, E.; Wang, J.; Vaezeafshar, R.; Katznelson, L.; Hwang, P.H. Safety analysis of long-term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2016, 6, 568–572. [Google Scholar] [CrossRef]
- Franzese, C.B. The Role of Biologics in the Treatment of Nasal Polyps. Immunol. Allergy Clin. N. Am. 2020, 40, 295–302. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]

| Baseline | 0–6 Mo | 6–12 Mo | 12–18 Mo | 18–24 Mo | 24–30 Mo | 30–36 Mo | n | p |
|---|---|---|---|---|---|---|---|---|
| 145.05 ± 114.33 | 55.15 ± 64.94 | 33 | <0.001 | |||||
| 145.05 ± 114.33 | 30.48 ± 50.19 | 33 | <0.001 | |||||
| 146.95 ± 115.62 | 22.88 ± 37.37 | 32 | <0.001 | |||||
| 154.23 ± 120.22 | 22.57 ± 46.57 | 28 | <0.001 | |||||
| 185.36 ± 142.60 | 19.76 ± 36.73 | 17 | <0.001 | |||||
| 189.68 ± 137.76 | 8.40 ± 26.56 | 10 | 0.003 |
| Baseline | 0–6 Mo | 6–12 Mo | 12–18 Mo | 18–24 Mo | 24–30 Mo | 30–36 Mo | n | p |
|---|---|---|---|---|---|---|---|---|
| 145.05 ± 114.33 | 55.15 ± 64.94 | 33 | <0.001 | |||||
| 145.05 ± 114.33 | 55.15 ± 64.94 | 30.48 ± 50.19 | 33 | <0.001 | ||||
| 146.95 ± 115.62 | 54.25 ± 65.77 | 31.44 ± 50.69 | 22.88 ± 37.37 | 32 | <0.001 | |||
| 154.23 ± 120.22 | 57.43 ± 66.54 | 35.93 ± 52.75 | 26.14 ± 38.93 | 22.57 ± 46.57 | 28 | <0.001 | ||
| 185.36 ± 142.60 | 74.82 ± 74.36 | 50.71 ± 54.11 | 29.65 ± 41.38 | 37.18 ± 55.53 | 19.76 ± 36.73 | 17 | <0.001 | |
| 189.68 ± 137.76 | 55.20 ± 60.36 | 60.80 ± 56.15 | 33.60 ± 43.38 | 54.80 ± 63.02 | 25.20 ± 40.58 | 8.40 ± 26.56 | 10 | <0.001 |
| Baseline | 0–6 Mo | 6–12 Mo | 12–18 Mo | 18–24 Mo | 24–30 Mo | 30–36 Mo | n | p |
|---|---|---|---|---|---|---|---|---|
| 145.05 ± 114.33 | 55.15 ± 64.94 | 33 | <0.001 * | |||||
| 55.15 ± 64.94 | 30.48 ± 50.19 | 33 | 0.029 * | |||||
| 31.44 ± 50.69 | 22.88 ± 7.37 | 32 | 0.412 | |||||
| 26.14 ± 38.93 | 22.57 ± 46.57 | 28 | 0.696 | |||||
| 37.18 ± 55.53 | 19.76 ± 36.73 | 17 | 0.090 | |||||
| 25.20 ± 40.58 | 8.40 ± 26.56 | 10 | 0.343 |
| Baseline | 0–6 Mo | 6–12 Mo | 12–18 Mo | 18–24 Mo | 24–30 Mo | 30–36 Mo | n | p |
|---|---|---|---|---|---|---|---|---|
| 8.61 ± 8.32 | 3.82 ± 4.98 | 33 | 0.005 | |||||
| 8.61 ± 8.32 | 2.55 ± 4.89 | 33 | 0.001 | |||||
| 8.88 ± 8.31 | 1.97 ± 4.07 | 32 | 0.000 | |||||
| 8.82 ± 8.46 | 1.00 ± 3.14 | 28 | <0.001 | |||||
| 8.18 ± 7.97 | 0.82 ± 2.32 | 17 | 0.001 | |||||
| 6.90 ± 5.82 | 0.70 ± 2.21 | 10 | 0.008 |
| Baseline | 0–6 Mo | 6–12 Mo | 12–18 Mo | 18–24 Mo | 24–30 Mo | 30–36 Mo | n | p |
|---|---|---|---|---|---|---|---|---|
| 4.94 ± 2.21 | 3.12 ± 1.69 | 33 | <0.001 | |||||
| 4.94 ± 2.21 | 2.24 ± 1.58 | 33 | <0.001 | |||||
| 4.91 ± 2.23 | 1.78 ± 1.66 | 32 | <0.001 | |||||
| 5.19 ± 2.08 | 1.62 ± 1.68 | 26 | <0.001 | |||||
| 4.94 ± 1.63 | 1.50 ± 1.54 | 18 | <0.001 | |||||
| 4.80 ± 1.48 | 2.00 ± 1.33 | 10 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.M.; Kwak, J.H.; Kim, M.K.; Tae, K.; Cho, S.H.; Jeong, J.H. The Long-Term Effects of Budesonide Nasal Irrigation in Chronic Rhinosinusitis with Asthma. J. Clin. Med. 2022, 11, 2690. https://doi.org/10.3390/jcm11102690
Jung SM, Kwak JH, Kim MK, Tae K, Cho SH, Jeong JH. The Long-Term Effects of Budesonide Nasal Irrigation in Chronic Rhinosinusitis with Asthma. Journal of Clinical Medicine. 2022; 11(10):2690. https://doi.org/10.3390/jcm11102690
Chicago/Turabian StyleJung, Seon Min, Jin Hye Kwak, Moo Keon Kim, Kyung Tae, Seok Hyun Cho, and Jin Hyeok Jeong. 2022. "The Long-Term Effects of Budesonide Nasal Irrigation in Chronic Rhinosinusitis with Asthma" Journal of Clinical Medicine 11, no. 10: 2690. https://doi.org/10.3390/jcm11102690
APA StyleJung, S. M., Kwak, J. H., Kim, M. K., Tae, K., Cho, S. H., & Jeong, J. H. (2022). The Long-Term Effects of Budesonide Nasal Irrigation in Chronic Rhinosinusitis with Asthma. Journal of Clinical Medicine, 11(10), 2690. https://doi.org/10.3390/jcm11102690






