Risk Assessment of CHD Using Retinal Images with Machine Learning Approaches for People with Cardiometabolic Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Retinal Imaging Acquisition and Analysis
2.3. Statistical Analysis
2.4. Sample Size Estimation
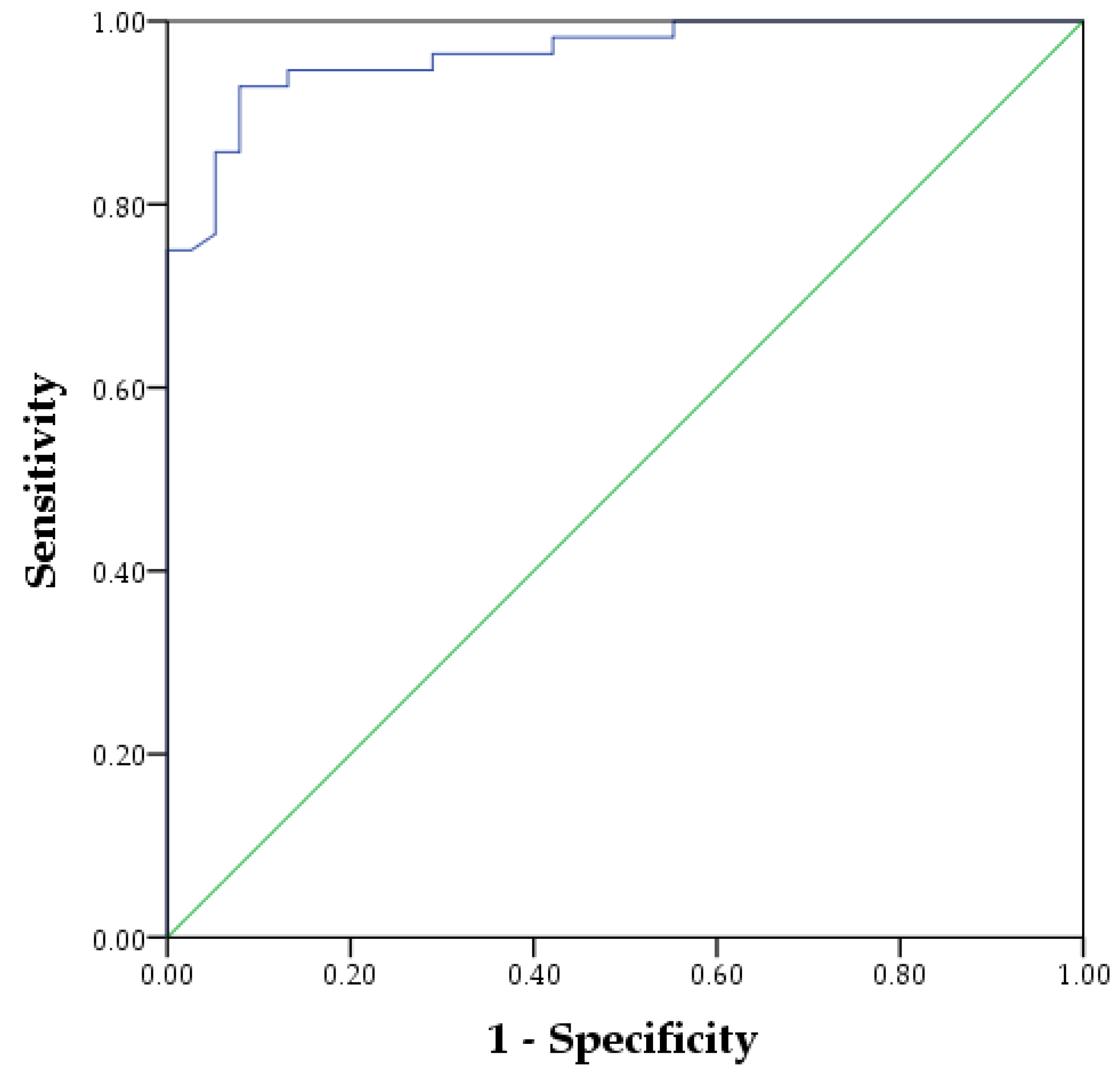
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health 2003, 16, 230. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef]
- Dalen, J.E.; Alpert, J.S.; Goldberg, R.J.; Weinstein, R.S. The epidemic of the 20(th) century: Coronary heart disease. Am. J. Med. 2014, 127, 807–812. [Google Scholar] [CrossRef]
- Gupta, R.; Mohan, I.; Narula, J. Trends in Coronary Heart Disease Epidemiology in India. Ann. Glob. Health 2016, 82, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.F.; Wang, Y.M.; Zhu, J.Z.; Zhou, Q.Y.; Wang, N.F. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur. J. Prev. Cardiol. 2016, 23, 530–543. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prevalence of coronary heart disease—United States, 2006–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1377–1381. [Google Scholar]
- Ferreira-González, I. The Epidemiology of Coronary Heart Disease. Rev. Española Cardiol. Engl. Ed. 2014, 67, 139–144. [Google Scholar] [CrossRef]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Yu, C.; Zhou, M.; Wang, L.; Zhang, Y.; Luo, L. Burden of Ischaemic heart disease and attributable risk factors in China from 1990 to 2015: Findings from the global burden of disease 2015 study. BMC Cardiovasc. Disord. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, Y.; Liu, X.; Zhang, H.; Abdulai, T.; Tu, R.; Tian, Z.; Qian, X.; Jiang, J.; Qiao, D.; et al. Prevalence and Influencing Factors of Coronary Heart Disease and Stroke in Chinese Rural Adults: The Henan Rural Cohort Study. Front. Public Health 2020, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.; Arnold, M.; Clarke, R.; Du, H.; Wan, E.; Kurmi, O.; Chen, Y.; Guo, Y.; Bian, Z.; Collins, R.; et al. Assessment of the Role of Carotid Atherosclerosis in the Association Between Major Cardiovascular Risk Factors and Ischemic Stroke Subtypes. JAMA 2019, 2, e194873. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission. China Health and Family Planning Statistical Yearbook 2017; Peking Union Medical College: Beijing, China, 2017. [Google Scholar]
- Moran, A.; Zhao, D.; Gu, D.; Coxson, P.; Chen, C.-S.; Cheng, J.; Liu, J.; He, J.; Goldman, L. The future impact of population growth and aging on coronary heart disease in China: Projections from the Coronary Heart Disease Policy Model-China. BMC Public Health 2008, 8, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lown, B.; Wolf, M. Approaches to sudden death from coronary heart disease. Circulation 1971, 44, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.Y.; Hu, L.W.; Jalaludin, B.; Knibbs, L.D.; Markevych, I.; Heinrich, J.; Bloom, M.S.; Morawska, L.; Lin, S.; Jalava, P.; et al. Association Between Residential Greenness, Cardiometabolic Disorders, and Cardiovascular Disease Among Adults in China. JAMA 2020, 3, e2017507. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Kokubo, Y.; Matsumoto, C. Hypertension Is a Risk Factor for Several Types of Heart Disease: Review of Prospective Studies. Adv. Exp. Med. Biol. 2017, 956, 419–426. [Google Scholar] [CrossRef]
- Xu, G.; You, D.; Wong, L.; Duan, D.; Kong, F.; Zhang, X.; Zhao, J.; Xing, W.; Han, L.; Li, L. Risk of all-cause and CHD mortality in women versus men with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019, 180, 243–255. [Google Scholar] [CrossRef]
- Reaven, G.M. Multiple CHD risk factors in type 2 diabetes: Beyond hyperglycaemia. Diabetes Obes. Metab. 2002, 4 (Suppl. 1), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. Fat, Sugar, Whole Grains and Heart Disease: 50 Years of Confusion. Nutrients 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Dyslipidemia as a risk factor for ischemic stroke. Curr. Top. Med. Chem. 2009, 9, 1291–1297. [Google Scholar] [CrossRef]
- Yu, J.N.; Cunningham, J.A.; Thouin, S.R.; Gurvich, T.; Liu, D. Hyperlipidemia. Prim. Care 2000, 27, 541–587. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Attia, J.; D’Este, C.; Yu, X.-H.; Wu, X.-G. A risk score predicted coronary heart disease and stroke in a Chinese cohort. J. Clin. Epidemiol. 2005, 58, 951–958. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.M.; Wilson, P.W.; Odell, P.M.; Kannel, W.B. An updated coronary risk profile. A statement for health professionals. Circulation 1991, 83, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.M.; Odell, P.M.; Wilson, P.W.; Kannel, W.B. Cardiovascular disease risk profiles. Am. Heart J. 1991, 121, 293–298. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Y.; D’Agostino, R.B., Sr.; Wu, Z.; Wang, W.; Sun, J.; Wilson, P.W.; Kannel, W.B.; Zhao, D. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA 2004, 291, 2591–2599. [Google Scholar] [CrossRef]
- Chambless, L.E.; Folsom, A.R.; Sharrett, A.R.; Sorlie, P.; Couper, D.; Szklo, M.; Nieto, F.J. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J. Clin. Epidemiol. 2003, 56, 880–890. [Google Scholar] [CrossRef]
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 2002, 105, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, M.; Chiodini, P.; Chambless, L.E.; Cesana, G.; Vanuzzo, D.; Panico, S.; Sega, R.; Pilotto, L.; Palmieri, L.; Giampaoli, S. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE Cohort Study prediction equation. Int. J. Epidemiol. 2005, 34, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M. The UKPDS risk engine: A model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin. Sci. 2001, 101, 671–679. [Google Scholar] [CrossRef]
- Pekkanen, J.; Tervahauta, M.; Nissinen, A.; Karvonen, M.J. Does the predictive value of baseline coronary risk factors change over a 30-year follow-up? Cardiology 1993, 82, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Benfante, R.; Reed, D. Is elevated serum cholesterol level a risk factor for coronary heart disease in the elderly? JAMA 1990, 263, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Blackburn, H.; Kromhout, D.; Nissinen, A.; Karvonen, M.; Aravanis, C.; Dontas, A.; Fidanza, F.; Giampaoli, S. The inverse relation of average population blood pressure and stroke mortality rates in the seven countries study: A paradox. Eur. J. Epidemiol. 1997, 13, 379–386. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Klein, B.E.K.; Tielsch, J.M.; Hubbard, L.; Nieto, F.J. Retinal Microvascular Abnormalities and their Relationship with Hypertension, Cardiovascular Disease, and Mortality. Surv. Ophthalmol. 2001, 46, 59–80. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Li, X.; Li, Y.; Zhao, L.; Chen, Z.; Li, Y.; Rao, X.; Zhou, B.; Detrano, R.; et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation 2006, 114, 2217–2225. [Google Scholar] [CrossRef] [Green Version]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef]
- Cheng, L.; Barlis, P.; Gibson, J.; Colville, D.; Hutchinson, A.; Gleeson, G.; Lamoureux, E.; VanGaal, W.; Savige, J. Microvascular retinopathy and angiographically-demonstrated coronary artery disease: A cross-sectional, observational study. PLoS ONE 2018, 13, e0192350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaee, A.; Asharin, M.R.; Dehghan, M.H.; Pourbehi, M.R.; Nasiri-Ahmadabadi, M.; Assadi, M. Retinal vessel abnormalities predict coronary artery diseases. Perfusion 2013, 28, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Moss, H.E. Retinal Vascular Changes are a Marker for Cerebral Vascular Diseases. Curr. Neurol. Neurosci. Rep. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yu, H.; Yang, Z.; Zee, B.; Lee, J.; Kuang, L. Prediction Factors of Recurrent Stroke among Chinese Adults Using Retinal Vasculature Characteristics. J. Stroke Cerebrovasc. Dis. 2017, 26, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leng, F.; Li, Z.; Tang, X.; Qian, H.; Li, X.; Zhang, Y.; Chen, X.; Du, H.; Liu, P. Retinal vascular abnormalities and their associations with cardiovascular and cerebrovascular diseases: A Study in rural southwestern Harbin, China. BMC Ophthalmol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007, 30, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Liew, G.; Wong, T.Y.; Smith, W.; Klein, R.; Leeder, S.R.; Mitchell, P. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart Br. Card. Soc. 2006, 92, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Wong, T.Y. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 2007, 114, 1884–1892. [Google Scholar] [CrossRef]
- Doubal, F.N.; de Haan, R.; MacGillivray, T.J.; Cohn-Hokke, P.E.; Dhillon, B.; Dennis, M.S.; Wardlaw, J.M. Retinal arteriolar geometry is associated with cerebral white matter hyperintensities on magnetic resonance imaging. Int. J. Stroke Off. J. Int. Stroke Soc. 2010, 5, 434–439. [Google Scholar] [CrossRef]
- Witt, N.; Wong, T.Y.; Hughes, A.D.; Chaturvedi, N.; Klein, B.E.; Evans, R.; McNamara, M.; Thom, S.A.; Klein, R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006, 47, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Thorac. Cardiovasc. Surg. 2015, 149, e5–e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zee, B.C.-y.; Lee, J.J.-w.; Li, E.Q. Method and Device for Retinal Image Analysis. U.S. Patent 8,787,638, 22 July 2014. [Google Scholar]
- Lai, M.; Lee, J.; Chiu, S.; Charm, J.; So, W.Y.; Yuen, F.P.; Kwok, C.; Tsoi, J.; Lin, Y.; Zee, B. A machine learning approach for retinal images analysis as an objective screening method for children with autism spectrum disorder. eClinicalMedicine 2020, 28, 100588. [Google Scholar] [CrossRef] [PubMed]
- Guo, V.Y.; Cao, B.; Wu, X.; Lee, J.J.W.; Zee, B.C. Prospective Association between Diabetic Retinopathy and Cardiovascular Disease-A Systematic Review and Meta-analysis of Cohort Studies. J. Stroke Cerebrovasc. Dis. 2016, 25, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Guo, V.Y.; Chan, J.C.; Chung, H.; Ozaki, R.; So, W.; Luk, A.; Lam, A.; Lee, J.; Zee, B.C. Retinal Information is Independently Associated with Cardiovascular Disease in Patients with Type 2 diabetes. Sci. Rep. 2016, 6, 19053. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.-E.; Chen, P.-H.; Lin, C.-J.; Joachims, T. Working set selection using second order information for training support vector machines. J. Mach. Learn. Res. 2005, 6, 1889–1918. [Google Scholar]
- Schölkopf, B.; Smola, A.J.; Bach, F. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond; MIT Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Lau, A.Y.; Mok, V.; Lee, J.; Fan, Y.; Zeng, J.; Lam, B.; Wong, A.; Kwok, C.; Lai, M.; Zee, B. Retinal image analytics detects white matter hyperintensities in healthy adults. Ann. Clin. Transl. Neurol. 2019, 6, 98–105. [Google Scholar] [CrossRef]
- Tedeschi-Reiner, E.; Strozzi, M.; Skoric, B.; Reiner, Z. Relation of Atherosclerotic Changes in Retinal Arteries to the Extent of Coronary Artery Disease. Am. J. Cardiol. 2005, 96, 1107–1109. [Google Scholar] [CrossRef]
- Tedeschi-Reiner, E.; Reiner, Z.; Sonicki, Z. Atherosclerosis of retinal arteries in men: Role of serum lipoproteins and apoproteins. Croat. Med. J. 2004, 45, 333–337. [Google Scholar] [PubMed]
- Theuerle, J.D.; Al-Fiadh, A.H.; Amirul Islam, F.M.; Patel, S.K.; Burrell, L.M.; Wong, T.Y.; Farouque, O. Impaired retinal microvascular function predicts long-term adverse events in patients with cardiovascular disease. Cardiovasc. Res. 2021, 117, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- McGeechan, K.; Liew, G.; Macaskill, P.; Irwig, L.; Klein, R.; Klein, B.E.; Wang, J.J.; Mitchell, P.; Vingerling, J.R.; Dejong, P.T.; et al. Meta-analysis: Retinal vessel caliber and risk for coronary heart disease. Ann. Intern. Med. 2009, 151, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, C. Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD. Open Life Sci. 2021, 16, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Cordina, R.; Leaney, J.; Golzan, M.; Grieve, S.; Celermajer, D.S.; Graham, S.L. Ophthalmological consequences of cyanotic congenital heart disease: Vascular parameters and nerve fibre layer. Clin. Exp. Ophthalmol. 2015, 43, 115–123. [Google Scholar] [CrossRef]
- Hart, W.E.; Goldbaum, M.; Côté, B.; Kube, P.; Nelson, M.R. Measurement and classification of retinal vascular tortuosity. Int. J. Med. Inform. 1999, 53, 239–252. [Google Scholar] [CrossRef]
- Vilela, M.A.; Amaral, C.E.; Ferreira, M.A.T. Retinal vascular tortuosity: Mechanisms and measurements. Eur. J. Ophthalmol. 2021, 31, 1497–1506. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, S.M.; Kang, D.W.; Kwon, S.U.; Suh, D.C.; Kim, J.S. Vascular tortuosity may be related to intracranial artery atherosclerosis. Int. J. Stroke 2015, 10, 1081–1086. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Zheng, Y.; Hsu, W.; Lee, M.L.; Lau, Q.P.; Mitchell, P.; Wang, J.J.; Klein, R.; Wong, T.Y. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology 2011, 118, 812–818. [Google Scholar] [CrossRef]
- Sasongko, M.B.; Wong, T.Y.; Nguyen, T.T.; Cheung, C.Y.; Shaw, J.E.; Wang, J.J. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia 2011, 54, 2409–2416. [Google Scholar] [CrossRef]
- Sandoval-Garcia, E.; McLachlan, S.; Price, A.H.; MacGillivray, T.J.; Strachan, M.W.J.; Wilson, J.F.; Price, J.F. Retinal arteriolar tortuosity and fractal dimension are associated with long-term cardiovascular outcomes in people with type 2 diabetes. Diabetologia 2021, 64, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Wong, T.Y.; Tyroler, H.A.; Davis, C.E.; Fuchs, F.D. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br. J. Ophthalmol. 2002, 86, 1002–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.H.; Pan, X.R.; Liu, P.A.; Li, G.W.; Howard, B.V.; Bennett, P.H. Coronary heart disease and diabetic retinopathy in newly diagnosed diabetes in Da Qing, China: The Da Qing IGT and Diabetes Study. Acta Diabetol. 1991, 28, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Butcher, A.; Martin, N.; Farmer, J.; Dobson, P.M.; Bartlett, W.A.; Jones, A.F. Cardiovascular risk assessment in patients with retinal vein occlusion. Br. J. Ophthalmol. 2002, 86, 774–776. [Google Scholar] [CrossRef] [Green Version]
- Rim, T.H.; Lee, C.J.; Tham, Y.C.; Cheung, N.; Yu, M.; Lee, G.; Kim, Y.; Ting, D.S.W.; Chong, C.C.Y.; Choi, Y.S.; et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit. Health 2021, 3, e306–e316. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, J.; Zhu, Y.; Ge, Z.; Hua, R.; Fu, M.; Li, C.; Wang, B.; Dong, L.; Zhao, X.; et al. Deep learning algorithm using fundus photographs for 10-year risk assessment of ischemic cardiovascular diseases in China. Sci. Bull. 2022, 67, 17–20. [Google Scholar] [CrossRef]


| Basic Characteristics | Control n = 128 | CHD n = 188 | p |
|---|---|---|---|
| Age (years) | 52.13 ± 11.78 | 63.89 ± 11.40 | <0.001 |
| Sex n, (%) | <0.001 | ||
| Male | 42(32.81%) | 103(55.79%) | |
| Female | 86(67.19%) | 85(45.21%) | |
| Smoking n, (%) | 0.891 | ||
| No | 115(89.84%) | 168(89.36%) | |
| Yes | 13(10.16%) | 20(10.64%) | |
| Drinking n, (%) | 0.100 | ||
| No | 114(89.06%) | 177(94.15%) | |
| Yes | 14(10.94%) | 11(5.85%) | |
| BMI group | 0.200 | ||
| <24 | 70(54.69%) | 89(47.34%) | |
| ≥24 | 58(45.31%) | 99(52.66%) | |
| Diabetes n, (%) | <0.001 | ||
| No | 97(75.78%) | 95(50.53%) | |
| Yes | 31(24.22%) | 93(49.47%) | |
| HbA1c (%) | 6.25 ± 1.41 | 6.66 ± 1.26 | 0.019 |
| Fasting glucose (mmol/L) | 5.16 ± 2.16 | 5.63 ± 1.95 | 0.050 |
| Hypertension n, (%) | <0.001 | ||
| No | 49(38.28%) | 34(18.09%) | |
| Yes | 79(61.72%) | 154(81.91%) | |
| SBP (mmHg) | 135.39 ± 22.05 | 133.87 ± 20.26 | 0.529 |
| DBP (mmHg) | 85.53 ± 14.39 | 80.64 ± 13.47 | 0.002 |
| Dyslipidemia n, (%) | 0.043 | ||
| No | 50(39.06%) | 53(28.19%) | |
| Yes | 78(60.94%) | 135(71.81%) | |
| TG (mmol/L) | 1.85 ± 1.34 | 1.90 ± 1.90 | 0.791 |
| TC (mmol/L) | 4.56 ± 0.98 | 4.32 ± 1.29 | 0.076 |
| HDL-C (mmol/L) | 1.20 ± 0.33 | 1.13 ± 0.31 | 0.073 |
| LDL-C (mmol/L) | 2.85 ± 0.90 | 2.67 ± 1.10 | 0.119 |
| Retinal Characteristics | Control n = 128 | CHD n = 188 | p |
|---|---|---|---|
| lCRVE | 18.34 ± 0.36 | 18.21 ± 0.38 | 0.002 |
| lMBCV | 1.21 ± 0.03 | 1.20 ± 0.03 | 0.014 |
| lMAasymmetry | 0.85 ± 0.01 | 0.85 ± 0.01 | <0.001 |
| lMVasymmetry | 0.75 ± 0.01 | 0.74 ± 0.01 | 0.008 |
| lAocclusion | 0.13 ± 0.08 | 0.16 ± 0.09 | 0.032 |
| lExudates | 0.23 ± 0.07 | 0.26 ± 0.08 | 0.001 |
| lTortuosity_av | 0.20 ± 0.07 | 0.22 ± 0.08 | 0.020 |
| lTortuosity_a | 0.14 ± 0.06 | 0.16 ± 0.07 | 0.013 |
| lTortuosity_v | 0.15 ± 0.06 | 0.18 ± 0.08 | <0.001 |
| rCRAE | 11.17 ± 0.26 | 11.10 ± 0.25 | 0.028 |
| rMBCV | 1.20 ± 0.02 | 1.20 ± 0.02 | 0.015 |
| rMAangle | 76.76 ± 1.44 | 76.32 ± 1.44 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Lee, J.J.-W.; Zhuo, Y.; Liu, S.; Thomas, R.L.; Owens, D.R.; Zee, B.C.-Y. Risk Assessment of CHD Using Retinal Images with Machine Learning Approaches for People with Cardiometabolic Disorders. J. Clin. Med. 2022, 11, 2687. https://doi.org/10.3390/jcm11102687
Qu Y, Lee JJ-W, Zhuo Y, Liu S, Thomas RL, Owens DR, Zee BC-Y. Risk Assessment of CHD Using Retinal Images with Machine Learning Approaches for People with Cardiometabolic Disorders. Journal of Clinical Medicine. 2022; 11(10):2687. https://doi.org/10.3390/jcm11102687
Chicago/Turabian StyleQu, Yimin, Jack Jock-Wai Lee, Yuanyuan Zhuo, Shukai Liu, Rebecca L. Thomas, David R. Owens, and Benny Chung-Ying Zee. 2022. "Risk Assessment of CHD Using Retinal Images with Machine Learning Approaches for People with Cardiometabolic Disorders" Journal of Clinical Medicine 11, no. 10: 2687. https://doi.org/10.3390/jcm11102687
APA StyleQu, Y., Lee, J. J.-W., Zhuo, Y., Liu, S., Thomas, R. L., Owens, D. R., & Zee, B. C.-Y. (2022). Risk Assessment of CHD Using Retinal Images with Machine Learning Approaches for People with Cardiometabolic Disorders. Journal of Clinical Medicine, 11(10), 2687. https://doi.org/10.3390/jcm11102687






