Effect of Manual Therapy Compared to Ibuprofen on Primary Dysmenorrhea in Young Women—Concentration Assessment of C-Reactive Protein, Vascular Endothelial Growth Factor, Prostaglandins and Sex Hormones
Abstract
:1. Introduction
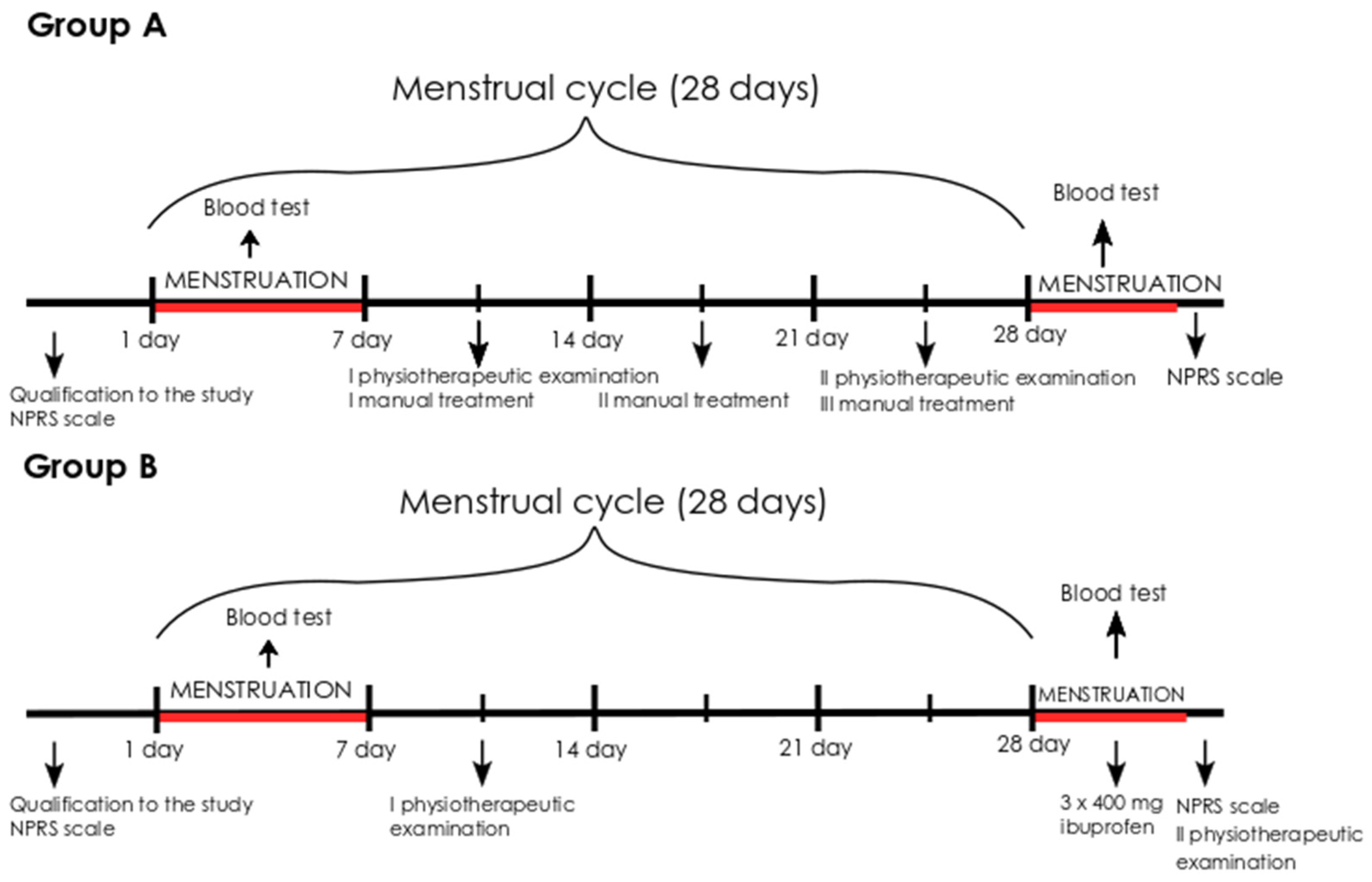
2. Materials and Methods
2.1. Data Collection
2.2. Eligibility Criteria
2.3. Sample Collection
2.4. Physiotherapy Evaluation
2.5. Intervention
2.5.1. Manual Therapy
2.5.2. Pharmacological Treatment
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Studied Women
3.2. Examined Factors before and after the Intervention
3.3. Severity of Dysmenorrhea
3.4. Muscle Dysfunctions
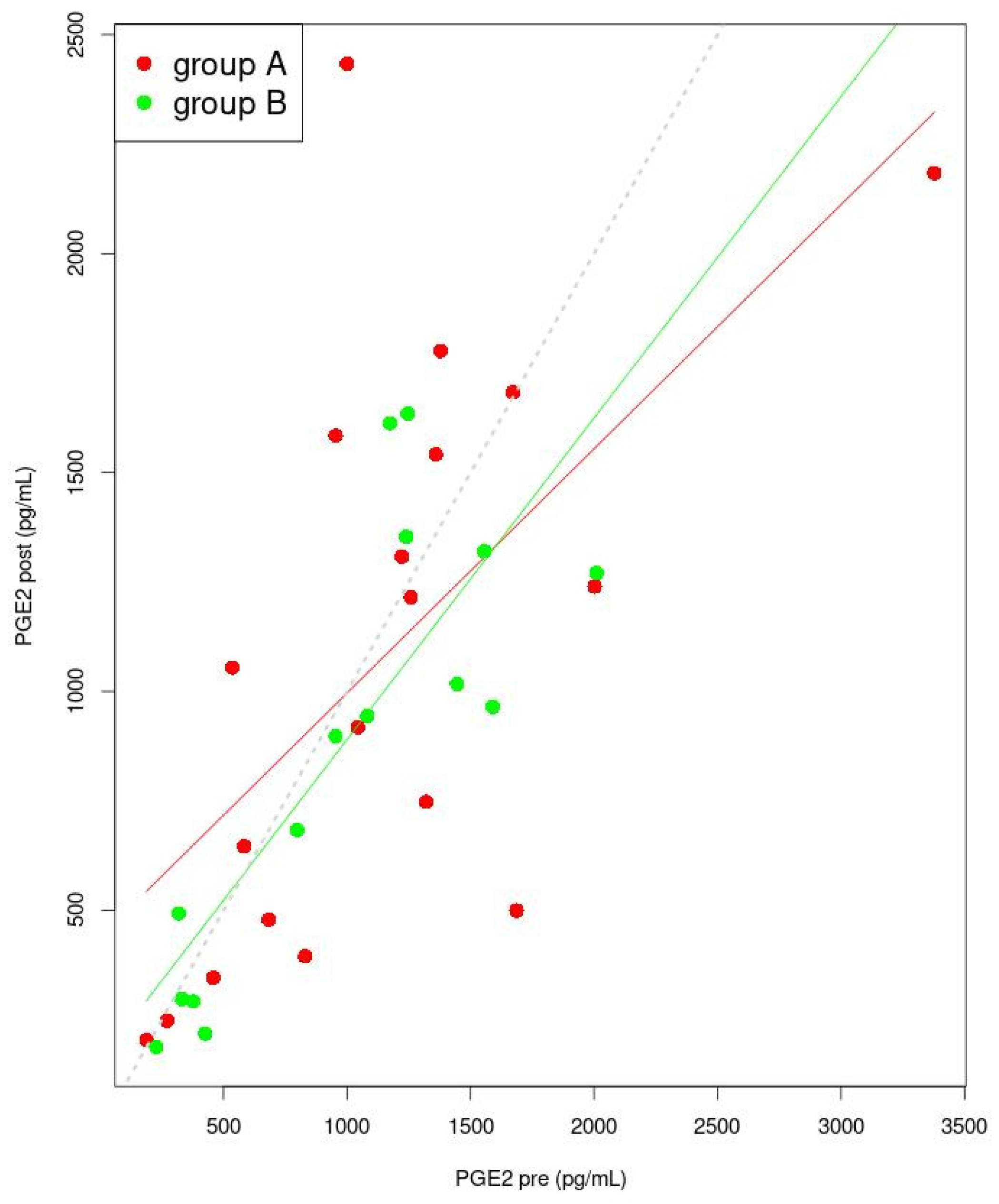
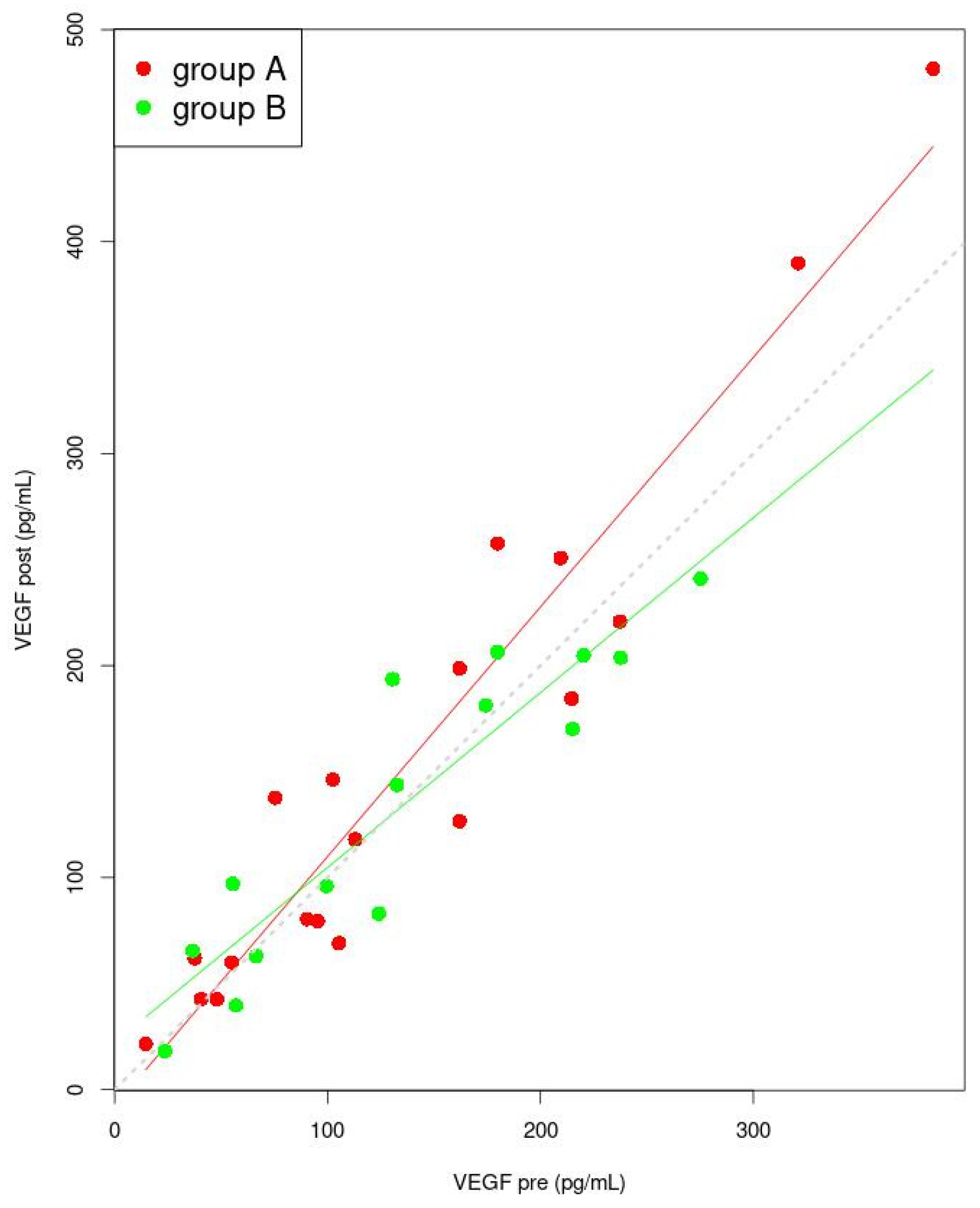
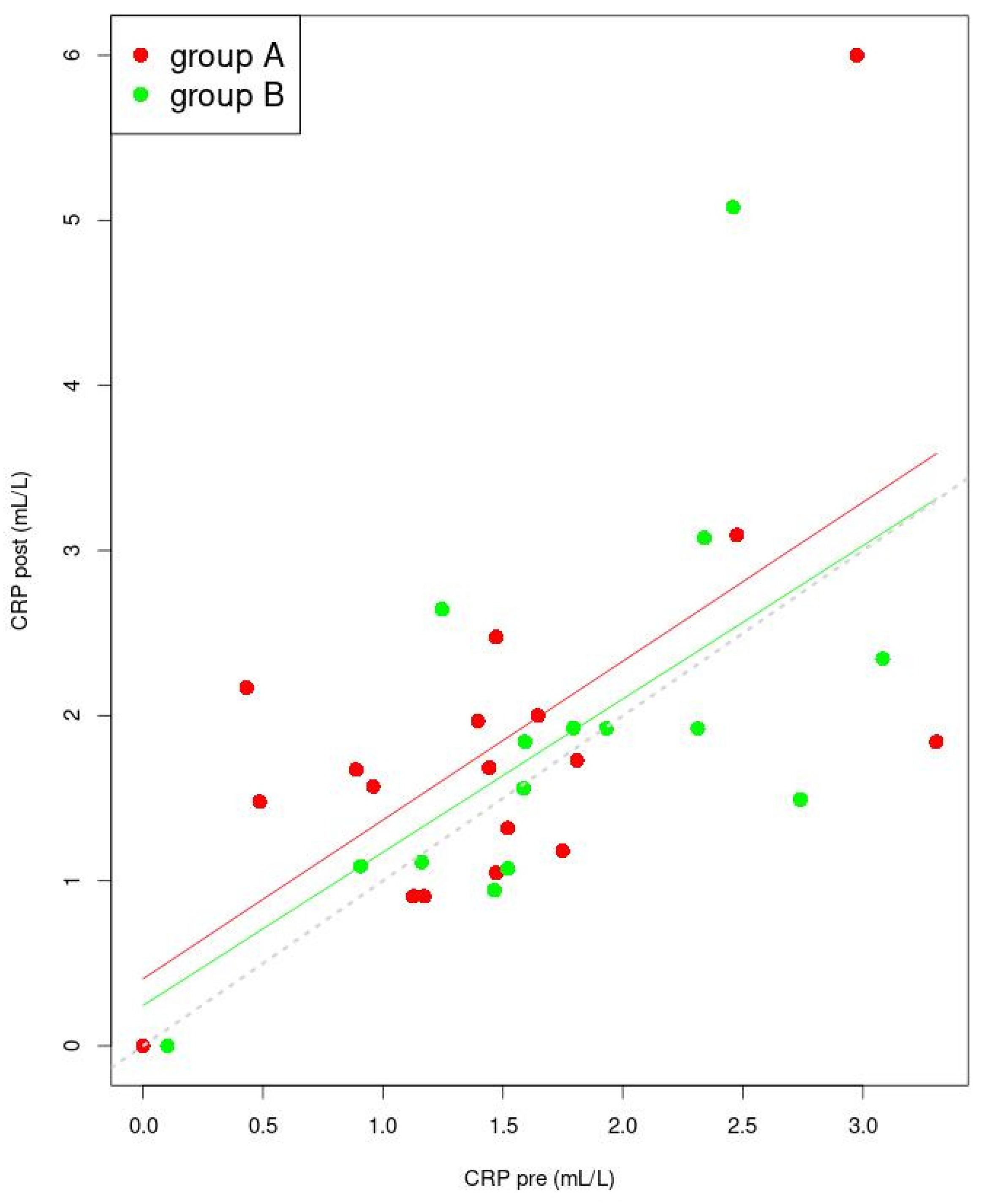
3.5. Correlation between Sex Hormones and Inflammatory Factor Concentrations
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, S.; Wong, B.; Kanumalla, R.; Goldstein, L. Osteopathic manipulative treatment and psychosocial management of dysmenorrhea. J. Am. Osteopath. Assoc. 2020, 120, 479–482. [Google Scholar] [CrossRef]
- Latthe, P.; Latthe, M.; Say, L.; Gülmezoglu, M.; Khan, K.S. WHO systematic review of prevalence of chronic pelvic pain: A neglected reproductive health morbidity. BMC Public Health 2006, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Barcikowska, Z.; Wójcik-Bilkiewicz, K.; Sobierajska-Rek, A.; Grzybowska, M.E.; Wąż, P.; Zorena, K. Dysmenorrhea and Associated Factors among Polish Women: A Cross-Sectional Study. Pain Res. Manag. 2020, 2020, 6161536. [Google Scholar] [CrossRef]
- Dawood, M.Y. Clinical Expert Series Primary Dysmenorrhea Advances in Pathogenesis and Management. Obstet. Gynaecol. 2006, 108, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.Y. Dysmenorrhea. Glob. Libr. Women’s Med. 2008, 7. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th Revision, 5th ed.; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Critchley, H.O.D.; Kelly, R.W.; Brenner, R.M.; Baird, D.T. The endocrinology of menstruation—A role for the immune system. Clin. Endocrinol. 2001, 55, 701–710. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D. Progesterone: A pivotal hormone at menstruation. Ann. N. Y. Acad. Sci. 2011, 1221, 88–97. [Google Scholar] [CrossRef]
- Maybin, J.A.; Hirani, N.; Brown, P.; Jabbour, H.N.; Critchley, H. The Regulation of Vascular Endothelial Growth Factorby Hypoxia and Prostaglandin F2α during Human Endometrial Repair. J. Clin. Endocrinol. Meta 2011, 96, 2475–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.P. Dysmenorrhea and Menorrhagia; Springer: Cham, Switzerland, 2018; pp. 75–86. [Google Scholar]
- Evans, J.; Salamonsen, L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harel, Z. Dysmenorrhea in adolescents and young adults: An update on pharmacological treatments and management strategies. Expert Opin. Pharmacother. 2012, 13, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Lundström, V.; Green, K. Endogenous levels of prostaglandin F2αand its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am. J. Obstet. Gynecol. 1978, 130, 640–646. [Google Scholar] [CrossRef]
- Maybin, J.; Critchley, H. Repair and regeneration of the human endometrium. Expert Rev. Obstet. Gynecol. 2009, 4, 283–298. [Google Scholar] [CrossRef]
- Wander, K.; Brindle, E.; O’Connor, K.A. C-reactive protein across the menstrual cycle. Am. J. Phys. Anthropol. 2008, 136, 138–146. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Wilchesky, M.; Mumford, S.L.; Whitcomb, B.W.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: The BioCycle Study. Am. J. Epidemiol. 2012, 175, 423–431. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Sirivichayakul, S.; Matsumoto, A.K.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Barbosa, D.S.; Moreira, E.G.; Maes, M. Menstruation distress is strongly associated with hormone-immune-metabolic biomarkers. J. Psychosom. Res. 2021, 142, 110355. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.; Farquhar, C.; Proctor, M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 7, CD001751. [Google Scholar] [CrossRef]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Wąż, P.; Zorena, K. An evaluation of the effectiveness of ibuprofen and manual therapy in young women with dysmenorrhea—A pilot study. Healthcare 2021, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Schiøtz, H.A.; Jettestad, M.; Al-Heeti, D. Treatment of dysmenorrhoea with a new TENS device (OVA). J. Obstet. Gynaecol. 2007, 27, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Cortés-Márquez, S.K.; Romero-Quezada, L.C.; Murguía-Cánovas, G.; Jaramillo-Díaz, A.P. Effect of a physiotherapy program in women with primary dysmenorrhea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, I.; Póvoa, A.M. Primary Dysmenorrhea: Assessment and Treatment. Rev. Bras. Ginecol. Obs. 2020, 42, 501–507. [Google Scholar] [CrossRef]
- Dobrzycka, A.; Wilk, I. Evaluation of the effectiveness of self-massage in dysmenorrhea Ocena efektywności automasażu w redukcji bólu menstruacyjnego kobiet. Med. Sci. Pulse 2017, 11, 26–32. [Google Scholar] [CrossRef]
- Posadzki, P.; Ernst, E. Spinal manipulation: An update of a systematic review of systematic reviews. N. Z. Med. J. 2011, 124, 55–71. [Google Scholar]
- Ruffini, N.; D’Alessandro, G.; Cardinali, L.; Frondaroli, F.; Cerritelli, F. Osteopathic manipulative treatment in gynecology and obstetrics: A systematic review. Complement. Ther. Med. 2016, 26, 72–78. [Google Scholar] [CrossRef]
- Howland, R.H. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Shermon, S.; Docherty, J.; Yao, S.; Capobianco, J. An Osteopathic Approach to Diagnosing and Treating Perimenstrual Disorders. Osteopath. Fam. Physician 2019, 11, 32–40. [Google Scholar]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef] [Green Version]
- Corner, J. Pain, Clinical Manual for Nursing Practice. J. Adv. Nurs. 1989, 14, 988. [Google Scholar]
- Simons, D.G.; Travell, J.G.; Simons, L.S. Myofascial Pain and Dysfunction: The Trigger Point Manual Upper Half of Body; William & Wilkins: Philadelphia, PA, USA, 1999; Volume 1. [Google Scholar]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual; The Lower Extremities; William & Wilkins: Philadelphia, PA, USA, 1992; Volume 2. [Google Scholar]
- Rocha, T.; Souza, H.; Brandão, D.C.; Rattes, C.; Ribeiro, L.; Campos, S.L.; Aliverti, A.; De Andrade, A.D. The Manual Diaphragm Release Technique improves diaphragmatic mobility, inspiratory capacity and exercise capacity in people with chronic obstructive pulmonary disease: A randomised trial. J. Physiother. 2015, 61, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitow, L. Sequential assessment and MET treatment of main postural muscles. In Muscle Energy Techniques; Elsevier Urban & Partner: Amsterdam, The Netherlands, 2011; pp. 131–198. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 16 March 2022).
- Mayo, J.L. A Healthy Menstrual Cycle. Clin. Nutr. Insights 1997, 5, 1–8. [Google Scholar]
- Henriet, P.; Gaide Chevronnay, H.P.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef]
- Ma, H.; Hong, M.; Duan, J.; Liu, P.; Fan, X.; Shang, E.; Su, S.; Guo, J.; Qian, D.; Tang, Y. Altered Cytokine Gene Expression in Peripheral Blood Monocytes across the Menstrual Cycle in Primary Dysmenorrhea: A Case-Control Study. PLoS ONE 2013, 8, e55200. [Google Scholar] [CrossRef]
- Shahriari, S.; Rezaei, A.; Jalalzadeh, S.M.; Mani, K.; Zamani, A. Effect of ibuprofen on IL-1β, TNF-α and PGE2 levels in periapical exudates: A double blinded clinical trial. Iran. J. Immunol. 2011, 8, 176–182. [Google Scholar]
- Kokjohn, K.; Schmid, D.M.; Triano, J.J.; Brennan, P.C. The effect of spinal manipulation on pain and prostaglandin levels in women with primary dysmenorrhea. J. Manip. Physiol. Ther. 1992, 15, 279–285. [Google Scholar]
- Dawood, M.Y.; Khan-Dawood, F.S. Clinical efficacy and differential inhibition of menstrual fluid prostaglandin F2αin a randomized, double-blind, crossover treatment with placebo, acetaminophen, and ibuprofen in primary dysmenorrhea. Am. J. Obstet. Gynecol. 2007, 196, 35.e1–35.e5. [Google Scholar] [CrossRef]
- Andrzejewski, W.; Kassolik, K.; Kobierzycki, C.; Grzegrzolka, J.; Ratajczak-Wielgomas, K.; Jablonska, K.; Halski, T.; Dziegiel, P.; Gworys, B.; Podhorska-Okolow, M. Increased skeletal muscle expression of VEGF induced by massage and exercise. Folia Histochem. Cytobiol. 2015, 53, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Toomey, D.P.; Murphy, J.F.; Conlon, K.C. COX-2, VEGF and tumour angiogenesis. Surgeon 2009, 7, 174–180. [Google Scholar] [CrossRef]
- Beharry, K.D.A.; Modanlou, H.D.; Hasan, J.; Gharraee, Z.; Abad-Santos, P.; Sills, J.H.; Jan, A.; Nageotte, S.; Aranda, J.V. Comparative effects of early postnatal ibuprofen and indomethacin on VEGF, IGF-I, and GH during rat ocular development. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Yu, S.; Su, H.; Zhu, S. Assessment of the effects of ischaemia/ hypoxia on angiogenesis in rat myofascial trigger points using colour Doppler flow imaging. PeerJ 2020, 8, e10481. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.B.H.; Klein, L.D.; Ziomkiewicz, A.; Nenko, I.; Jasienska, G.; Bribiescas, R.G. Relationships between biomarkers of inflammation, ovarian steroids, and age at menarche in a rural polish sample. Am. J. Hum. Biol. 2013, 25, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tang, J.; Xue, Y.; Wang, Q.; Li, S.; Zhou, Y.; Zhang, W. Comparison between manual acupuncture and electroacupuncture for hot flashes and sex hormone of perimenopausal syndrome. Zhongguo Zhen Jiu 2017, 37, 247–252. [Google Scholar] [CrossRef]
- Ajeena, I.M.; Al-Haris, N.; Al-Allak, M.M.; Sleiman, Z.; Al-Kefae, H.N. How transcutaneous electrical nerve stimulation (TENS) improves fertility in healthy women, the role of estradiol hormone. Int. J. Pharm. Res. 2019, 11, 227–231. [Google Scholar] [CrossRef]
- de Kruijf, M.; Stolk, L.; Zillikens, M.C.; de Rijke, Y.B.; Bierma-Zeinstra, S.M.; Hofman, A.; Huygen, F.J.; Uitterlinden, A.G.; van Meurs, J.B. Lower sex hormone levels are associated with more chronic musculoskeletal pain in community-dwelling elderly women. Pain 2016, 157, 1425–1431. [Google Scholar] [CrossRef]
- Kannan, P.; Cheung, K.K.; Lau, B.W.M. Does aerobic exercise induced-analgesia occur through hormone and inflammatory cytokine-mediated mechanisms in primary dysmenorrhea? Med. Hypotheses 2019, 123, 50–54. [Google Scholar] [CrossRef]
- Sherif, B.; Al-Zohyri, A.; Shihab, S. Effects of Some Non Steroidal Anti-inflammatory Drugs on Ovulation in Women with Mild Musculoskeletal Pain (A Clinical Study). IOSR J. Pharm. Biol. Sci. 2014, 9, 43–49. [Google Scholar] [CrossRef]
- Barassi, G.; Bellomo, R.G.; Porreca, A.; Di Felice, P.A.; Prosperi, L.; Saggini, R. Somato-Visceral Effects in the Treatment of Dysmenorrhea: Neuromuscular Manual Therapy and Standard Pharmacological Treatment. J. Altern. Complement. Med. 2017, 24, 291–299. [Google Scholar] [CrossRef]
- Schwerla, F.; Wirthwein, P.; Rütz, M.; Resch, K.-L. Osteopathic treatment in patients with primary dysmenorrhoea: A randomised controlled trial. Int. J. Osteopath. Med. 2014, 17, 222–231. [Google Scholar] [CrossRef]
- Zecchillo, D.; Acquati, A.; Aquino, A.; Pisa, V.; Uberti, S.; Ratti, S. Osteopathic Manipulative Treatment of Primary Dysmenorrhea and Related Factors: A Randomized Controlled Trial. Int. J. Med. Res. Health Sci. 2017, 6, 165–174. [Google Scholar]
- Holtzman, D.A.; Petrocco-Napuli, K.L.; Burke, J.R. Prospective Case Series on the Effects of Lumbosacral Manipulation on Dysmenorrhea. J. Manip. Physiol. Ther. 2008, 31, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Trybulec, B.; Wyżycka, E. The use of selected techniques of manual therapy in conservative treatment of primary dysmenorrhea. Med. Rev. 2015, 14, 162–172. [Google Scholar] [CrossRef]
- Kim, M.-J.; Baek, I.-H.; Goo, B.-O. The relationship between pelvic alignment and dysmenorrhea. J. Phys. Ther. Sci. 2016, 28, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Sarajari, S.; Oblinger, M.M. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp. Neurol. 2010, 224, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Pickar, J.G. Neurophysiological effects of spinal manipulation. Spine J. 2002, 2, 357–371. [Google Scholar] [CrossRef]



| All Patients (n = 35) | Group A Manual Therapy (n = 20) | Group B Ibuprofen Therapy (n = 15) | p | |
|---|---|---|---|---|
| Age (years) | 23 (22; 24.5) | 23.3 ± 2.1 | 23 (21.5; 25) | 0.978 a |
| Body Mass Index (kg/m2) | 21.7 ± 2.8 | 21.9 ± 2.6 | 21.5 ± 3.2 | 0.660 b |
| Severity of dysmenorrhea in NPRS | 8 (7; 8) | 8 (7; 8) | 8 (7; 9) | 0.484 a |
| Duration of menstrual cycle (days) | 28 (28; 30.5) | 28 (28; 29) | 29.5 ± 2.6 | 0.413 a |
| Duration of menstruation (days) | 6 (5; 6) | 5 (5; 6) | 6 (5.5; 6) | 0.016 a |
| Duration of dysmenorrhea (days) | 2 (2; 3) | 2 (2; 3) | 3 (2; 3.5) | 0.046 a |
| Group A Manual Therapy (n = 20) | Group B Ibuprofen Therapy (n = 15) | p | ||
|---|---|---|---|---|
| PGE2 (pg/mL) | Pre | 1044 (633.4; 1368.8) | 985 ± 554 | 0.614 a |
| Post | 1079.08 ± 664.89 | 878.9 ± 499.8 | 0.340 b | |
| p | 0.587 c | 0.201 c | ||
| PGF2α (pg/mL) | Pre | (1245.3; 4615) | 2450 (1595; 3457.5) | 0.791 a |
| Post | 2689.3 ± 1904 | 2030 (1482.5; 3317.5) | 0.945 a | |
| p | 0.409 c | 0.798 c | ||
| VEGF (pg/mL) | Pre | 139.4 ± 99.6 | 135.2 ± 79.3 | 0.895 b |
| Post | 126.6 (65.5; 209.7) | 133.8 ± 71.3 | 0.938 a | |
| p | 0.141 c | 0.754 c | ||
| CRP (mL/L) | Pre | 1.386 ± 0.879 | 1.749 ± 0.764 | 0.215 b |
| Post | 1.673 (1.115; 1.984) | 1.869 ± 1.165 | 0.662 a | |
| p | 0.098 c | 0.977 c | ||
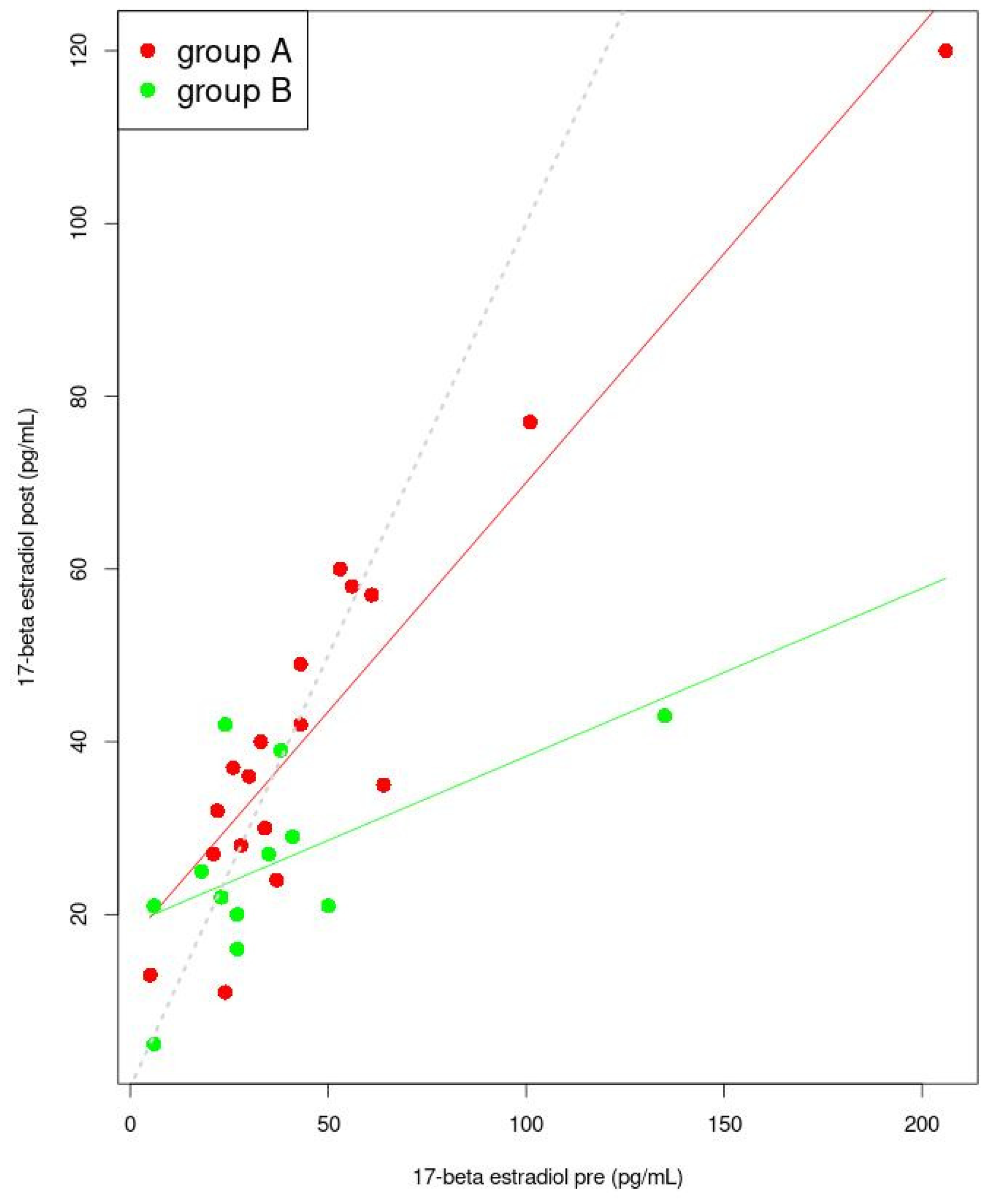
| Estradiol-17β (pg/mL) | Pre | 34 (25; 54.5) | 27 (19.25; 37.75) | 0.229 a |
| Post | 36 (27.5; 53) | 25.462 ± 10.76 | 0.036 a | |
| p | 0.776 c | 0.307 c | ||
| Progesterone (ng/mL) | Pre | 0.415 (0.262; 0.54) | 0.35 (0.25; 0.47) | 0.376 a |
| Post | 0.25 (0.18; 0.33) | 0.246 ± 0.133 | 0.302 a | |
| p | 0.016 c | 0.028 c |
| Estimate | Std. Error | t | p | |
|---|---|---|---|---|
| (Intercept) | 438.410 | 234.764 | 1.867 | 0.079 |
| Group A | 0.558 | 0.174 | 3.212 | 0.005 |
| Multiple R-squared: 0.378 | Adjusted R-squared: 0.341 | |||
| (Intercept) | 155.505 | 162.945 | 0.954 | 0.357 |
| Group B | 0.734 | 0.1454 | 5.053 | 0.0002 |
| Multiple R-squared: 0.662 | Adjusted R-squared: 0.636 | |||
| Estimate | Std. Error | t | p | |
|---|---|---|---|---|
| (Intercept) | −7.827 | 14.732 | −0.531 | 0.602 |
| Group A | 1.177 | 0.087 | 13.572 | 0.0000000002 |
| Multiple R-squared: 0.916 | Adjusted R-squared: 0.910 | |||
| (Intercept) | 22.242 | 15.449 | 1.44 | 1.74 |
| Group B | 0.825 | 0.099 | 8.30 | 0.000001 |
| Multiple R-squared: 0.841 | Adjusted R-squared: 0.829 | |||
| Estimate | Std. Error | t | p | |
|---|---|---|---|---|
| (Intercept) | 0.406 | 0.230 | 0.946 | 0.357 |
| Group A | 0.962 | 0.264 | 3.647 | 0.002 |
| Multiple R-squared: 0.439 | Adjusted R-squared: 0.406 | |||
| (Intercept) | 0.245 | 0.637 | 0.385 | 0.707 |
| Group B | 0.928 | 0.337 | 2.767 | 0.016 |
| Multiple R-squared: 0.371 | Adjusted R-squared: 0.322 | |||
| Estimate | Std. Error | t Value | p | |
|---|---|---|---|---|
| (Intercept) | 16.981 | 3.473 | 4.889 | 0.0002 |
| Group A | 0.530 | 0.053 | 10.024 | 0.00000003 |
| Multiple R-squared: 0.863 | Adjusted R-squared: 0.854 | |||
| (Intercept) | 0.0002 | 4.059 | 4.648 | 0.0009 |
| Group B | 0.194 | 0.084 | 2.315 | 0.043 |
| Multiple R-squared: 0.349 | Adjusted R-squared: 0.284 | |||
| Group A Manual Therapy (n = 20) | Group B Ibuprofen Therapy (n = 15) | p | ||
|---|---|---|---|---|
| Severity of dysmenorrhea | Pre | 8 (7; 8) | 8 (7; 9) | 0.484 a |
| Post | 4.9 ± 2.4 | 3.9 ± 2.8 | 0.265 b | |
| p | 0.000 c | 0.002 c |
| Group A Manual Therapy (n = 20) | Group B Ibuprofen Therapy (n = 15) | p | ||
|---|---|---|---|---|
| Number of muscles with dysfunction | Pre | 12 (11; 13) | 12 (12; 13) | 0.641 a |
| Post | 8.75 ± 2.552 | 12 (11; 12.5) | 0.002 a | |
| p | 0.0005 a | 0.112 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcikowska, Z.; Grzybowska, M.E.; Wąż, P.; Jaskulak, M.; Kurpas, M.; Sotomski, M.; Starzec-Proserpio, M.; Rajkowska-Labon, E.; Hansdorfer-Korzon, R.; Zorena, K. Effect of Manual Therapy Compared to Ibuprofen on Primary Dysmenorrhea in Young Women—Concentration Assessment of C-Reactive Protein, Vascular Endothelial Growth Factor, Prostaglandins and Sex Hormones. J. Clin. Med. 2022, 11, 2686. https://doi.org/10.3390/jcm11102686
Barcikowska Z, Grzybowska ME, Wąż P, Jaskulak M, Kurpas M, Sotomski M, Starzec-Proserpio M, Rajkowska-Labon E, Hansdorfer-Korzon R, Zorena K. Effect of Manual Therapy Compared to Ibuprofen on Primary Dysmenorrhea in Young Women—Concentration Assessment of C-Reactive Protein, Vascular Endothelial Growth Factor, Prostaglandins and Sex Hormones. Journal of Clinical Medicine. 2022; 11(10):2686. https://doi.org/10.3390/jcm11102686
Chicago/Turabian StyleBarcikowska, Zofia, Magdalena Emilia Grzybowska, Piotr Wąż, Marta Jaskulak, Monika Kurpas, Maksymilian Sotomski, Małgorzata Starzec-Proserpio, Elżbieta Rajkowska-Labon, Rita Hansdorfer-Korzon, and Katarzyna Zorena. 2022. "Effect of Manual Therapy Compared to Ibuprofen on Primary Dysmenorrhea in Young Women—Concentration Assessment of C-Reactive Protein, Vascular Endothelial Growth Factor, Prostaglandins and Sex Hormones" Journal of Clinical Medicine 11, no. 10: 2686. https://doi.org/10.3390/jcm11102686
APA StyleBarcikowska, Z., Grzybowska, M. E., Wąż, P., Jaskulak, M., Kurpas, M., Sotomski, M., Starzec-Proserpio, M., Rajkowska-Labon, E., Hansdorfer-Korzon, R., & Zorena, K. (2022). Effect of Manual Therapy Compared to Ibuprofen on Primary Dysmenorrhea in Young Women—Concentration Assessment of C-Reactive Protein, Vascular Endothelial Growth Factor, Prostaglandins and Sex Hormones. Journal of Clinical Medicine, 11(10), 2686. https://doi.org/10.3390/jcm11102686







