Abstract
Background: Brain natriuretic peptide serum levels (BNP) on admission are frequently elevated in patients with symptomatic chronic subdural hematoma (cSDH) and predict unfavorable long-term functional outcomes. However, the reasons for these elevated levels remain unclear. Therefore, we aimed to identify the predictors of BNP elevation. Methods: Patients with unilateral symptomatic cSDH who were surgically treated in our department between November 2016 and May 2020 were enrolled. Patients’ symptoms and neurological deficits were prospectively assessed using a study questionnaire. On initial computer tomography, hematoma volumes and midline shift (MLS) values were measured to analyze the degree of brain compression. Results: In total, 100 patients were analyzed. Linear regression analysis showed that higher BNP levels were significantly associated with smaller hematoma volumes (p = 0.003) and littler MLS values (p = 0.022). Multivariate analysis revealed that presence of a neurological deficit (p = 0.041), a hematoma volume < 140 mL (p = 0.047), advanced age (p = 0.023), and head trauma within 24 h of admission (p = 0.001) were independent predictors of BNP elevation. Conclusion: In symptomatic cSDH, BNP elevation is related, among others, to the presence of neurological deficits and smaller hematoma volumes. Whether BNP elevation may coincide with the early stage of hematoma growth, i.e., immaturity of cSDH neomembrane, requires further investigations.
1. Introduction
Chronic subdural hematoma (cSDH) represents a common and frequent neurosurgical condition [1] with a growing incidence [2] and challenging outcomes after surgical management [3]. In contrast to the rapid expansion of an acute SDH, the irregular occurrence of microbleeds from the cSDH neomembrane generates inconsistent hematoma expansion, which often results in neurological deficits for cases of rapid expansion due to the brain being unable to adjust to this mass effect.
During the last two decades, several studies have shown that patient brain natriuretic peptide levels on admission (BNP) were elevated in many acute central nervous system (CNS) diseases, including stroke [4,5,6], hypertensive intracerebral hemorrhage (ICH) [7], aneurysmal subarachnoid hemorrhage (aSAH) [7,8,9,10,11] and traumatic brain injury (TBI) [12,13,14]. BNP has also been used as an indicator of disease severity [10,14] and as a predictor of long-term functional outcomes [12,15,16,17]. Although cSDH usually occurs at least three weeks after mild head trauma and does not represent an acute event relative to the aforementioned CNS diseases, we showed in a previous report that almost two-thirds of these patients unexpectedly presented with elevated BNP [18]. In a later report, we also demonstrated that BNP was a reliable predictor of postoperative long-term functional outcomes [19]. However, the reasons behind these increases in BNP levels remain unexplained.
Recent histopathological studies have demonstrated that immature cSDH neomembranes exhibit more bleeding and exudation and are associated with worse clinical presentations and the rapid expansions of small hematomas [20,21]. Based on these findings, we investigated possible predictors of elevated BNP in patients with symptomatic cSDH.
2. Materials and Methods
2.1. Study Population
All procedures were performed following the Declaration of Helsinki and the ethical standards of the institutional review board that approved this study (Medical Faculty, University of Duisburg-Essen, registration number: 15-6632-BO). Written informed consent was obtained from each patient or in cases of altered state of consciousness from the next of kin. We screened 280 patients with symptomatic cSDH who were surgically treated in our neurosurgical department between November 2016 and May 2020 (flow diagram in Figure 1). The term symptomatic was defined to include progressive headache, neurological impairments, seizures, and/or changes in behavior.
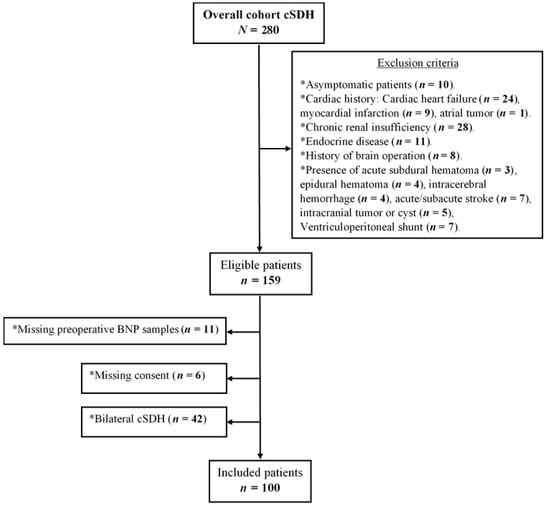
Figure 1.
Study flow diagram.
2.2. Inclusion Criteria
Patients ≥ 18 years diagnosed with a symptomatic cSDH and who underwent surgical removal of the hematoma were included. Hematomas had to be unilateral and isolated to allow for accurate assessment and analysis of brain compression.
2.3. Exclusion Criteria
Patients with cardiac or renal insufficiencies, a history of brain surgery, stroke, a ventriculoperitoneal shunt, intracranial tumors, or asymptomatic cSDH were excluded.
2.4. Treatment Protocol
The diagnosis of cSDH was made using cranial computed tomography (CCT, Somatom Definition AS, Siemens Healthcare GmbH, Erlangen, Germany; slice thickness = 5 mm) before surgery in all cases immediately after assessing BNP samples. Patients received a burr-hole trepanation and the insertion of a subdural drain for 48 h. Postoperatively, they remained in the intensive care unit (ICU) or intermediate care unit for 48 h. For these cases, a control CCT scan was performed on the second postoperative day, and the subdural drain was removed. However, some patients did not require any drainage or underwent operation through a minicraniotomy in case of septated cSDH or decompressive craniectomy in case of cSDH with acute bleeding and brain edema, without insertion of a subdural drain. For these cases, a CCT scan was performed on the next day. Perioperative antibiotic prophylaxis was ensured using a single injection of cefazolin (or erythromycin in case of penicillin allergy).
2.5. BNP Sample Collection and Management
Preoperative BNP levels were assessed on admission using an electrochemiluminescence immunoassay (Siemens, ADVIA Centauer®, Washington, DC, USA). The measuring range (provided by the manufacturer) was 2–5000 pg/mL.
2.6. Collection of Patient Data
Medical records were assessed by the first author (M.C.) using a study questionnaire on admission and included age, sex, comorbidities, such as atrial fibrillation, history of coronary heart disease, antiplatelet/anticoagulant therapy, and the presence of dementia. Patient symptoms and neurological deficits were assessed and patient neurological conditions using the Glasgow coma scale (GCS). Muscle force grading was done based on the medical research council manual muscle testing scale [22]. Hemiparesis was described as “severe” when the patient’s muscle strength was ≤ grade 3/5. Otherwise, hemiparesis was considered as “mild” (grade 4/5).
2.7. Radiological Measurements
Computer-assisted volumetric analysis was used on initial CCT to measure hematoma volumes. Hematoma margins were traced using iPlan software (BrainLab, Munich, Germany) for each axial CT-slice, and the volume was automatically calculated by the software in cubic centimeters (i.e., mL). Hematoma volume was assessed in all patients. Midline shift (MLS) was measured on initial CCT at the insular cortex’s level showing the basal ganglia and the thalamus above the basal cisterns. The midline was traced vertically from the frontal crest to the internal occipital crest. A perpendicular horizontal line was traced from the septum pellucidum to the midline. This distance was considered to be MLS and measured in millimeters. The presence of any hyperdensity areas on initial CCT, representing the microbleeds/hemorrhages of the outer membrane and/or septations of the hematoma, were documented. Volumetric analysis and Hounsfield units of the hyperdensity areas were measured using the iPlan software.
2.8. Statistical Analyses
All analyses were performed using IBM SPSS Statistics (version 25 for Windows, Chicago, IL, USA). Categorical data are presented as frequencies and percentages. Non-normally distributed continuous variables are expressed using the median and interquartile range (IQR). BNP values were log10-transformed (log10BNP) to assume a normal distribution when assessing linearity with hematoma volume values using a scatterplot. To assess statistical associations between log10BNP and hematoma volumes, a linear regression was performed. The Mann–Whitney U test was used to determine differences between two groups of a categorical variable on a non-normally distributed continuous variable (BNP). The factors associated with increased BNP using the univariate analysis that had p-values < 0.1 were then used in the multivariate analysis. A multiple regression analysis was then used to determine predictors for elevated BNP levels. p-values < 0.05 using two-sided testing were considered significant.
3. Results
3.1. Cohort Patient Characteristics and Operative Technique
In total, 100 patients (median age, 76 years, IQR 18 years; male/female, 3.8/1) were included in the study (flow diagram in Figure 1). Patients’ characteristics are summarized in Table 1.

Table 1.
Patient characteristics and operative technique.
3.2. Symptoms and Neurological Deficits on Admission
Patients’ consultations occurred due to symptoms and/or neurological deficits. The onset of nonspecific symptoms (headache, dizziness, change in behavior, cognitive deterioration, gait/balance disturbance) occurred relatively slowly and progressively over weeks/months. In contrast, seizures and neurological signs, such as alterations in the state of consciousness (GCS score < 15) and neurological deficits, such as aphasia, dysarthria, fine motor dysfunction and hemiparesis, resulted in more urgent consultations. The occurrence rates of symptoms and neurological deficits are summarized in Table 2.

Table 2.
Symptoms and neurological deficits in a symptomatic chronic subdural hematoma (cSDH).
3.3. Radiological Findings
Linear regression was used to better understand the effect of hematoma volumes and MLS on BNP. To assess linearity, a scatterplot of log10BNP values against hematoma volumes and MLS values with a superimposed regression line was created. Visual inspection of these plots indicated an inverse linear relationship between hematoma volumes and log10BNP and between MLS and log10BNP (Figure 2). Higher log10BNP values were significantly associated with smaller hematoma volumes (p = 0.003) and littler MLS values (p = 0.022). The presence of hyperdensity areas, their volumes and mean Hounsfield units on initial CCT scans were not statistically associated with BNP values (Table 3). Furthermore, there was no statistical association between hematoma volumes and the presence of neurological deficits (Mann–Whitney U Test: hemiparesis, p = 0.264; mild hemiparesis, p = 0.283; severe hemiparesis, p = 0.756; dysarthria, p = 0.152).
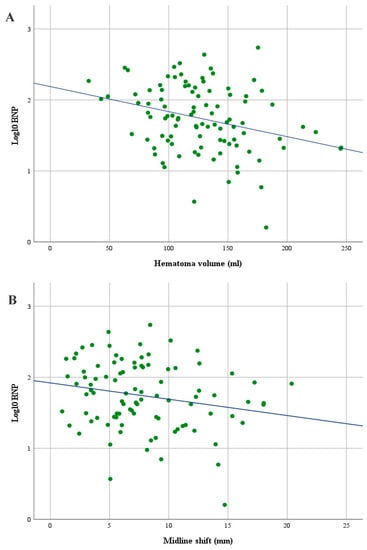
Figure 2.
Scatterplot showing an inverse linear relationship between hematoma volumes and log10 brain natriuretic peptide serum levels (BNP) (A) and between midline shift (MLS) and log10BNP (B) in patients with symptomatic cSDH.

Table 3.
Relationship between BNP and clinical and radiographic parameters.
3.4. Predictors of Elevated BNP Serum Levels
Elevated BNP values were significantly associated with advanced age (p = 0.0005), the presence of neurological deficits (hemiparesis and/or dysarthria) (p = 0.001), and head trauma within 24 h of admission (p = 0.024). In contrast, patients presenting with larger hematoma volumes, and greater MLS showed significantly lower BNP values (p = 0.006 and p = 0.022, respectively) (Table 3). As BNP was significantly associated with hematoma volumes, ROC curves analyses with different hematoma volume cutoff-values were performed. The ROC curve analysis with the best discrimination capacity was selected. The corresponding ROC curve analysis identified a hematoma volume cutoff-value of 140 mL and showed an acceptable discriminative capacity according to Hosmer et al. [23] with an area under the curve of 0.70 (95% CI: 0.58–0.80). After adjusting for all confounders, a multiple regression analysis revealed that the presence of a neurological deficit (p = 0.041), a hematoma volume < 140 mL (p = 0.047), advanced age (p = 0.023), and head trauma within 24 h of admission (p = 0.001) were independent predictors of increased BNP values. (Table 4). The multiple regression model statistically significantly predicted BNP levels, F (8, 91) = 5.482, p < 0.0005, R2 = 0.325.

Table 4.
Predictors of Increased BNP.
4. Discussion
This study is the first to investigate the reasons behind BNP elevation in patients with symptomatic cSDH. We found that higher BNP levels are significantly associated with smaller hematoma volumes and littler MLS values. In addition, elevated BNP levels in cSDH could be, among others, independently predicted by the presence of a neurological deficit (hemiparesis and/or dysarthria) and smaller hematoma volumes.
The natural history of cSDH has been described as an initial occurrence of mild head trauma (mostly in elderly patients with brain atrophy) resulting in injury to the bridging veins of the dural border cells layer [24] and/or arachnoidea with subsequent leakage of blood and/or cerebrospinal fluid (CSF), respectively, in the new formed subdural space [25]. The spontaneous resolution of both fluid accumulations is common due to the highly active fibrinolytic systems contained in CSF [26]. However, a delay in absorption can occur due to advanced age or cortical atrophy, and the subsequent inflammatory and angiogenic responses of the body can lead to the formation of a neomembrane that encloses the hematoma [25]. The cSDH will continue to grow due to fluid exudation resulting from the hyperpermeability of the new fragile capillaries of the hematoma neomembrane and intermittent microhemorrhages of these blood vessels into the hematoma cavity [27]. The high concentration of tissue plasminogen activator within the neomembrane causes a local hyperfibrinolysis within the hematoma cavity and abnormal clotting of the hematoma [28].
The relationship between hematoma volumes and NT-proBNP (a non-active prohormone that is released from the same molecule that produces BNP) was first investigated in patients with hypertensive ICH in a prospective and multicenter study design, revealing significantly higher levels of NT-proBNP in patients with intracerebral hematoma volumes > 30 mL [29]. In addition, larger hematoma volumes were associated with increased ICP [29]. In patients with severe TBI [12,13,14], higher plasma levels of both NT-proBNP and BNP were also significantly associated with elevated ICP. Therefore, these data suggest that elevated BNP levels in acute CNS diseases are related to the increased ICP due to a higher degree of brain compression. Some authors suggested that the posttraumatic dysfunction of the autonomic nervous system, which leads to increased sympathetic activity and catecholamine hypersecretion, may represent the bridge between the brain and the heart [30], as an increase in catecholamine release could result in cardiac wall abnormalities and dysfunction [11,31] that may explain the BNP increase.
In contrast, in patients with cSDH, we found that higher BNP levels were, among others, independently predicted by the presence of neurological deficits and smaller hematoma volumes. These paradoxical results may be explained by the fact that ICP elevation in patients with cSDH remains an unpredictable event, which may occur many days/weeks after the initial trauma, but not in the few hours following admission, as in case of spontaneous ICH or severe TBI. In fact, due to the irregular occurrence of microbleedings and fluid exudation and inconsistency of their severity from one patient to another, some patients could experience “several microbleedings” of their small hematoma (originating from the outer membrane or hematoma septations) in a relatively short period. Consequently, even a small volume excess may lead to an elevation of the ICP and the occurrence of neurological deficits. Accordingly, Tomita et al. recently demonstrated that the assumption “thicker hematomas are associated with more severe motor weakness” in cSDH patients was logical but inaccurate. Indeed, the authors found that some patients with very thick cSDH had a minor motor weakness, suggesting that “hematoma thickness” was not a decisive factor in determining motor weakness severity [32]. These findings are in line with our results, as no statistical association was found between hematoma volumes and neurological deficits, in particular hemiparesis and its severity. Furthermore, the authors showed that “hematoma tension” on the motor cortex instead, calculated by Laplace law using hematoma thickness and hematoma pressure (intraoperative measurement using glass manometers), strongly correlated with the severity of the motor weakness [32].
The relationship between BNP levels and hematoma volumes in patients with cSDH has not yet been addressed. Recently published histopathological examinations [21] have revealed that immature cSDH membranes (type II neomembranes) that represent an early stage in the inflammatory cycle and exhibit more exudation and bleeding are associated with smaller, unstable hematomas and worse clinical presentations [20]. In contrast, patients with more mature hematoma neomembranes have already passed this phase and show larger and more stable hematomas, with a more gradual onset of symptoms [27]. Interestingly, our results showed that higher BNP levels in symptomatic cSDH, in contrast to acute CNS diseases, were significantly associated with smaller hematoma volumes and littler MLS values, suggesting that BNP elevation might coincide with the early stage of hematoma growth, i.e., the immaturity of cSDH neomembrane. This would then explains the inverse relationship between BNP values and hematoma volumes, as larger hematomas are more stable and evolve more slowly, allowing for adequate brain adaptation to the mass effect and a more gradual onset of symptoms [27]. Additionally, smaller hematoma volumes and the presence of neurological deficits on admission independently predicted higher BNP levels. These results also fit with our recent study findings regarding the prediction of poor functional long-term outcome through higher BNP levels on admission, which may promote using our recent predictive scoring system for cSDH long-term surgical outcome (the FLOP-score) [19] in the daily clinical practice.
Study Limitations
Our findings do not represent the whole spectrum of patients with cSDH, only patients without premorbid conditions, such as chronic cardiac and renal insufficiencies and stroke. Further prospective studies with the larger patient–sample sizes and histopathological analyses of cSDH neomembranes must validate our findings.
5. Conclusions
Elevated BNP levels in cases of symptomatic cSDH are related, among others, to the presence of neurological deficits and smaller hematoma volumes, two arguments in favor of cSDH immaturity prompting hematoma expansion. Further histopathological studies must also consider analyzing BNP values on admission to investigate whether BNP can be used as a reliable predictor for neomembrane immaturity.
Author Contributions
M.C. conceptualized and designed the study, acquired, analyzed and interpreted the data, drafted the initial manuscript, and reviewed and revised the final version of the manuscript. R.J., A.P., M.D.-O., O.G. and K.H.W. analyzed and interpreted the data and reviewed and revised the manuscript’s final version. U.S. revised the final version of the manuscript. H.M. conceptualized and designed the study, analyzed and interpreted the data, revised the manuscript for important intellectual content and reviewed and revised the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was secured for this study.
Institutional Review Board Statement
All procedures were performed following the Declaration of Helsinki and the ethical standards of the institutional review board that approved this study (Medical Faculty, University of Duisburg-Essen, Registration number: 15-6632-BO).
Informed Consent Statement
Written informed consent was obtained from each patient or in cases of altered state of consciousness from the next of kin.
Data Availability Statement
The data presented in the study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohno, K.; Suzuki, R.; Masaoka, H.; Matsushima, Y.; Inaba, Y.; Monma, S. Chronic subdural haematoma preceded by persistent traumatic subdural fluid collection. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Balser, D.; Farooq, S.; Mehmood, T.; Reyes, M.; Samadani, U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J. Neurosurg. 2015, 123, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.-A.; Aboukaïs, R.; Reyns, N.; Bourgeois, P.; Labreuche, J.; Duhamel, A.; Lejeune, J.P. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J. Clin. Neurosci. 2015, 22, 1895–1900. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamaguchi, T.; Seida, M.; Yamada, S.; Imae, S.; Tanaka, Y.; Yamamoto, K.; Ohno, K. Plasma concentrations of brain natriuretic peptide in patients with acute ischemic stroke. Cerebrovasc. Dis. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; De Castro, S.; Toni, D.; Sacchetti, M.L.; Biraschi, F.; Prencipe, M.; Fiorelli, M. Determinants of plasma levels of brain natriuretic peptide after acute ischemic stroke or TIA. J. Neurol. Sci. 2007, 260, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Perea-Gainza, M.; Delgado, P.; Ribó, M.; Chacón, P.; Rosell, A.; Quintana, M.; Palacios, M.E.; Molina, C.A.; Alvarez-Sabín, J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008, 39, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Jabbarli, R.; Pierscianek, D.; Oppong, M.D.; Sato, T.; Dammann, P.; Wrede, K.H.; Kaier, K.; Köhrmann, M.; Forsting, M.; Kleinschnitz, C.; et al. Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: A systematic review. Neurosurg. Rev. 2018, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berendes, E.; Walter, M.; Cullen, P.; Prien, T.; Van Aken, H.; Horsthemke, J.; Schulte, M.; von Wild, K.; Scherer, R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 1997, 349, 245–249. [Google Scholar] [CrossRef]
- McGirt, M.J.; Blessing, R.; Nimjee, S.M.; Friedman, A.H.; Alexander, M.J.; Laskowitz, D.T.; Lynch, J.R. Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery 2004, 54, 1369–1374. [Google Scholar] [CrossRef]
- Tsubokawa, T.; Shiokawa, Y.; Kurita, H.; Kaneko, N. High plasma concentration of brain natriuretic peptide in patients with ruptured anterior communicating artery aneurysm. Neurol. Res. 2004, 26, 893–896. [Google Scholar] [CrossRef]
- Tung, P.P.; Olmsted, E.; Kopelnik, A.; Banki, N.M.; Drew, B.J.; Ko, N.; Lawton, M.T.; Smith, W.; Foster, E.; Young, W.L.; et al. Plasma B-type natriuretic peptide levels are associated with early cardiac dysfunction after subarachnoid hemorrhage. Stroke 2005, 36, 1567–1569. [Google Scholar] [CrossRef]
- Sviri, G.; Soustiel, J.; Zaaroor, M. Alteration in brain natriuretic peptide (BNP) plasma concentration following severe traumatic brain injury. Acta Neurochir. 2006, 148, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Stegmaier, J.; Bogner, V.; Buhmann, S.; Mussack, T.; Kreimeier, U.; Mutschler, W.; Biberthaler, P. Intrathecal and systemic concentration of NT-proBNP in patients with severe traumatic brain injury. J. Neurotrauma 2006, 23, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sha, H.; Sun, Y.; Gao, L.; Liu, H.; Yuan, Q.; Zhang, T.; Zhu, J.; Zhou, L.; Hu, J. N-terminal pro-B-type natriuretic peptide in patients with isolated traumatic brain injury: A prospective cohort study. J. Trauma Acute Care Surg. 2011, 71, 820–825. [Google Scholar] [CrossRef]
- Chaudhuri, J.R.; Sharma, V.K.; Mridula, K.R.; Balaraju, B.; Bandaru, V.C.S.S. Association of plasma brain natriuretic peptide levels in acute ischemic stroke subtypes and outcome. J. Stroke Cerebrovasc. Dis. 2015, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- McAteer, A.; Hravnak, M.; Chang, Y.; Crago, E.A.; Gallek, M.J.; Yousef, K.M. The relationships between BNP and neurocardiac injury severity, noninvasive cardiac output, and outcomes after aneurysmal subarachnoid hemorrhage. Biol. Res. Nurs. 2017, 19, 531–537. [Google Scholar] [CrossRef]
- Gupta, H.V.; Finlay, C.W.; Jacob, S.; Raina, S.K.; Lee, R.W.; Hinduja, A. Can Admission BNP Level Predict Outcome After Intravenous Thrombolysis in Acute Ischemic Stroke? Neurologist 2019, 24, 6–9. [Google Scholar] [CrossRef]
- Chihi, M.; Gembruch, O.; Oppong, M.D.; Helsper, M.; Hütter, B.; Jabbarli, R.; Wrede, K.H.; Sure, U.; Maslehaty, H. Analysis of Brain Natriuretic Peptide Serum Levels in Patients With Symptomatic Chronic Subdural Hematoma: A Potential Reliable Biomarker. J. Neurotrauma 2020, 37, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Chihi, M.; Gembruch, O.; Oppong, M.D.; Rauschenbach, L.; Rauscher, S.; Jabbarli, R.; Wrede, K.H.; Sure, U.; Maslehaty, H. Role of brain natriuretic peptide in the prediction of long-term surgical outcome of chronic subdural hematoma. J. Neurol. Sci. 2020, 420, 117240. [Google Scholar] [CrossRef]
- Gandhoke, G.S.; Kaif, M.; Choi, L.; Williamson, R.W.; Nakaji, P. Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features. J. Clin. Neurosci. 2013, 20, 1398–1401. [Google Scholar] [CrossRef]
- Nagahori, T.; Nishijima, M.; Takaku, A. Histological study of the outer membrane of chronic subdural hematoma: Possible mechanism for expansion of hematoma cavity. No Shinkei Geka Neurol. Surg. 1993, 21, 697–701. [Google Scholar]
- Naqvi, U. Muscle strength grading. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Haines, D.E.; Harkey, H.L.; Al-Mefty, O. The “subdural” space: A new look at an outdated concept. Neurosurgery 1993, 32, 111–120. [Google Scholar] [CrossRef]
- Lee, K.; Bae, W.; Doh, J.; Bae, H.; Yun, I. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998, 12, 901. [Google Scholar]
- Astrup, T. Assay and content of tissue thromboplastin in different organs. Thromb. Haemost. 1965, 14, 401–416. [Google Scholar] [CrossRef]
- Edlmann, E.; Giorgi-Coll, S.; Whitfield, P.C.; Carpenter, K.; Hutchinson, P. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J. Neuroinflammation 2017, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Katano, H.; Kamiya, K.; Mase, M.; Tanikawa, M.; Yamada, K. Tissue plasminogen activator in chronic subdural hematomas as a predictor of recurrence. J. Neurosurg. 2006, 104, 79–84. [Google Scholar] [CrossRef]
- Li, F.; Chen, Q.-X.; Xiang, S.-G.; Yuan, S.-Z.; Xu, X.-Z. N-Terminal Pro-Brain Natriuretic Peptide Concentrations After Hypertensive Intracerebral Hemorrhage: Relationship With Hematoma Size, Hyponatremia, and Intracranial Pressure. J. Intensive Care Med. 2018, 33, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Yang, G.L.; McGuire, J.L.; Robson, M.J.; Foreman, B.; Ngwenya, L.B.; Lorenz, J.N. Autonomic dysfunction following traumatic brain injury: Translational insights. Neurosurg. Focus 2019, 47, E8. [Google Scholar] [CrossRef] [PubMed]
- Elrifai, A.M.; Bailes, J.E.; Shih, S.-R.; Dianzumba, S.; Brillman, J. Characterization of the cardiac effects of acute subarachnoid hemorrhage in dogs. Stroke 1996, 27, 737–742. [Google Scholar] [CrossRef]
- Tomita, Y.; Yamada, S.M.; Yamada, S.; Matsuno, A. Subdural tension on the brain in patients with chronic subdural hematoma is related to hemiparesis but not to headache or recurrence. World Neurosurg. 2018, 119, e518–e526. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).