Can Deep Learning Using Weight Bearing Knee Anterio-Posterior Radiograph Alone Replace a Whole-Leg Radiograph in the Interpretation of Weight Bearing Line Ratio?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
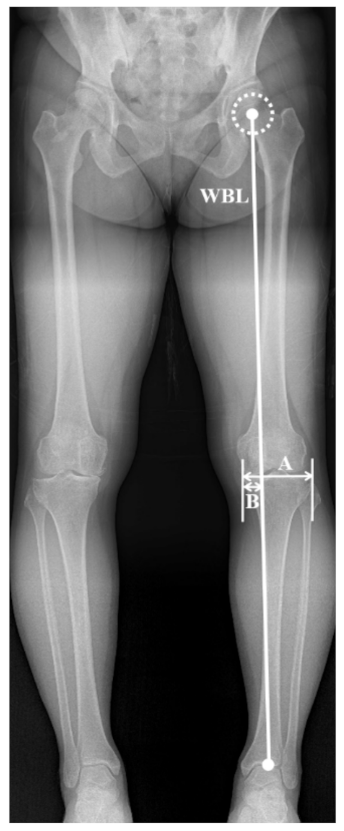
2.2. WBL Ratio Measurement and Labeling
2.3. Image Preprocessing
2.4. DL Algorithm
2.5. Statistical Analysis
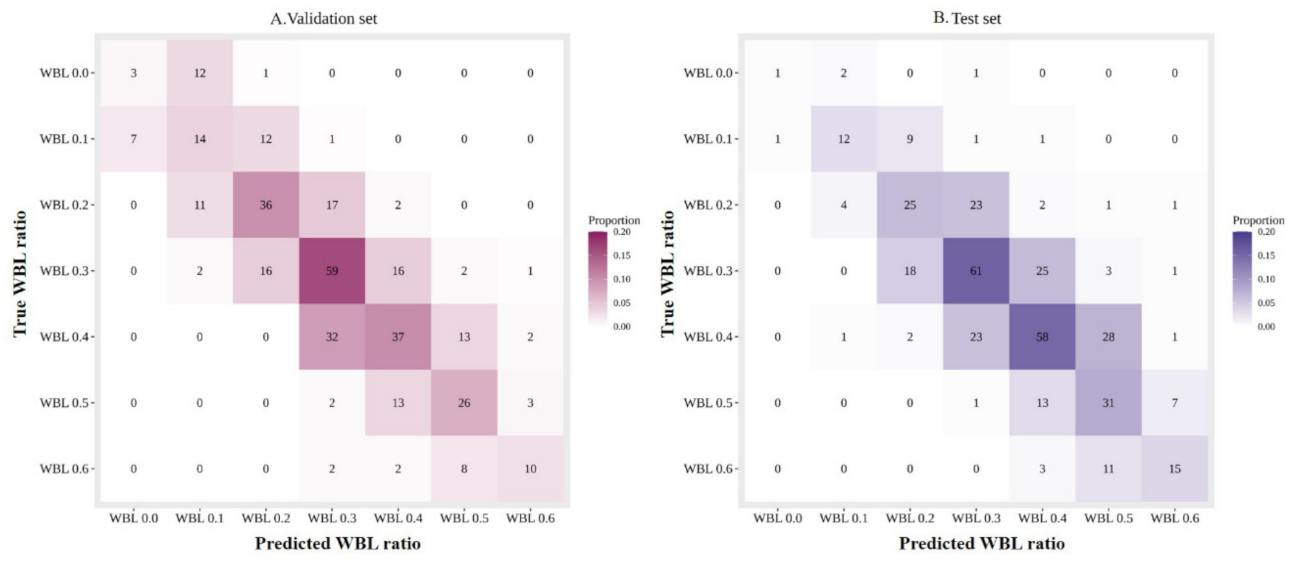
3. Results
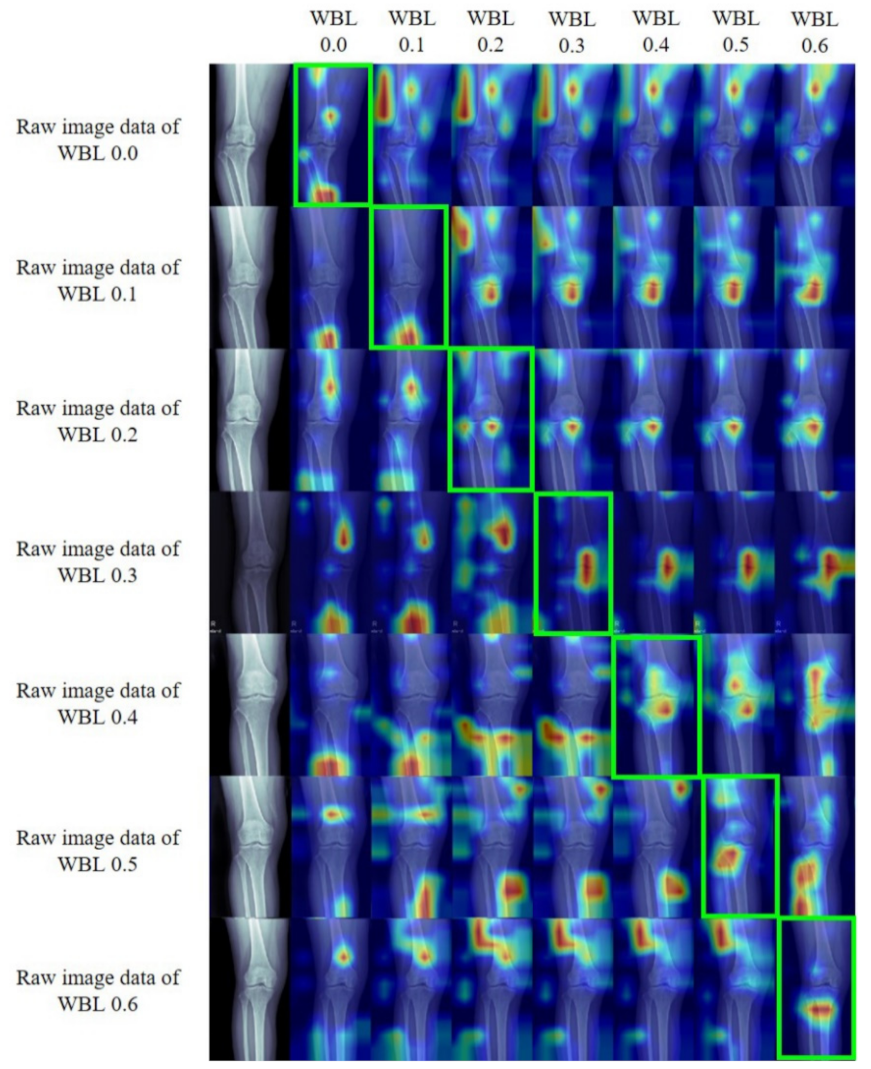
Gradient-Weighted Class Activation Mapping (Grad-CAM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wittenauer, R.; Smith, L.; Aden, K.J. Background Paper 6.12 Osteoarthritis; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Hunter, D.J.; Schofield, D.J.; Callander, E.J. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Nevitt, M.; Losina, E.; Kraus, V. Biomarkers for osteoarthritis: Current position and steps towards further validation. Best Pract. Res. Clin. Rheumatol. 2014, 28, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.R.; Conrad, B.P.; Fregly, B.J.; Vincent, H.K. The pathophysiology of osteoarthritis: A mechanical perspective on the knee joint. PM R 2012, 4, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Bastick, A.N.; Belo, J.N.; Runhaar, J.; Bierma-Zeinstra, S.M.A. What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin. Orthop. Relat. Res. 2015, 473, 2969–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, T.M.; Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. 2005, 33, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Hinman, R.S.; Wrigley, T.V.; Bennell, K.L. Varus malalignment and its association with impairments and functional limitations in medial knee osteoarthritis. Arthritis Rheum. 2008, 59, 935–942. [Google Scholar] [CrossRef]
- Cerejo, R.; Dunlop, D.D.; Cahue, S.; Channin, D.; Song, J.; Sharma, L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002, 46, 2632–2636. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, B.K.; Lee, S.H.; Park, H.G.; Jun, D.-S.; Moon, D.H. Effect of foot rotation on the mechanical axis and correlation between knee and whole leg radiographs. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2542–2547. [Google Scholar] [CrossRef]
- Iseki, Y.; Takahashi, T.; Takeda, H.; Tsuboi, I.; Imai, H.; Mashima, N.; Watanabe, S.; Yamamoto, H. Defining the load bearing axis of the lower extremity obtained from anterior-posterior digital radiographs of the whole limb in stance. Osteoarthr. Cartil. 2009, 17, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.-S.; Lee, S.H.; Lee, Y.S. Does coronal knee and ankle alignment affect recurrence of the varus deformity after high tibial osteotomy? Knee Surg. Relat. Res. 2018, 30, 311. [Google Scholar] [CrossRef]
- Takeuchi, R.; Ishikawa, H.; Aratake, M.; Bito, H.; Saito, I.; Kumagai, K.; Akamatsu, Y.; Saito, T. Medial opening wedge high tibial osteotomy with early full weight bearing. Arthrosc. J. Arthrosc. Relat. Surg. 2009, 25, 46–53. [Google Scholar] [CrossRef]
- Lee, O.-S.; Lee, E.S.; Lee, Y.S. Disparity between preoperative target correction amount and postoperative correction amount in open wedge high tibial osteotomy. Knee Surg. Relat. Res. 2019, 31, 126. [Google Scholar] [PubMed]
- Lee, Y.S.; Lee, B.K.; Kwon, J.H.; Kim, J.I.; Reyes, F.J.V.; Suh, D.W.; Nha, K.-W. Serial assessment of weight-bearing lower extremity alignment radiographs after open-wedge high tibial osteotomy. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 319–325. [Google Scholar] [CrossRef]
- Brouwer, G.; Tol, A.V.; Bergink, A.; Belo, J.; Bernsen, R.; Reijman, M.; Pols, H.; Bierma-Zeinstra, S.M. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007, 56, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Hinman, R.S.; May, R.L.; Crossley, K.M. Is there an alternative to the full-leg radiograph for determining knee joint alignment in osteoarthritis? Arthritis Rheum. 2006, 55, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, B.; Vasta, S.; Amendola, A.; Marbach, B.U.-E.; Gao, Y.; Papalia, R.; Denaro, V. Assessing lower limb alignment: Comparison of standard knee xray vs long leg view. Iowa Orthop. J. 2015, 35, 49–54. [Google Scholar]
- Cabitza, F.; Locoro, A.; Banfi, G. Machine learning in orthopedics: A literature review. Front. Bioeng. Biotechnol. 2018, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Obermeyer, Z.; Emanuel, E.J. Predicting the future—Big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016, 375, 1216. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Summers, R.M. Machine learning and radiology. Med. Image Anal. 2012, 16, 933–951. [Google Scholar] [CrossRef] [Green Version]
- Tiulpin, A.; Thevenot, J.; Rahtu, E.; Lehenkari, P.; Saarakkala, S. Automatic knee osteoarthritis diagnosis from plain radiographs: A deep learning-based approach. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, K.J.; Choi, D.; Lee, J.I.; Choi, H.G.; Lee, Y.S. Can additional patient information improve the diagnostic performance of deep learning for the interpretation of knee osteoarthritis severity. J. Clin. Med. 2020, 9, 3341. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.S.; Dougherty, E.R. Effect of separate sampling on classification accuracy. Bioinformatics 2014, 30, 242–250. [Google Scholar] [CrossRef]
- Nha, K.-W.; Lee, Y.S.; Hwang, D.-H.; Kwon, J.H.; Chae, D.J.; Park, Y.J.; Kim, J.I. Second-look arthroscopic findings after open-wedge high tibia osteotomy focusing on the posterior root tears of the medial meniscus. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2018; pp. 7132–7141. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Yao, L.; Prosky, J.; Poblenz, E.; Covington, B.; Lyman, K. Weakly supervised medical diagnosis and localization from multiple resolutions. arXiv 2018, arXiv:1803.07703. [Google Scholar]
- Pan, H.; Han, H.; Shan, S.; Chen, X. Mean-variance loss for deep age estimation from a face. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2018; pp. 5285–5294. [Google Scholar]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. In Proceedings of the 13th International Conference on Artificial Intelligence and Statistics, Sardinia, Italy, 13–15 May 2010; pp. 249–256. [Google Scholar]
- Hinton, G.; Srivastava, N.; Swersky, K. Neural networks for machine learning lecture 6a overview of mini-batch gradient descent. Cited on 2012, 14, 1–31. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Visual explanations from deep networks via gradient-based localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Khan, F.A.; Koff, M.F.; Noiseux, N.O.; Bernhardt, K.A.; O’Byrne, M.M.; Larson, D.R.; Amrami, K.K.; Kaufman, K.R. Effect of local alignment on compartmental patterns of knee osteoarthritis. J. Bone Jt. Surg. Am. Vol. 2008, 90, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Gao, L.; Shi, X.; Allen, K.; Yang, L. Fully automatic knee osteoarthritis severity grading using deep neural networks with a novel ordinal loss. Comput. Med. Imaging Graph. 2019, 75, 84–92. [Google Scholar] [CrossRef]

| Training Set | Validation Set | Test Set | Total | p-Value | |
|---|---|---|---|---|---|
| Age (year) | 64.8 ± 9.22 | 64.3 ± 9.41 | 65.1 ± 9.02 | 64.8 ± 12.8 | 0.449 |
| Gender (M/F) | 573/2676 | 61/301 | 82/304 | 716/3281 | 0.186 |
| BMI (kg/m2) | 25.6 ± 3.17 | 25.7 ± 2.49 | 25.4 ± 2.42 | 25.6 ± 3.10 | 0.284 |
| WBL ratio | 0.32 ± 0.16 | 0.32 ± 0.17 | 0.35 ± 0.14 | 0.32 ± 0.16 | <0.001 * |
| 0.0 | 148 | 16 | 4 | 168 | |
| 0.1 | 307 | 34 | 24 | 365 | |
| 0.2 | 595 | 66 | 56 | 717 | |
| 0.3 | 859 | 96 | 108 | 1063 | |
| 0.4 | 754 | 84 | 113 | 951 | |
| 0.5 | 392 | 44 | 52 | 488 | |
| 0.6 | 194 | 22 | 29 | 245 | |
| Total | 3249 | 362 | 386 | 3997 |
| Validation Set | Test Set | |
|---|---|---|
| MAE | 0.054 | 0.054 |
| CS (0.1) | 0.953 (345/362, 0.924–0.970) | 0.951 (367/386, 0.924–0.970) |
| CS (0.0) | 0.511 (185/362, 0.458–0.564) | 0.526 (203/386, 0.474–0.577) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.-D.; Choi, H.-G.; Lee, K.-J.; Choi, D.-J.; Yoo, H.-J.; Lee, Y.-S. Can Deep Learning Using Weight Bearing Knee Anterio-Posterior Radiograph Alone Replace a Whole-Leg Radiograph in the Interpretation of Weight Bearing Line Ratio? J. Clin. Med. 2021, 10, 1772. https://doi.org/10.3390/jcm10081772
Moon H-D, Choi H-G, Lee K-J, Choi D-J, Yoo H-J, Lee Y-S. Can Deep Learning Using Weight Bearing Knee Anterio-Posterior Radiograph Alone Replace a Whole-Leg Radiograph in the Interpretation of Weight Bearing Line Ratio? Journal of Clinical Medicine. 2021; 10(8):1772. https://doi.org/10.3390/jcm10081772
Chicago/Turabian StyleMoon, Hyun-Doo, Han-Gyeol Choi, Kyong-Joon Lee, Dong-Jun Choi, Hyun-Jin Yoo, and Yong-Seuk Lee. 2021. "Can Deep Learning Using Weight Bearing Knee Anterio-Posterior Radiograph Alone Replace a Whole-Leg Radiograph in the Interpretation of Weight Bearing Line Ratio?" Journal of Clinical Medicine 10, no. 8: 1772. https://doi.org/10.3390/jcm10081772
APA StyleMoon, H.-D., Choi, H.-G., Lee, K.-J., Choi, D.-J., Yoo, H.-J., & Lee, Y.-S. (2021). Can Deep Learning Using Weight Bearing Knee Anterio-Posterior Radiograph Alone Replace a Whole-Leg Radiograph in the Interpretation of Weight Bearing Line Ratio? Journal of Clinical Medicine, 10(8), 1772. https://doi.org/10.3390/jcm10081772







