Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Sample
2.2. Flow Cytometry (FCM) Analysis
2.3. Cytokine Assay
2.4. RT-PCR Analysis
2.5. Cell Culture and Cytokine Induction
2.6. Assay for Autologous T-Cell Proliferation
2.7. Animal Models and Treatments
2.8. Statistical Analysis
3. Results
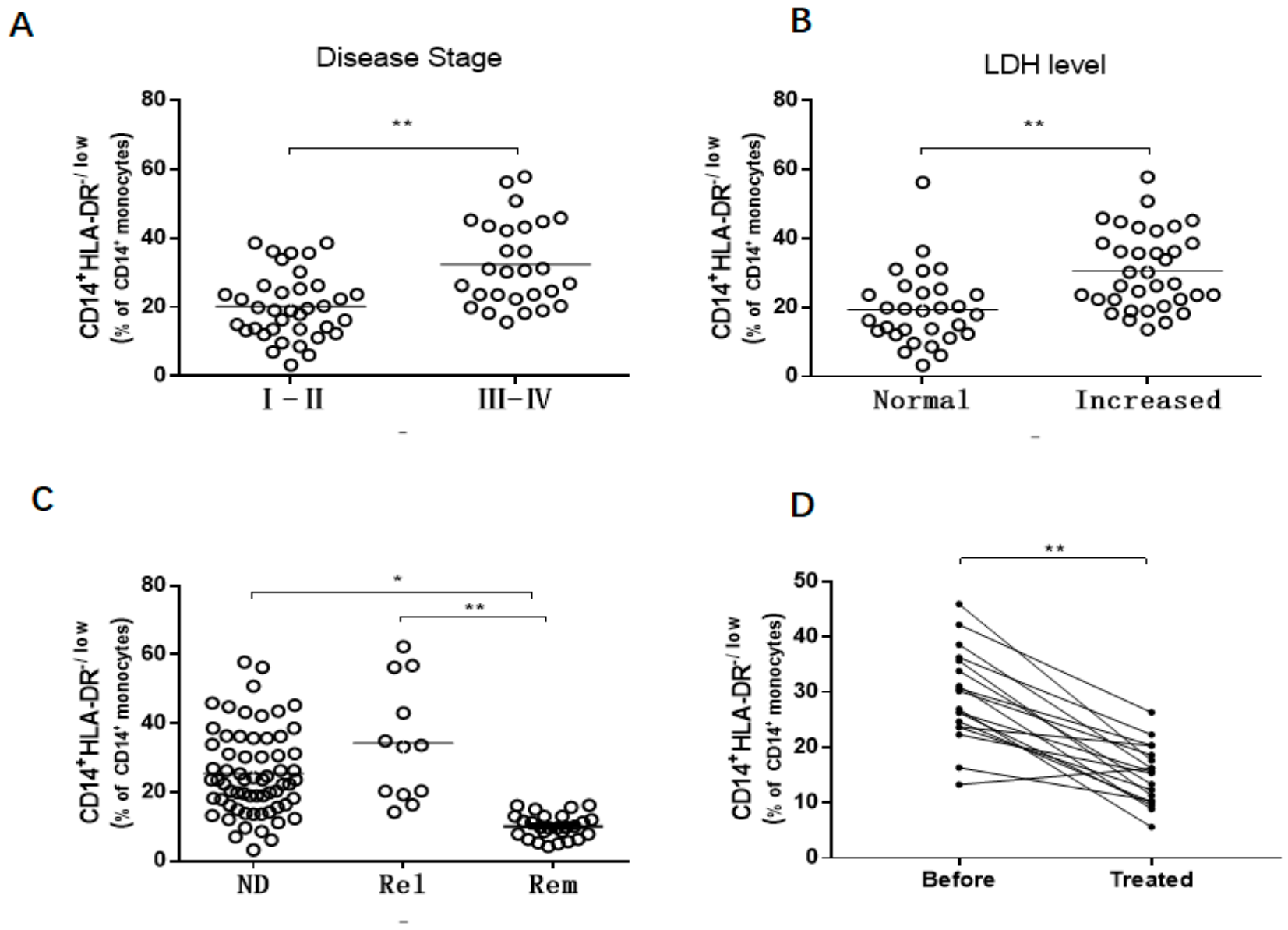
3.1. Increased M-MDSCs in Newly Diagnosed and Relapsed DLBCL Patients
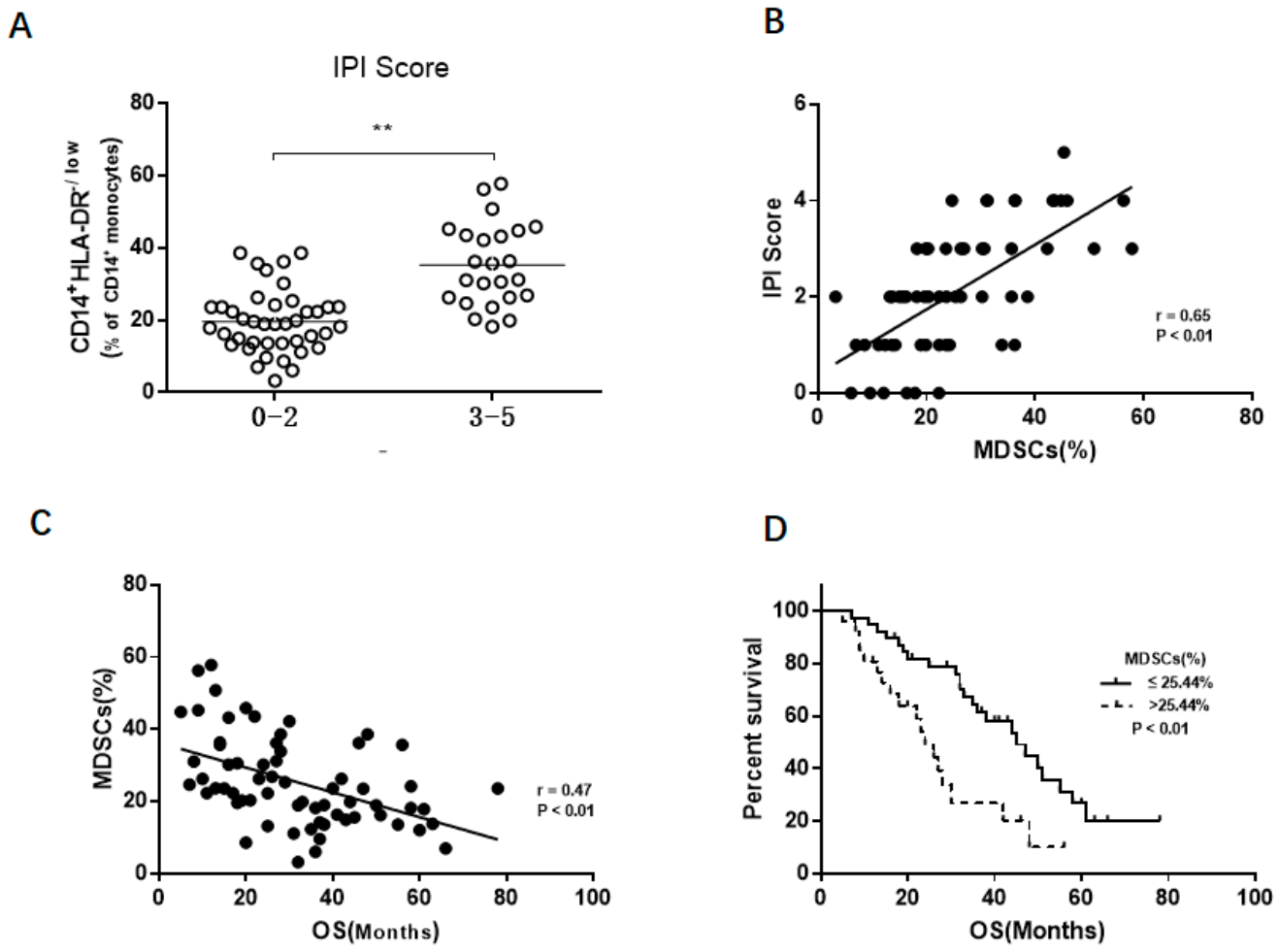
3.2. M-MDSCs Levels Correlate with Disease Progression in Newly Diagnosed DLBCL Patients
3.3. The Association Between M-MDSCs and Prognosis of DLBCL Patient
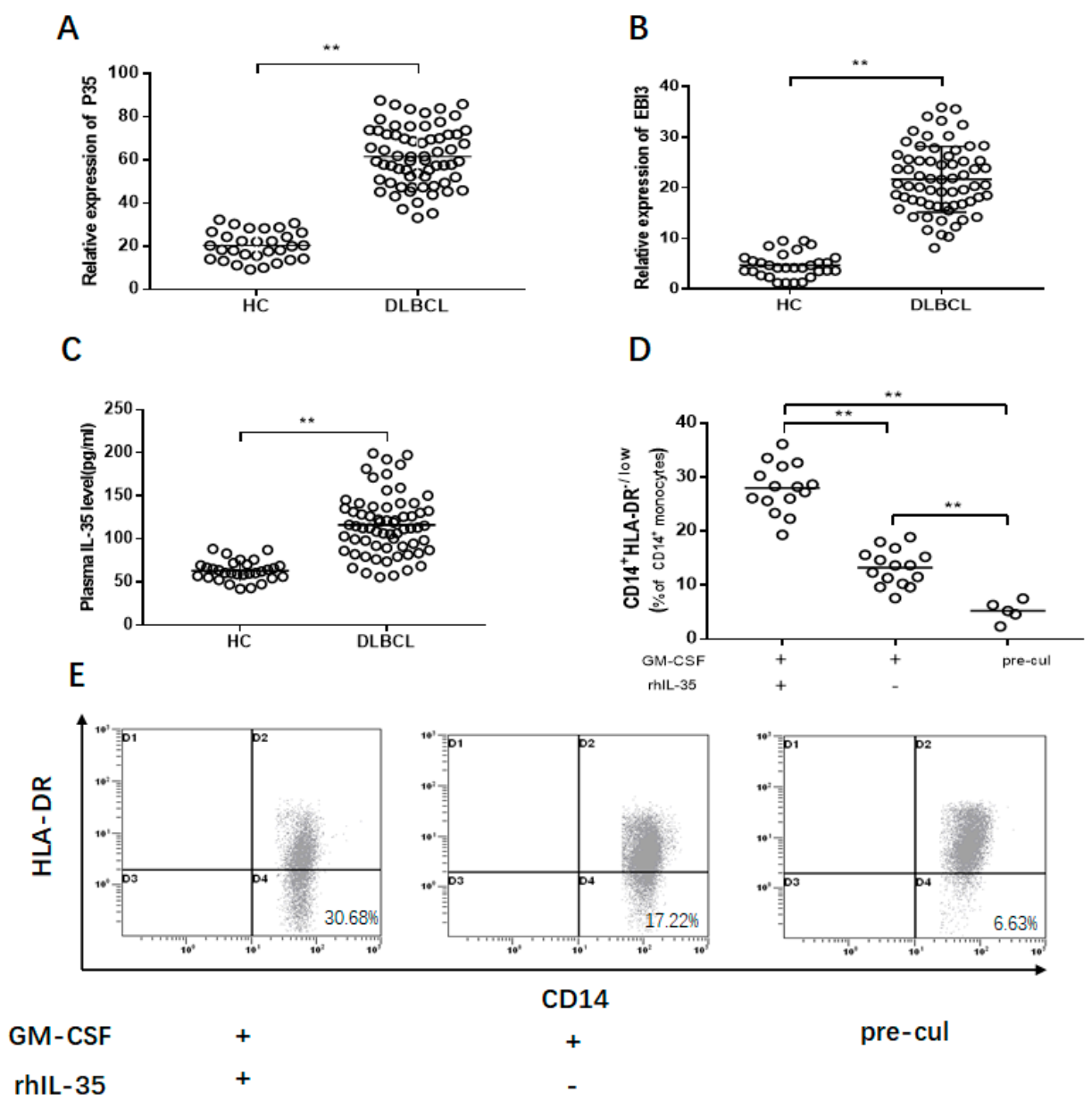
3.4. Increased IL-35 Induce the M-MDSCs Expansion
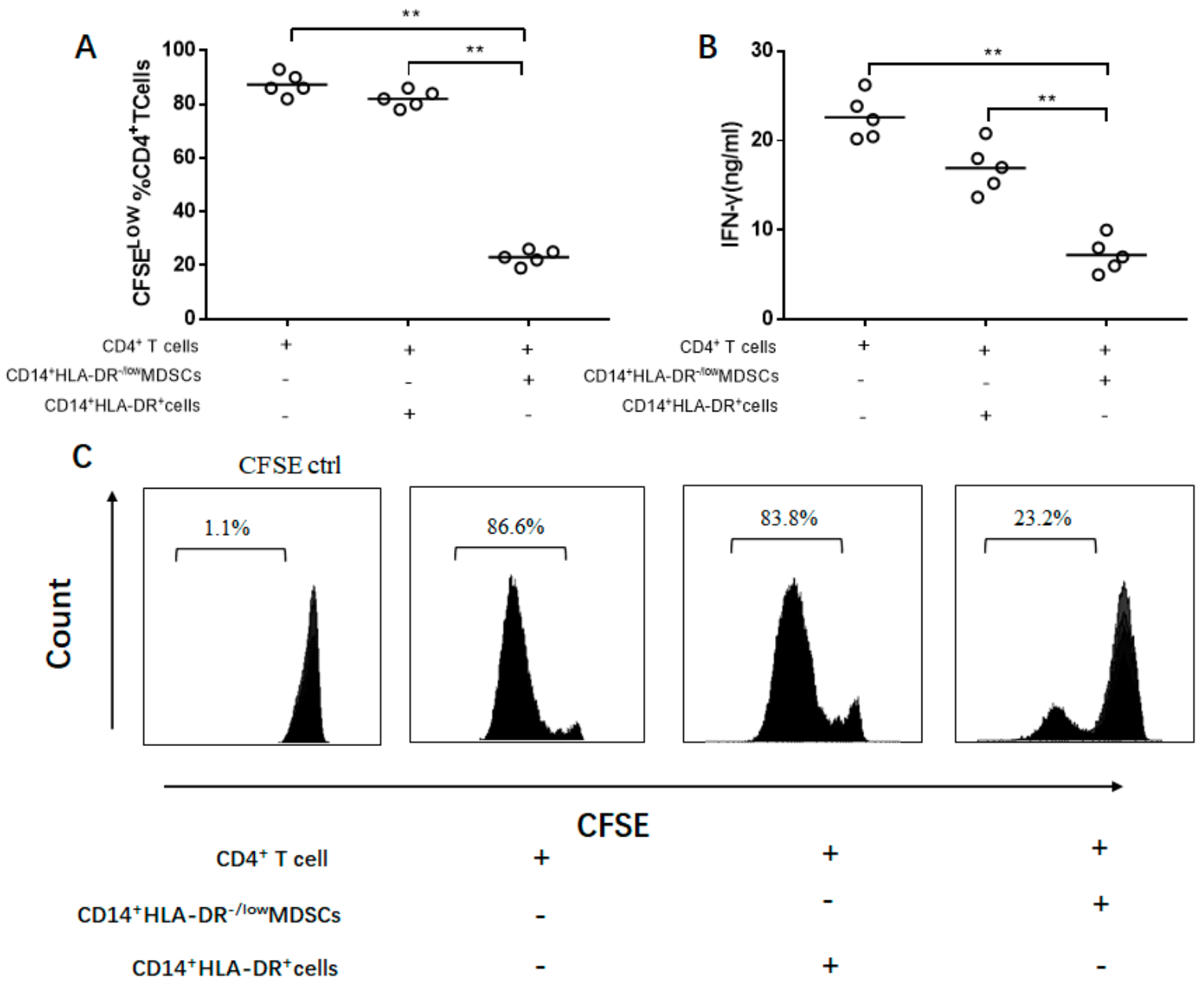
3.5. IL-35-Induced M-MDSCs Suppress CD4+ T Cell Response
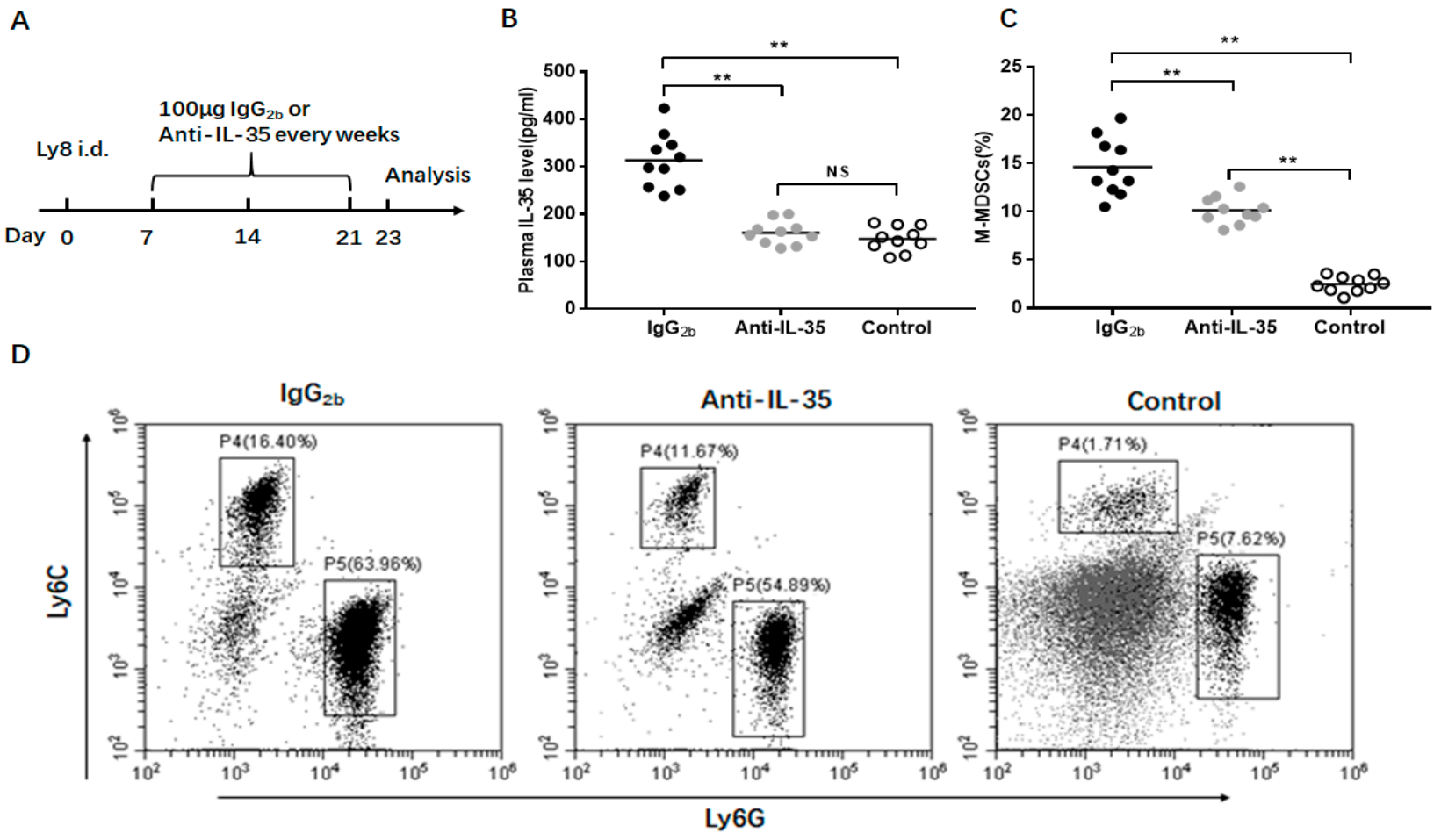
3.6. Anti-IL-35 Treatment Block M-MDSC Expansion In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Coiffier, B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non-Hodgkin’s lymphoma. Semin. Oncol. 2002, 29, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA A Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Yokoyama, M.; Yamamoto, W.; Watanabe, R.; Shimazu, Y.; Masaki, Y.; Tsunoda, S.; Hashimoto, C.; Murayama, K.; Yano, T.; et al. The standard international prognostic index for predicting the risk of CNS involvement in DLBCL without specific prophylaxis. Leuk. Lymphoma 2017, 59, 97–104. [Google Scholar] [CrossRef]
- Chan, A.; Dogan, A. Prognostic and Predictive Biomarkers in Diffuse Large B-cell Lymphoma. Surg. Pathol. Clin. 2019, 12, 699–707. [Google Scholar] [CrossRef]
- Pyzer, A.R.; Cole, L.; Rosenblatt, J.; Avigan, D.E. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int. J. Cancer 2016, 139, 1915–1926. [Google Scholar] [CrossRef]
- Chen, M.-F.; Tsai, M.-S.; Chen, W.-C.; Chen, P.-T. Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2018, 7, 294. [Google Scholar] [CrossRef]
- Chang, C.-J.; Yang, Y.-H.; Chiu, C.-J.; Lu, L.-C.; Liao, C.-C.; Liang, C.-W.; Hsu, C.-H.; Cheng, A.-L. Targeting tumor-infiltrating Ly6G+ myeloid cells improves sorafenib efficacy in mouse orthotopic hepatocellular carcinoma. Int. J. Cancer 2018, 142, 1878–1889. [Google Scholar] [CrossRef]
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Iwata, T.; Kondo, Y.; Kimura, O.; Morosawa, T.; Fujisaka, Y.; Umetsu, T.; Kogure, T.; Inoue, J.; Nakagome, Y.; Shimosegawa, T. PD-L1+MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci. Rep. 2016, 6, 39296. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.-L.; Ye, S.-B.; Ouyang, L.-Y.; Chen, Y.-S.; He, J.; Huang, H.-Q.; Zeng, Y.-X.; Zhang, X.-S.; Li, J. Myeloid-derived suppressor cells inhibit T cell proliferation in human extranodal NK/T cell lymphoma: A novel prognostic indicator. Cancer Immunol. Immunother. 2015, 64, 1587–1599. [Google Scholar] [CrossRef]
- Yin, J.; Wang, C.; Huang, M.; Mao, X.; Zhou, J.; Zhang, Y. Circulating CD14(+) HLA-DR(-/low) myeloid-derived suppressor cells in leukemia patients with allogeneic hematopoietic stem cell transplantation: Novel clinical potential strategies for the prevention and cellular therapy of graft-versus-host disease. Cancer Med. 2016, 5, 1654–1669. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Wang, H.; Xiong, S.; Li, Y.; Tao, Q.; Xiao, W.; Qin, H.; Wang, Y.; Zhai, Z. Tumor-induced CD14+HLA-DR−/low myeloid-derived suppressor cells correlate with tumor progression and outcome of therapy in multiple myeloma patients. Cancer Immunol. Immunother. 2015, 64, 389–399. [Google Scholar] [CrossRef]
- Xiao, P.; Wan, X.; Cui, B.; Liu, Y.; Qiu, C.; Rong, J.; Zheng, M.; Song, Y.; Chen, L.; He, J.; et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. OncoImmunology 2015, 5, e1063772. [Google Scholar] [CrossRef]
- Lechner, M.G.; Liebertz, D.J.; Epstein, A.L. Characterization of Cytokine-Induced Myeloid-Derived Suppressor Cells from Normal Human Peripheral Blood Mononuclear Cells. J. Immunol. 2010, 185, 2273–2284. [Google Scholar] [CrossRef]
- Wang, J.; Tao, Q.; Wang, H.; Wang, Z.; Wu, F.; Pan, Y.; Tao, L.; Xiong, S.; Wang, Y.; Zhai, Z. Elevated IL-35 in bone marrow of the patients with acute myeloid leukemia. Hum. Immunol. 2015, 76, 681–686. [Google Scholar] [CrossRef]
- Tao, Q.; Pan, Y.; Wang, Y.; Wang, H.; Xiong, S.; Lili, T.; Wang, J.; Tao, L.; Wang, Z.; Wu, F.; et al. Regulatory T cells-derived IL-35 promotes the growth of adult acute myeloid leukemia blasts. Int. J. Cancer 2015, 137, 2384–2393. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.-Q.; Liu, Z.; Shen, R.; Zhang, G.; Xu, J.; Basu, S.; Feng, Y.; Bai, X.-F. Tumor-Derived IL-35 Promotes Tumor Growth by Enhancing Myeloid Cell Accumulation and Angiogenesis. J. Immunol. 2013, 190, 2415–2423. [Google Scholar] [CrossRef]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef]
- Bayne, L.J.; Beatty, G.L.; Jhala, N.; Clark, C.E.; Rhim, A.D.; Stanger, B.Z.; Vonderheide, R.H. Tumor-Derived Granulocyte-Macrophage Colony-Stimulating Factor Regulates Myeloid Inflammation and T Cell Immunity in Pancreatic Cancer. Cancer Cell 2012, 21, 822–835. [Google Scholar] [CrossRef]
- Kanai, K.; Park, A.-M.; Yoshida, H.; Tsunoda, I.; Yoshie, O. IL-35 Suppresses Lipopolysaccharide-Induced Airway Eosinophilia in EBI3-Deficient Mice. J. Immunol. 2016, 198, 119–127. [Google Scholar] [CrossRef]
- Shu, C.-C.; Pan, S.-W.; Feng, J.-Y.; Wang, J.-Y.; Chan, Y.-J.; Yu, C.-J.; Su, W.-J. The Clinical Significance of Programmed Death-1, Regulatory T Cells and Myeloid Derived Suppressor Cells in Patients with Nontuberculous Mycobacteria-Lung Disease. J. Clin. Med. 2019, 8, 736. [Google Scholar] [CrossRef]
- Malek, E.; de Lima, M.; Letterio, J.J.; Kim, B.-G.; Finke, J.H.; Driscoll, J.J.; Giralt, S.A. Myeloid-derived suppressor cells: The green light for myeloma immune escape. Blood Rev. 2016, 30, 341–348. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. GammadeltaT17 cells promote the accumulation and expression of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef]
- Gonda, K.; Shibata, M.; Ohtake, T.; Matsumoto, Y.; Tachibana, K.; Abe, N.; Ohto, H.; Sakurai, K.; Takenoshita, S. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol. Lett. 2017, 14, 1766–1774. [Google Scholar] [CrossRef]
- Haverkamp, J.M.; Smith, A.M.; Weinlich, R.; Dillon, C.P.; Qualls, J.E.; Neale, G.; Koss, B.; Kim, Y.; Bronte, V.; Herold, M.J.; et al. Myeloid-Derived Suppressor Activity Is Mediated by Monocytic Lineages Maintained by Continuous Inhibition of Extrinsic and Intrinsic Death Pathways. Immunity 2014, 41, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Azzaoui, I.; Uhel, F.; Rossille, D.; Pangault, C.; Dulong, J.; Le Priol, J.; Lamy, T.; Houot, R.; Le Gouill, S.; Cartron, G.; et al. T-cell defect in diffuse large B-cell lymphomas involves expression of myeloid-derived suppressor cells. Blood 2016, 128, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, X.; Liu, X.; Yang, P.; Xu, J.; Chai, Y.; Guo, Q.; Wang, Z.; Zhang, L. Prognostic Significance of Monocytes and Monocytic Myeloid-Derived Suppressor Cells in Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Cell. Physiol. Biochem. 2016, 39, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, X.; Zhang, X.; Chai, Y.; Guo, Q.; Li, L.; Yue, L.; Bai, J.; Wang, Z.; Zhang, L. Prognostic significance of peripheral monocytic myeloid-derived suppressor cells and monocytes in patients newly diagnosed with diffuse large b-cell lymphoma. Int. J. Clin. Exp. Med. 2015, 8, 15173–15181. [Google Scholar]
- Wang, K.; Gong, H.; Chai, R.; Yuan, H.; Chen, Y.; Liu, J. RETRACTED: Aberrant frequency of IL-35 producing B cells in colorectal cancer patients. Cytokine 2018, 102, 206–210. [Google Scholar] [CrossRef]
- Long, J.; Guo, H.; Cui, S.; Zhang, H.; Liu, X.; Li, D.; Han, Z.; Xi, L.; Kou, W.; Xu, J.; et al. IL-35 expression in hepatocellular carcinoma cells is associated with tumor progression. Oncotarget 2016, 7, 45678–45686. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]

| State of Disease at Sample Draw | No. of Patients | Average Age (Range) | Gender(M/F) |
|---|---|---|---|
| Newly diagnosed | 65 | 58.7(28–80) | 40/25 |
| Disease stage | |||
| I-II | 37 | 57.8(30–80) | 20/17 |
| III-IV | 28 | 59.1(28–78) | 20/8 |
| B symptoms | |||
| YES | 17 | 52.3(28–69) | 9/8 |
| NO | 48 | 61.4(32–80) | 31/17 |
| GCB | |||
| YES | 38 | 58.9(28–76) | 23/15 |
| NO | 27 | 58.5(30–80) | 17/10 |
| LDH | |||
| Normal | 30 | 60.4(30–80) | 20/10 |
| Increased | 35 | 57.3(28–78) | 20/15 |
| IPI score | |||
| 0–2 | 41 | 55.8(28–76) | 24/17 |
| 3–5 | 24 | 63.7(32–80) | 16/8 |
| Relapsed | 12 | 58.3(36–62) | 9/3 |
| Remission | 26 | 62.3(31–76) | 14/12 |
| Healthy donors | 30 | 60.1(30–78) | 18/12 |
| Factor | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (year) | ||||||
| (≤60 vs. >60) | 1.511 | 0.811–2.817 | 0.194 | |||
| Gender | ||||||
| (Male vs. Female) | 1.127 | 0.601–2.112 | 0.709 | |||
| Disease stage | ||||||
| (I-II vs. III-IV) | 3.026 | 1.591–5.755 | 0.001 | 2.184 | 0.916–5.205 | 0.078 |
| B symptoms | ||||||
| (No vs. Yes) | 1.339 | 0.710–2.523 | 0.367 | |||
| GCB | ||||||
| (No vs. Yes) | 1.060 | 0.576–1.981 | 0.855 | |||
| LDH | ||||||
| (Normal vs. Increased) | 0.348 | 0.136–0.887 | 0.027 | 1.461 | 0.529–4.035 | 0.464 |
| IPI score | ||||||
| (0–2 vs. 3–5) | 2.976 | 1.549–5.717 | 0.001 | 0.271 | 0.103–0.712 | 0.008 |
| MDSCs | ||||||
| (≤25.44% vs. >25.44%) | 2.707 | 1.409–5.199 | 0.003 | 2.682 | 1.198–6.004 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jiang, R.; Li, Q.; Wang, H.; Tao, Q.; Zhai, Z. Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients. J. Clin. Med. 2021, 10, 1768. https://doi.org/10.3390/jcm10081768
Wang Z, Jiang R, Li Q, Wang H, Tao Q, Zhai Z. Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients. Journal of Clinical Medicine. 2021; 10(8):1768. https://doi.org/10.3390/jcm10081768
Chicago/Turabian StyleWang, Zhitao, Rui Jiang, Qian Li, Huiping Wang, Qianshan Tao, and Zhimin Zhai. 2021. "Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients" Journal of Clinical Medicine 10, no. 8: 1768. https://doi.org/10.3390/jcm10081768
APA StyleWang, Z., Jiang, R., Li, Q., Wang, H., Tao, Q., & Zhai, Z. (2021). Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients. Journal of Clinical Medicine, 10(8), 1768. https://doi.org/10.3390/jcm10081768






