PEComa—A Rare Liver Tumor
Abstract
1. Introduction
2. Materials and Methods
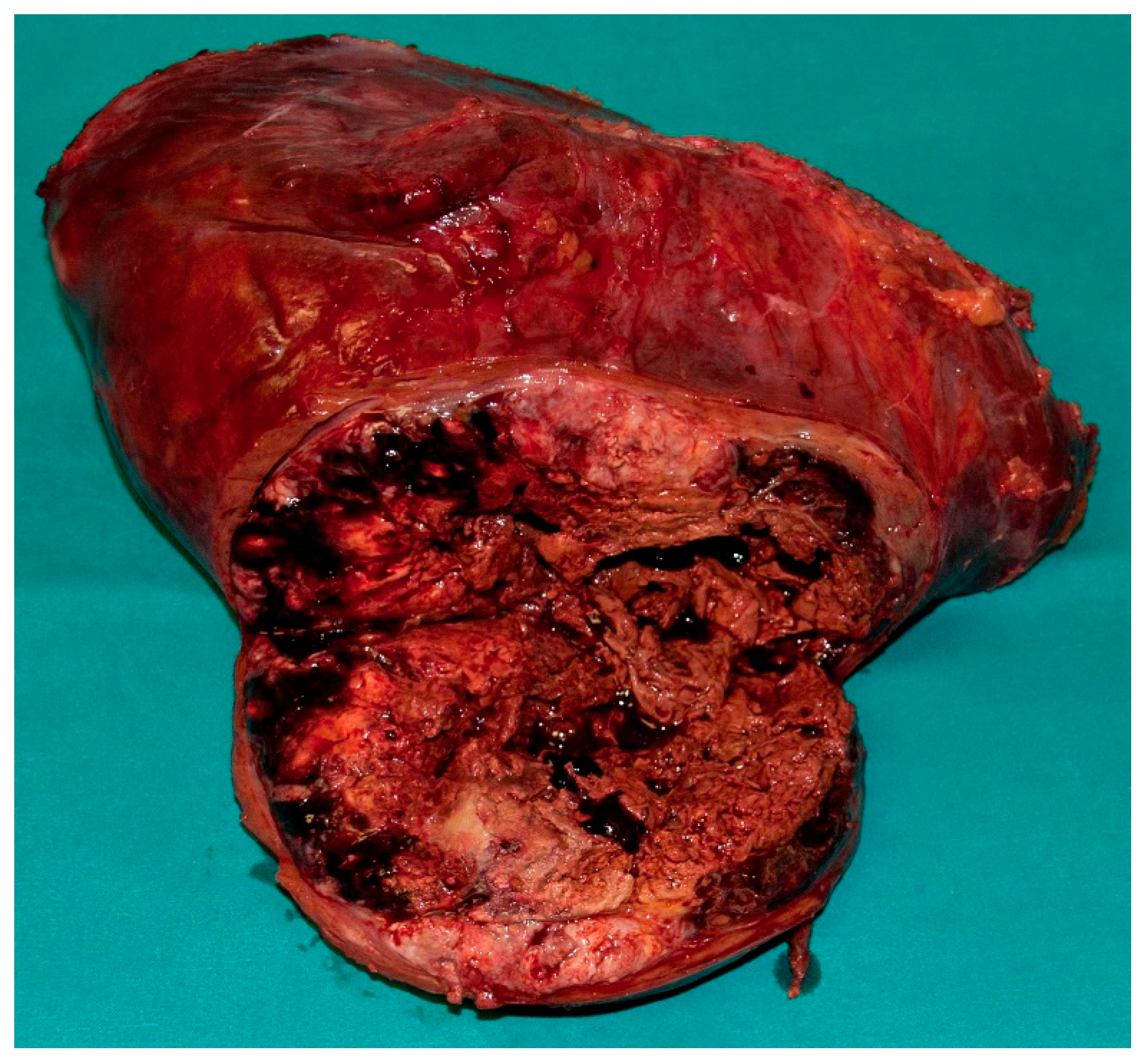
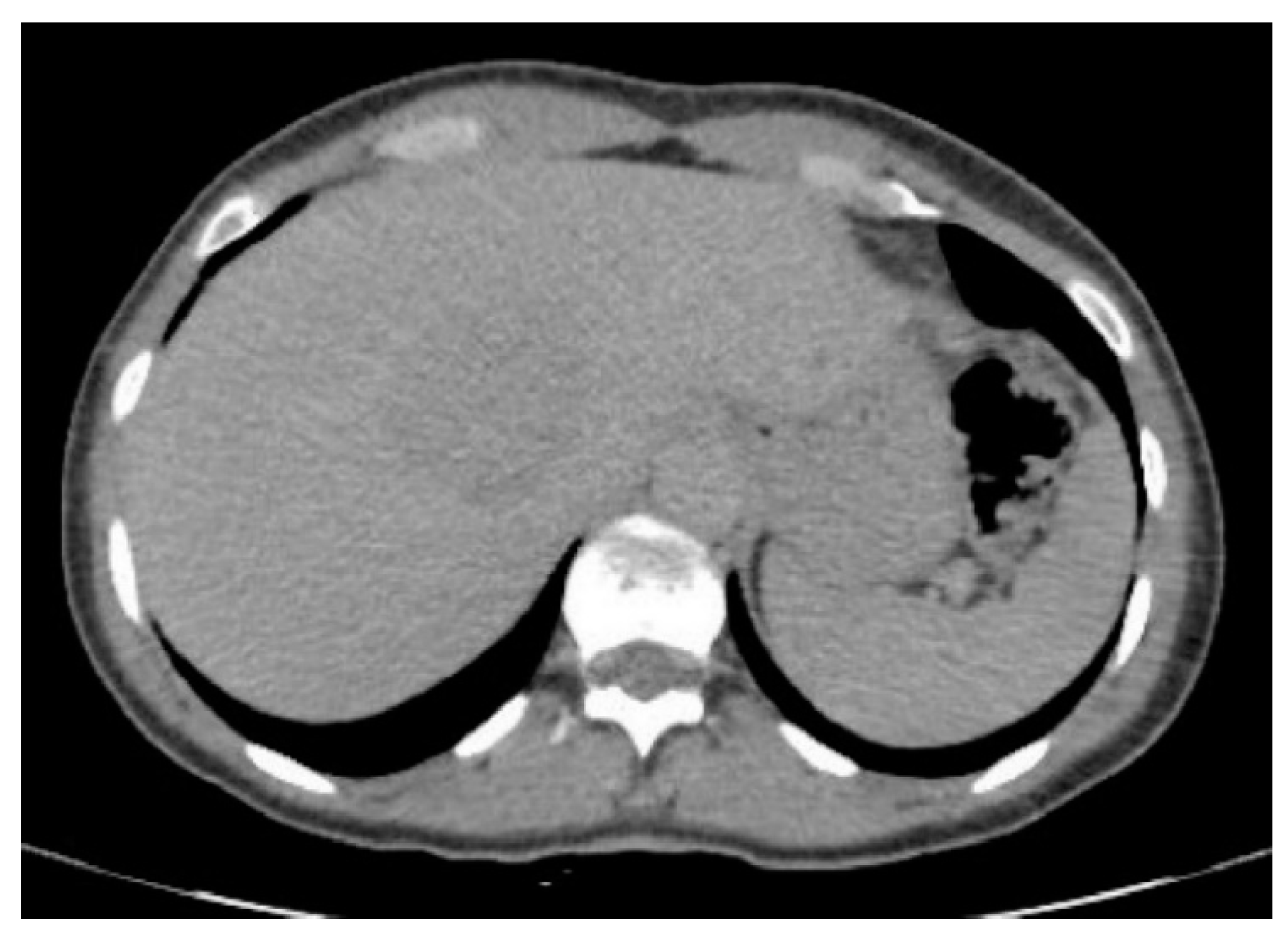
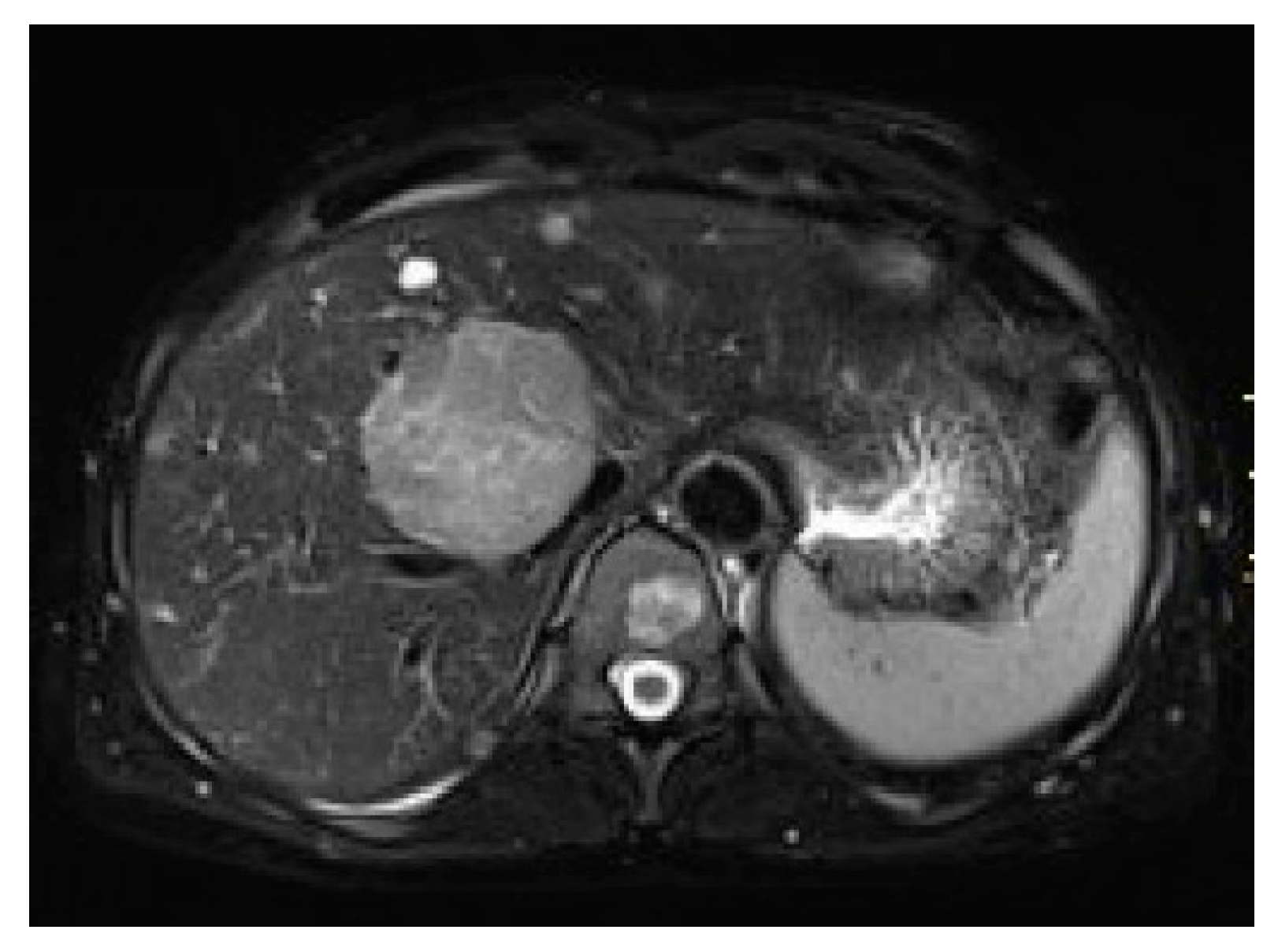
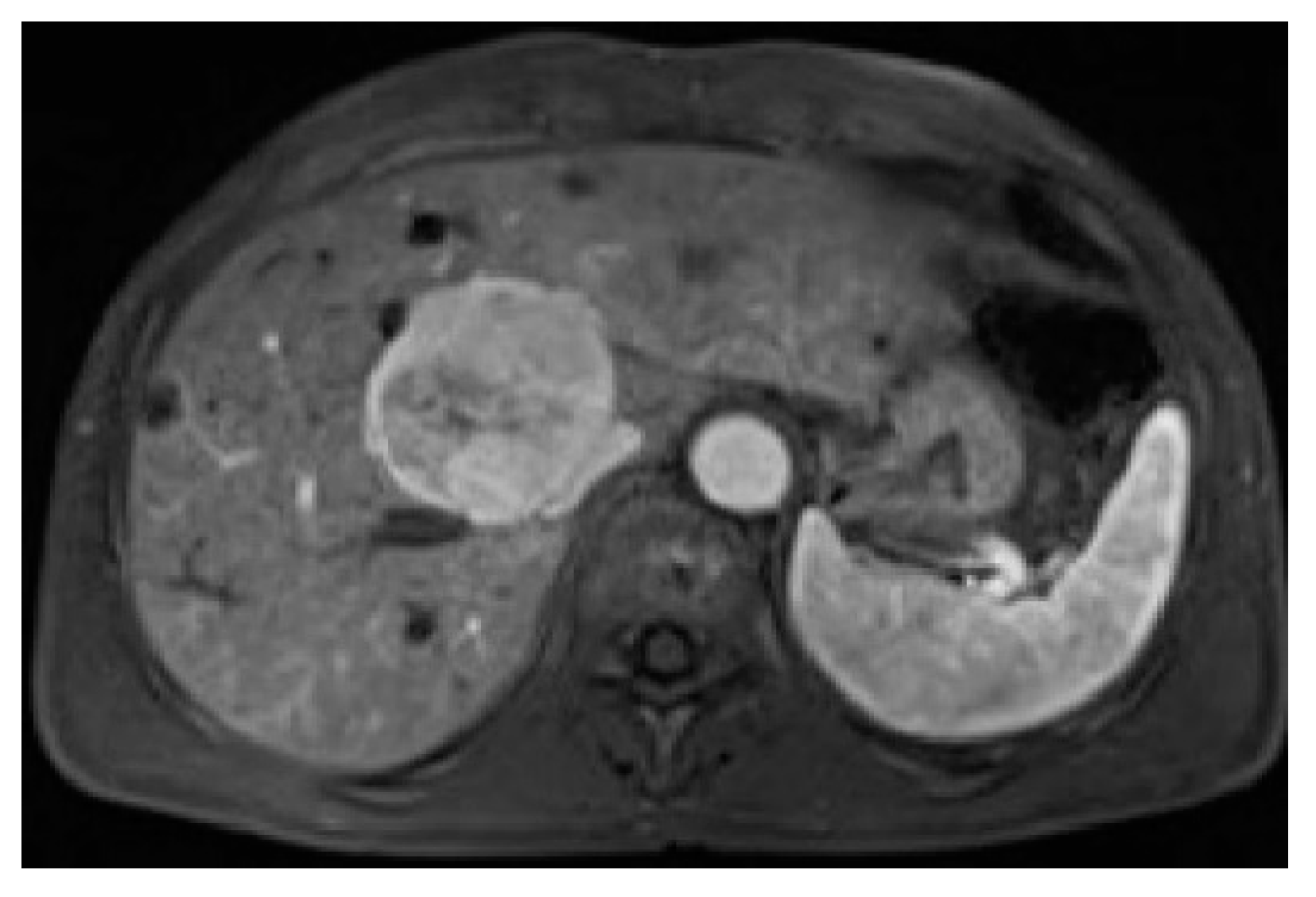
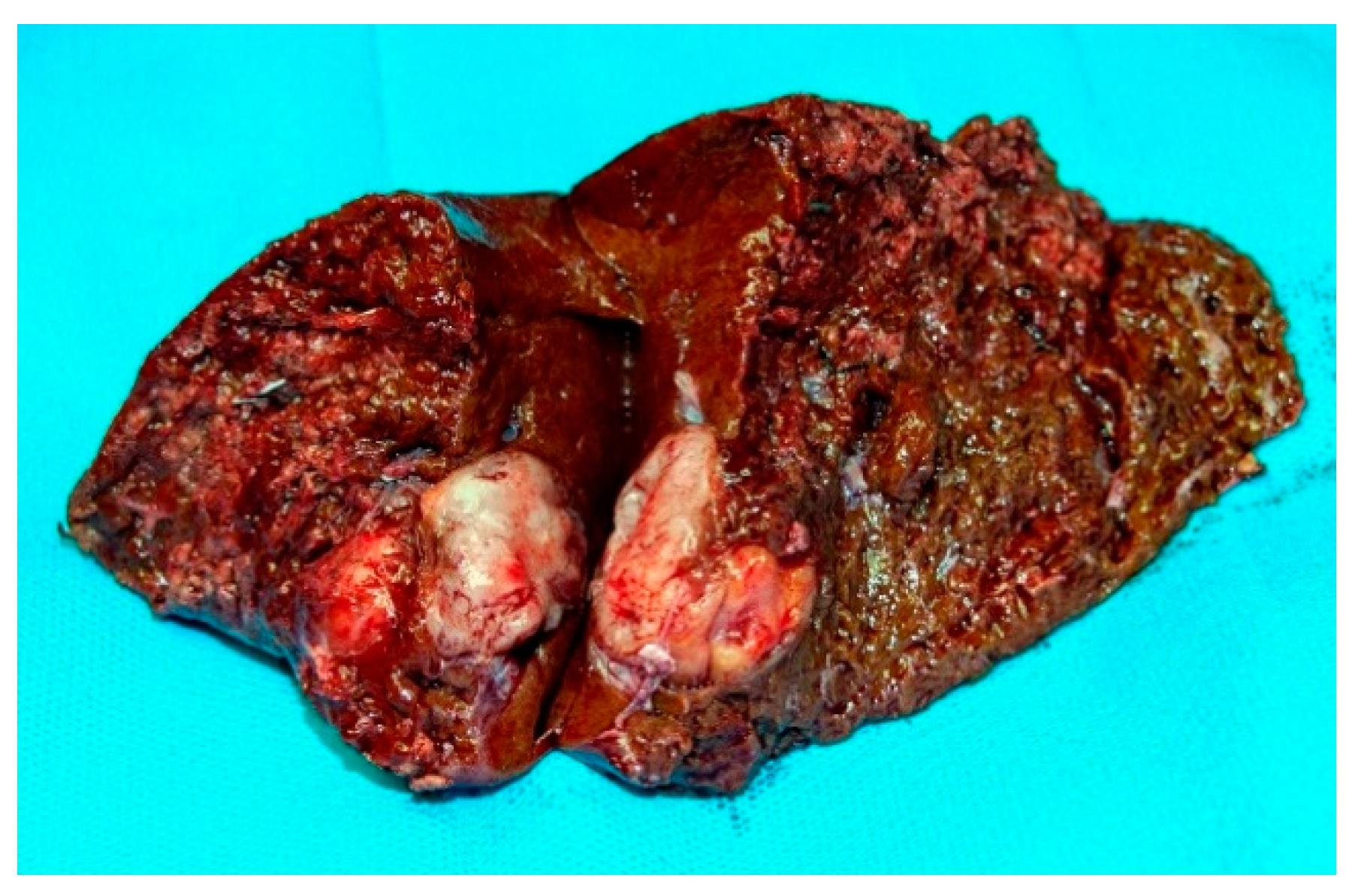
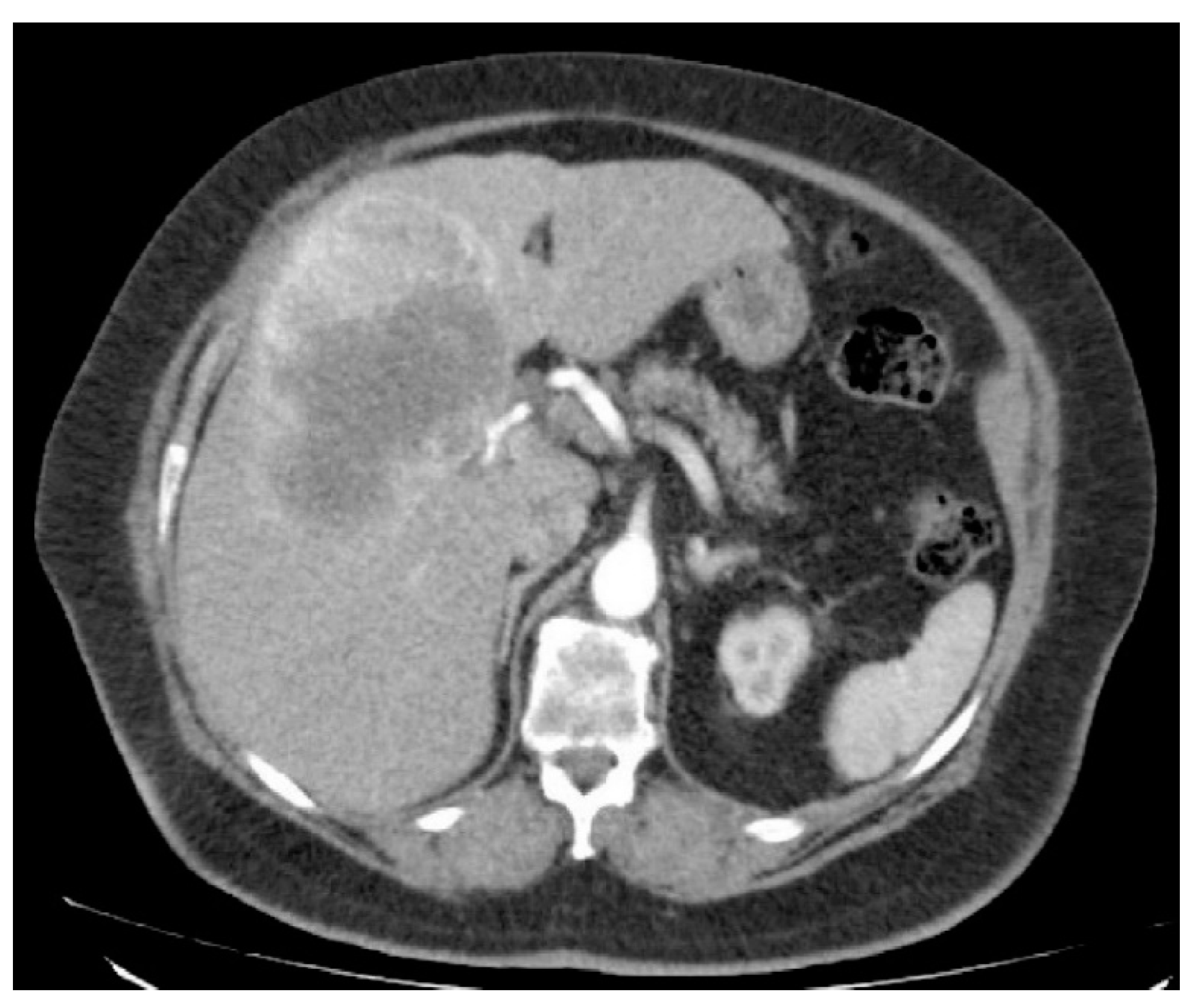
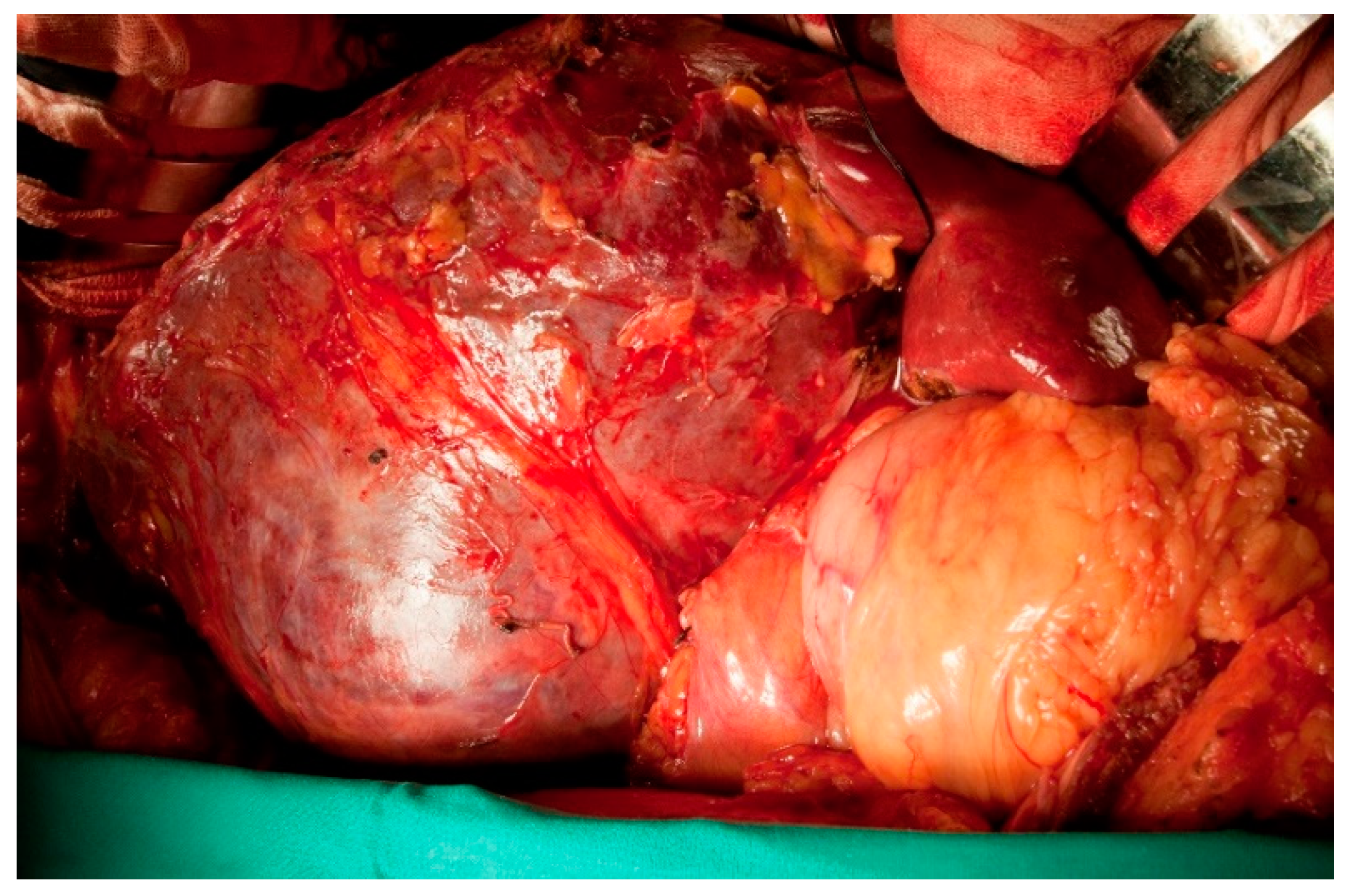
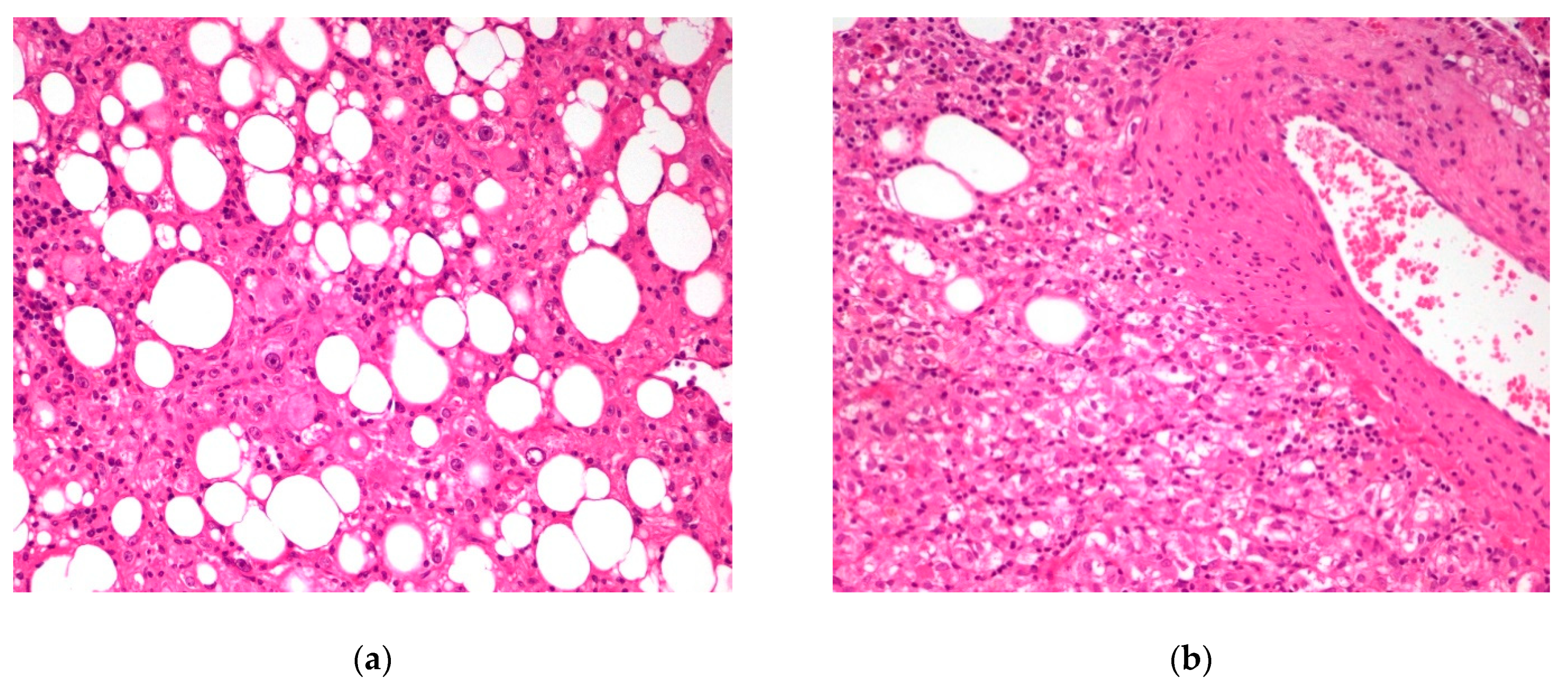
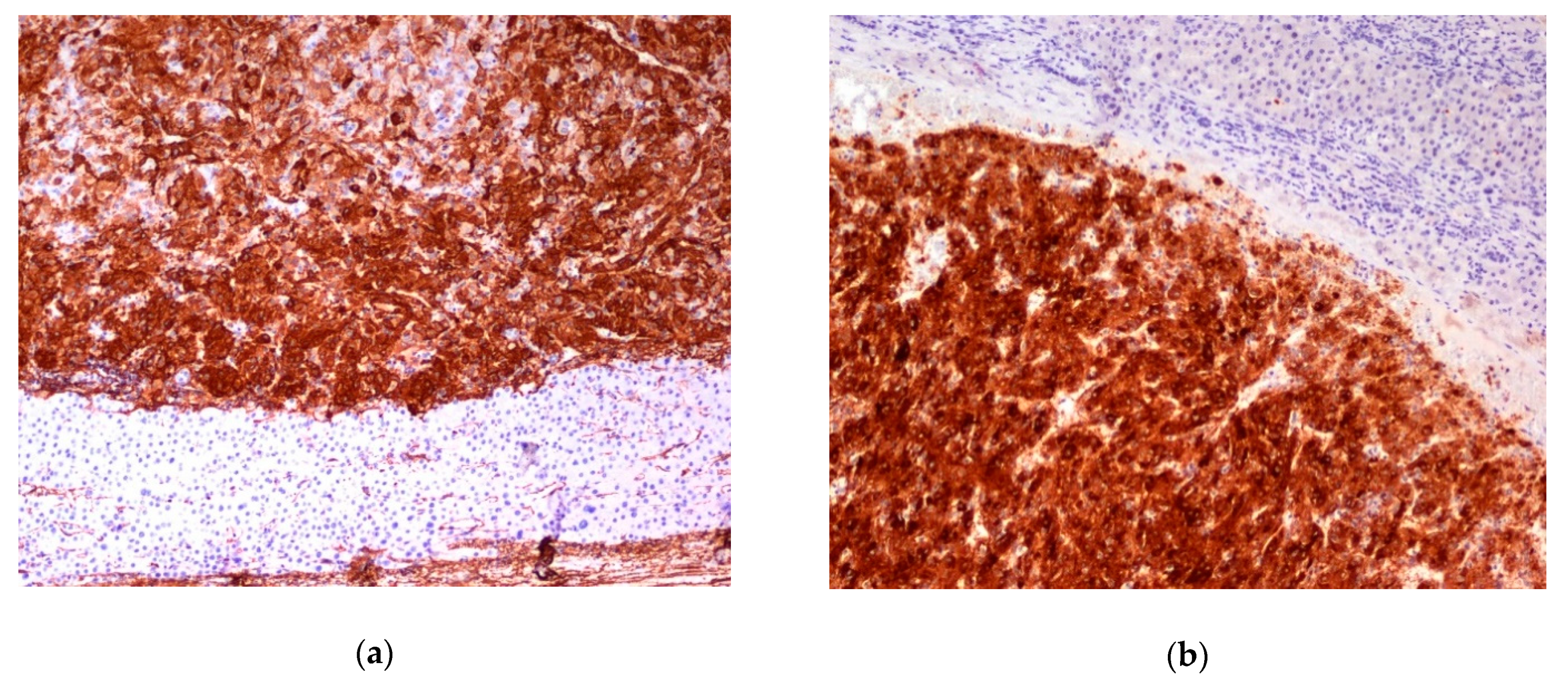
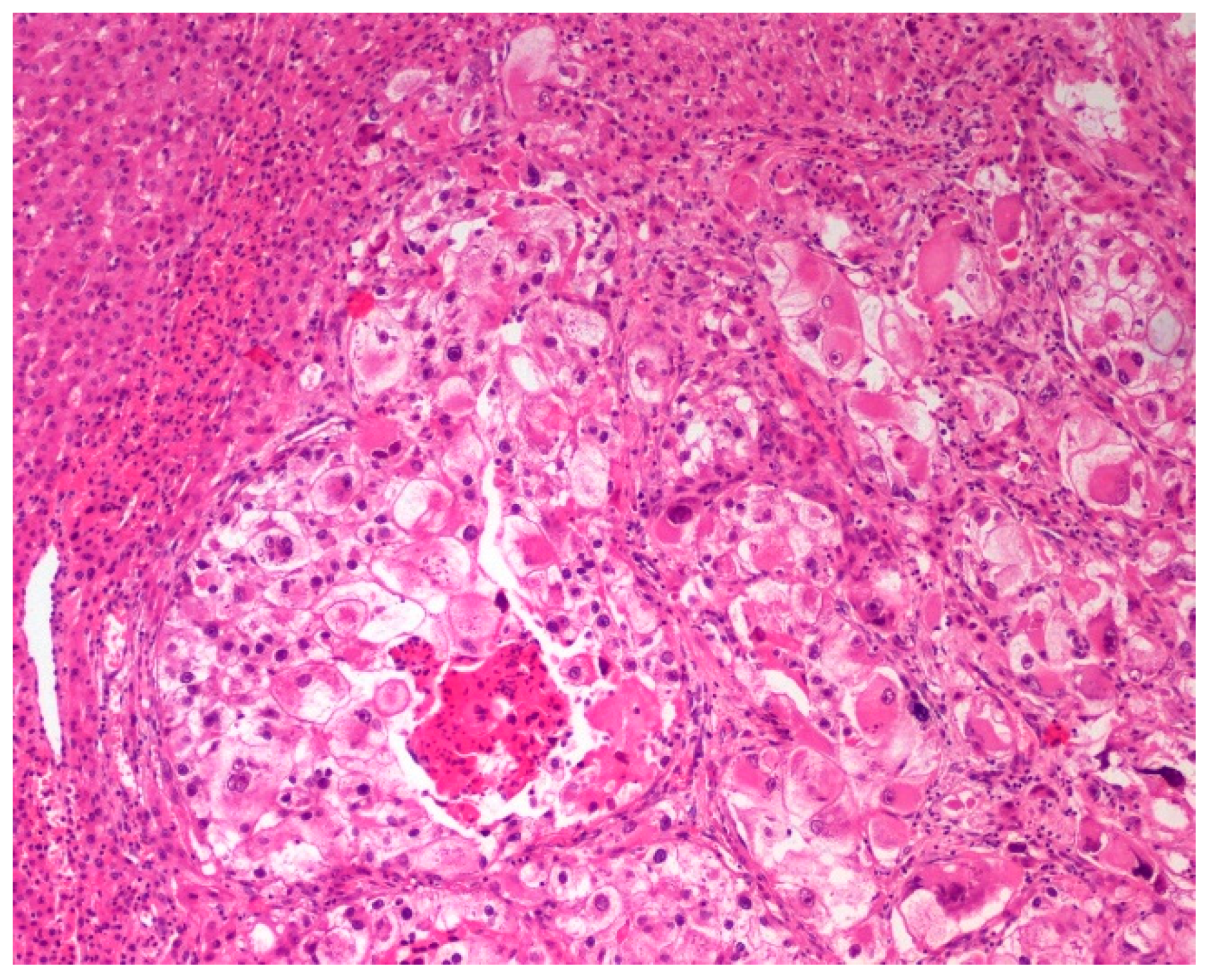
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonetti, F.; Pea, M.; Martignoni, G.; Zamboni, G. PEC and sugar. Am. J. Surg. Pathol. 1992, 16, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Chung, M.G.; Jung, D.H.; Oh, J.H. Perivascular epithelioid cell tumor (PEComa) in the transverse colon of an adolescent: A case report. Tumori 2007, 93, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. World Health Organization Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013; pp. 230–231. [Google Scholar]
- Doyle, L.A.; Argani, P.; Hornick, J.L. PEComa. In World Health Organization Classification of Tumours of Soft Tissue and Bone, 5th ed.; IARC Press: Lyon, France, 2020; pp. 312–314. [Google Scholar]
- Martignoni, G.; Pea, M.; Reghellin, D.; Zamboni, G.; Bonetti, F. PEComas: The past, the present and the future. Virchows Arch. 2008, 452, 119–132. [Google Scholar] [CrossRef]
- Tan, Y.; Xiao, E.H. Hepatic perivascular epithelioid cell tumor (PEComa): Dynamic CT, MRI, ultrasonography, and pathologic features—Analysis of 7 cases and review of the literature. Abdom. Imaging 2012, 37, 781. [Google Scholar] [CrossRef] [PubMed]
- Khaja, F.; Carilli, A.; Baidas, S.; Sriharan, A.; Norford, S. PEComa a perivascular epithelioid cell tumor in the liver—A case report and review of the literature. Case Rep. Med. 2013, 2013, 904126. [Google Scholar] [CrossRef]
- Fang, S.H.; Zhou, L.N.; Jin, M.; Hu, J.B. Perivascular epithelioid cell tumor of the liver: A report of two cases and review of the literature. World J. Gastroenterol. 2007, 13, 5537–5539. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Zhuang, Y.; Peng, J.; Huang, F.; Zhang, S. Hepatic perivascular epithelioid cell neoplasm: A clinical and pathological experience in diagnosis and treatment. Mol. Clin. Oncol. 2017, 6, 487–493. [Google Scholar] [CrossRef]
- Hekimoglu, K.; Haberal, M. Liver Perivascular Epithelioid Cell Tumor with an Unusual Location: Diagnostic Characteristics with Multidetector Computed Tomography and Magnetic Resonance Imaging. J. Clin. Imaging Sci. 2017, 7, 36. [Google Scholar] [CrossRef]
- Son, H.-J.; Kang, D.W.; Kim, J.H.; Han, H.Y.; Lee, M.K. Hepatic perivascular epithelioid cell tumor (PEComa): A case report with a review of literatures. Clin. Mol. Hepatol. 2017, 23, 80–86. [Google Scholar] [CrossRef]
- Lan, Y.Z.; Hua, X.E. Hepatic Multiple Perivascular Epithelioid Cell Neoplasm. Mol. Clin. Oncol. 2016, 4, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.; Katz, S.C.; Libbey, N.P.; Somasundar, P.S. Hepatic PEComa: A potential pitfall in the evaluation of hepatic neoplasms. BMJ. Case Rep. 2014, 2014, bcr2014204122. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, P.; Gao, H.; Zhai, W. Hepatic perivascular epithelioid cell tumor (PEComa): Analyses of 13 cases and review of the literature. Int. J. Clin. Exp. Pathol. 2018, 11, 2759–2767. [Google Scholar]
- Selvaggi, F.; Risio, D.; Claudi, R.; Cianci, R.; Angelucci, D.; Pulcini, D.; D’Aulerio, A.; Legnini, M.; Cotellese, R.; Innocenti, P. Malignant PEComa: A case report with emphasis on clinical and morphological criteria. BioMed Cent. Surg. 2011, 11, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Akitake, R.; Kimura, H.; Sekoguchi, S.; Nakamura, H.; Seno, H.; Chiba, T.; Fujimoto, S. Perivascular epithelioid cell tumor (PEComa) of the liver diagnosed by contrast-enhanced ultrasonography. Intern. Med. 2009, 48, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Abhirup, B.; Kaushal, K.; Sanket, M.; Ganesh, N. Malignant hepatic perivascular epithelioid cell tumor (PEComa)—Case report and a brief review. J. Egypt. Natl. Cancer Inst. 2015, 27, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Braga, A.C.; Pinto, A.; Van de Vijver, K.; Cornejo, K.; Pesci, A.; Zhang, L.; Morales-Oyarvide, V.; Kiyokawa, T.; Zannoni, G.F.; et al. Uterine PEComas: A Morphologic, Immunohistochemical, and Molecular Analysis of 32 Tumors. Am. J. Surg. Pathol. 2018, 42, 1370–1383. [Google Scholar] [CrossRef]
- Lin, K.-H.; Chang, N.-J.; Liou, L.-R.; Su, M.-S.; Tsao, M.-J.; Huang, M.-L. Successful management of perivascular epithelioid cell tumor of the rectum with recurrent liver metastases. A case report. Medicine 2018, 97, 31–39. [Google Scholar]
- Jiao, S.; Li, G.; Zhang, D.; Xu, Y.; Liu, J.; Li, G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int. J. Surg. 2020, 80, 243–255. [Google Scholar] [CrossRef]
- Ju, M.; Yopp, A.C. The Utility of Anatomical Liver Resection in Hepatocellular Carcinoma: Associated with Improved Outcomes or Lack of Supportive Evidence? Cancers 2019, 11, 1441. [Google Scholar] [CrossRef]
- Feng, X.; Su, Y.; Zheng, S.; Xia, F.; Ma, K.; Yan, J.; Li, X.; Tang, W.; Wang, S.; Bie, P.; et al. A double blinded prospective randomized trial comparing the effect of anatomic versus non-anatomic resection on hepatocellular carcinoma recurrence. HPB 2017, 19, 667–674. [Google Scholar] [CrossRef]
- Cucchetti, A.; Cescon, M.; Ercolani, G.; Bigonzi, E.; Torzilli, G.; Pinna, A.D. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann. Surg. Oncol. 2012, 19, 3697–3705. [Google Scholar] [CrossRef]
- McDonald, J.D.; Gupta, S.; Shindorf, M.L.; Gamble, L.A.; Ruff, S.M.; Drake, J.; Heller, T.; Wan, J.Y.; Dickson, P.V.; Glazer, E.S.; et al. Elevated Serum α-Fetoprotein is Associated with Abbreviated Survival for Patients with Fibrolamellar Hepatocellular Carcinoma Who Undergo a Curative Resection. Ann. Surg. Oncol. 2020, 27, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Oliva, E. Perivascular epithelioid cell tumors (PEComa) of the gynecologic tract. Genes Chromosomes Cancer. 2021, 60, 168–179. [Google Scholar] [CrossRef]
- Agaram, N.P.; Sung, Y.S.; Zhang, L.; Chen, C.L.; Chen, H.W.; Singer, S.; Dickson, M.A.; Berger, M.F.; Antonescu, C.R. Dichotomy of Genetic Abnormalities in PEComas with Therapeutic Implications. Am. J. Surg. Pathol. 2015, 39, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Delcambre, C.; Hostein, I.; Cazeau, A.L.; Marty, M.; Avril, A.; Coindre, J.M.; Bui, B. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann. Oncol. 2010, 21, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G. Histology- and non-histology-driven therapy for treatment of soft tissue sarcomas. Ann. Oncol. 2012, 23, x167–x169. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zou, Y.; Lv, Y.; Wang, C. Hepatic perivascular epithelioid cell tumor treated by transarterial embolization plus radiofrequency ablation: A case report and literature review. Medicine 2017, 96, e6969. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, M.; Ziarkiewicz-Wróblewska, B.; Wróblewski, T.; Podgórska, J.; Grzybowski, J.; Gierej, B.; Krawczyk, P.; Nyckowski, P.; Kornasiewicz, O.; Patkowski, W.; et al. PEComa—A Rare Liver Tumor. J. Clin. Med. 2021, 10, 1756. https://doi.org/10.3390/jcm10081756
Krawczyk M, Ziarkiewicz-Wróblewska B, Wróblewski T, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, Nyckowski P, Kornasiewicz O, Patkowski W, et al. PEComa—A Rare Liver Tumor. Journal of Clinical Medicine. 2021; 10(8):1756. https://doi.org/10.3390/jcm10081756
Chicago/Turabian StyleKrawczyk, Marek, Bogna Ziarkiewicz-Wróblewska, Tadeusz Wróblewski, Joanna Podgórska, Jakub Grzybowski, Beata Gierej, Piotr Krawczyk, Paweł Nyckowski, Oskar Kornasiewicz, Waldemar Patkowski, and et al. 2021. "PEComa—A Rare Liver Tumor" Journal of Clinical Medicine 10, no. 8: 1756. https://doi.org/10.3390/jcm10081756
APA StyleKrawczyk, M., Ziarkiewicz-Wróblewska, B., Wróblewski, T., Podgórska, J., Grzybowski, J., Gierej, B., Krawczyk, P., Nyckowski, P., Kornasiewicz, O., Patkowski, W., Remiszewski, P., Zając, K., & Grąt, M. (2021). PEComa—A Rare Liver Tumor. Journal of Clinical Medicine, 10(8), 1756. https://doi.org/10.3390/jcm10081756