Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Extracted Data
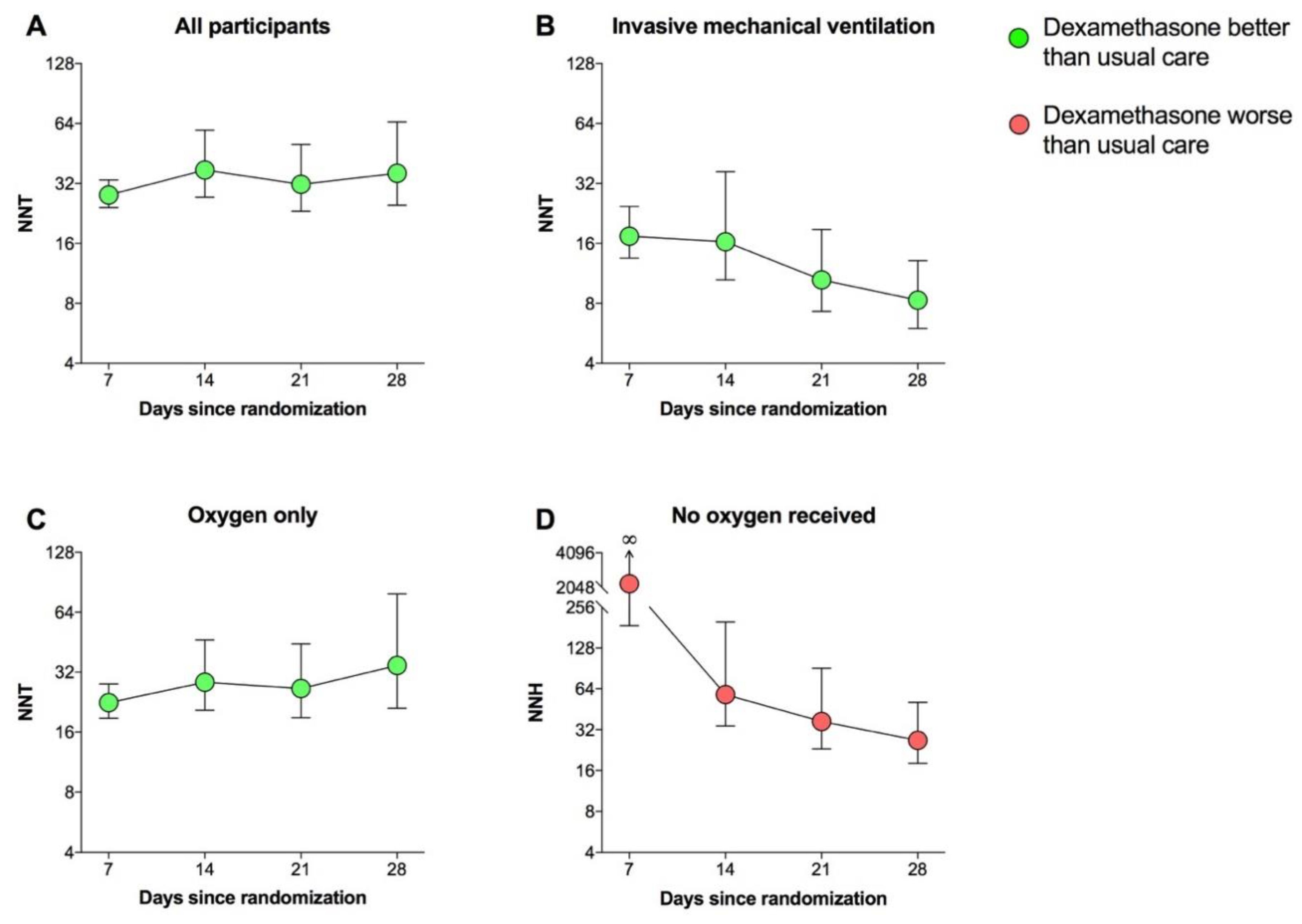
3.2. NNT
3.3. NNH
3.4. Subset Analyses
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, C.; Chotirmall, S.H.; Rello, J.; Alba, G.A.; Ginns, L.C.; Krishnan, J.A.; Rogers, R.; Bendstrup, E.; Burgel, P.-R.; Chalmers, J.D. Updated guidance on the management of COVID-19: From an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur. Respir. Rev. 2020, 29, 200287. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Cazzola, M.; Matera, M.G.; Rogliani, P. Adding a LAMA to ICS/LABA therapy: A meta-analysis of triple combination therapy in COPD. Chest 2019, 155, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M. Application of number needed to treat (NNT) as a measure of treatment effect in respiratory medicine. Treat. Respir. Med. 2006, 5, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Fergusson, D.A. Exaggeration of treatment benefits using the “event-based” number needed to treat. Can. Med. Assoc. J. 2008, 179, 669–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suissa, S. Number needed to treat in COPD: Exacerbations versus pneumonias. Thorax 2013, 68, 540–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, D.G.; Andersen, P.K. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999, 319, 1492–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, D.G. Confidence intervals for the number needed to treat. BMJ 1998, 317, 1309–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Citrome, L. Relative vs. absolute measures of benefit and risk: What’s the difference? Acta Psychiatr. Scand. 2010, 121, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. The tyranny of the P-value: Effect size matters. Klin. Psikofarmakol. Bul. 2011, 21, 91–92. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology 2020, 161, bqaa127. [Google Scholar] [CrossRef] [PubMed]
- Kalidhindi, R.S.R.; Borkar, N.A.; Ambhore, N.S.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Sex steroids skew ACE2 expression in human airway: A contributing factor to sex differences in COVID-19? Am. J. Physiol. Lung Cell. Mol. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Rice, T.W. Corticosteroids in COVID-19 ARDS: Evidence and hope during the pandemic. JAMA 2020, 324, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
| Days since Randomization | Treatment | All Participants | Invasive Mechanical Ventilation | Oxygen Only | No Oxygen Received | ||||
|---|---|---|---|---|---|---|---|---|---|
| Alive | Dead | Alive | Dead | Alive | Dead | Alive | Dead | ||
| 7 | Dexamethasone | 1903 | 201 | 290 | 34 | 1135 | 144 | 478 | 23 |
| Usual care | 3754 | 567 | 572 | 111 | 2195 | 409 | 987 | 47 | |
| 14 | Dexamethasone | 1725 | 379 | 248 | 76 | 1036 | 243 | 441 | 60 |
| Usual care | 3427 | 894 | 481 | 202 | 2018 | 586 | 928 | 106 | |
| 21 | Dexamethasone | 1659 | 445 | 232 | 92 | 1006 | 273 | 421 | 80 |
| Usual care | 3271 | 1050 | 424 | 259 | 1950 | 654 | 897 | 137 | |
| 28 | Dexamethasone | 1622 | 482 | 229 | 95 | 981 | 298 | 412 | 89 |
| Usual care | 3211 | 1110 | 400 | 283 | 1922 | 682 | 889 | 145 | |
| Days Since Randomization | NNT | NNH | ||
|---|---|---|---|---|
| All Participants | Invasive Mechanical Ventilation | Oxygen Only | No Oxygen Received | |
| 7 | 28.0 (24.2–33.3) * | 17.4 (24.5–13.5) * | 22.5 (18.8–27.9) * | 2204.4 (187.4–∞) |
| 14 | 37.4 (27.3–59.2) * | 16.3 (10.5–36.6) * | 28.5 (20.6–46.5) * | 58.0 (34,198.5) * |
| 21 | 31.7 (23.2–50.2) * | 10.5 (7.3–18.8) * | 26.5 (18.9–44.4) * | 36.8 (23.1–90.6) * |
| 28 | 36.0 (24.9–65.1) * | 8.3 (6.0–13.1) * | 34.6 (21.1–79.0) * | 26.7 (18.1–50.9) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Aiello, M.; Frizzelli, A.; Rogliani, P.; Chetta, A. Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom. J. Clin. Med. 2021, 10, 1607. https://doi.org/10.3390/jcm10081607
Calzetta L, Aiello M, Frizzelli A, Rogliani P, Chetta A. Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom. Journal of Clinical Medicine. 2021; 10(8):1607. https://doi.org/10.3390/jcm10081607
Chicago/Turabian StyleCalzetta, Luigino, Marina Aiello, Annalisa Frizzelli, Paola Rogliani, and Alfredo Chetta. 2021. "Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom" Journal of Clinical Medicine 10, no. 8: 1607. https://doi.org/10.3390/jcm10081607
APA StyleCalzetta, L., Aiello, M., Frizzelli, A., Rogliani, P., & Chetta, A. (2021). Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom. Journal of Clinical Medicine, 10(8), 1607. https://doi.org/10.3390/jcm10081607








