Clinical Performance of a Novel Two-Piece Abutment Concept: Results from a Prospective Study with a 1-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design, Setting, and Participants
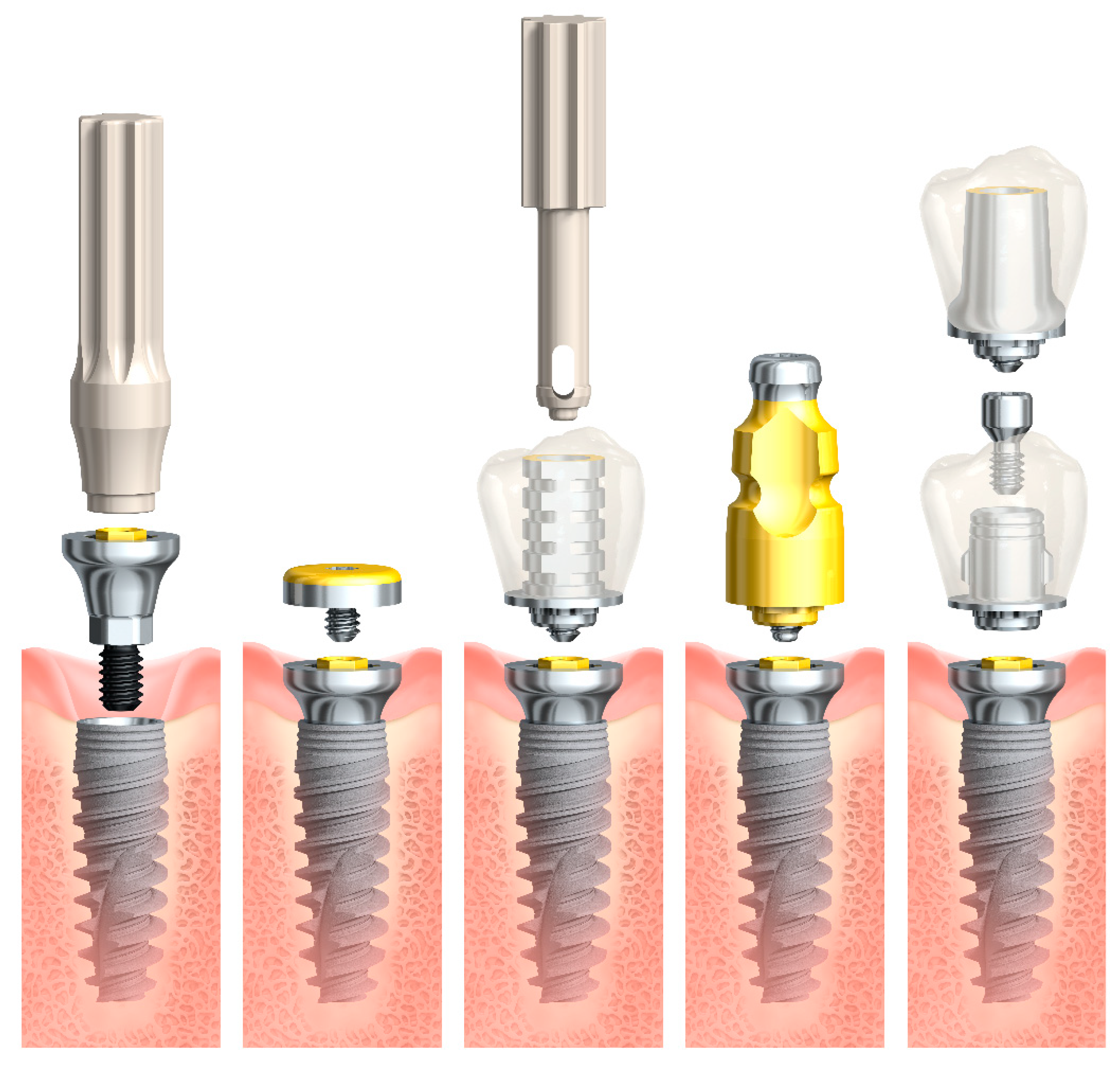
2.3. Surgical and Prosthetic Protocol
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patient Enrollment and Follow-Up
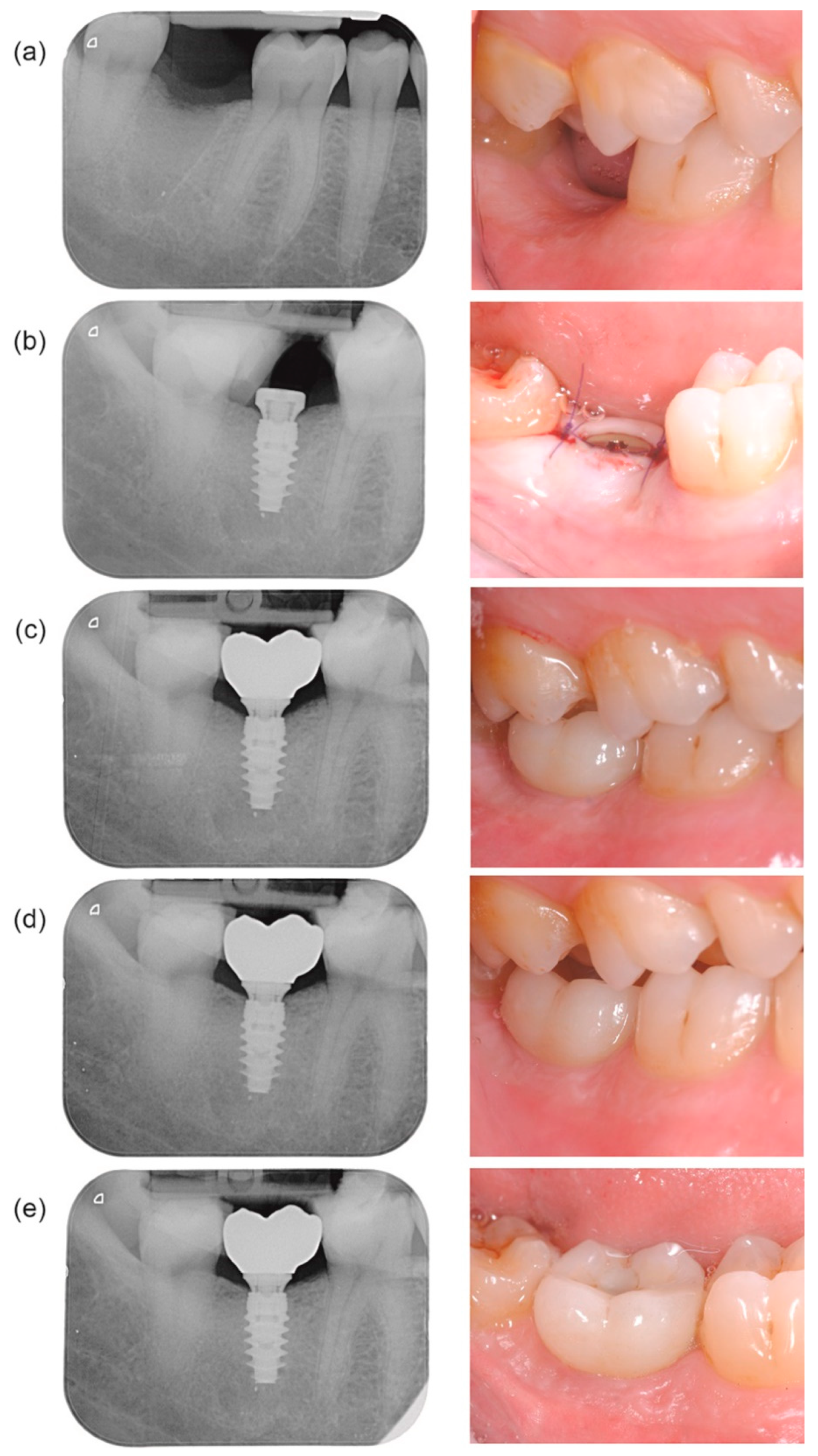
3.2. Digital Impression and Prosthetic Delivery
3.3. Outcome Measures
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; Fabianelli, A.; Gloria, A.; Martorelli, M.; Lanzotti, A.; Watts, D.C. Mechanical behavior of bulk direct composite versus block composite and lithium disilicate indirect Class II restorations by CAD-FEM modeling. Dent. Mater. 2017, 33, 690–701. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; Garcia-Godoy, F.; Gloria, A.; Lanzotti, A.; Maietta, S.; Martorelli, M. The effects of cavity-margin-angles and bolus stiffness on the mechanical behavior of indirect resin composite class II restorations. Dent. Mater. 2017, 33, e39–e47. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Maietta, S.; Martorelli, M.; Lanzotti, A.; Watts, D.C.; Ausiello, P. FE analysis of conceptual hybrid composite endodontic post designs in anterior teeth. Dent. Mater. 2018, 34, 1063–1071. [Google Scholar] [CrossRef]
- Souza, A.B.; Alshihri, A.; Kammerer, P.W.; Araujo, M.G.; Gallucci, G.O. Histological and micro-CT analysis of peri-implant soft and hard tissue healing on implants with different healing abutments configurations. Clin. Oral Implants Res. 2018, 29, 1007–1015. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is Marginal Bone Loss around Oral Implants the Result of a Provoked Foreign Body Reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.M.; Babay, N.; Alzoman, H.; Basudan, S.; Anil, S.; Jansen, J.A. Bone morphology changes around two types of bone-level implants installed in fresh extraction sockets—A histomorphometric study in Beagle dogs. Clin. Oral Implants Res. 2015, 26, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Fernández-Jiménez, A.; O’Valle, F.; Monje, A.; Silvestre, F.J.; Juodzbalys, G.; Sánchez-Fernández, E.; Catena, A. Influence of the crown-implant connection on the preservation of peri-implant bone: A retrospective multifactorial analysis. Int. J. Oral Maxillofac. Implants 2015, 30, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for Optimizing the Soft Tissue Seal around Osseointegrated Implants. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Canullo, L.; Fedele, G.R.; Iannello, G.; Jepsen, S. Platform switching and marginal bone-level alterations: The results of a randomized-controlled trial. Clin. Oral Implants Res. 2010, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hermann, F.; Lerner, H.; Palti, A. Factors influencing the preservation of the periimplant marginal bone. Implant. Dent. 2007, 16, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin. Implant Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.W.; Seo, J.M. A technique for minimizing subgingival residual cement by using rubber dam for cement-retained implant crowns. J. Prosthet. Dent. 2017, 117, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez Del Amo, F.; Lin, G.H.; Monje, A.; Galindo-Moreno, P.; Wang, H.L. Influence of Soft Tissue Thickness Upon Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 690–699. [Google Scholar] [CrossRef]
- Tallarico, M.; Caneva, M.; Meloni, S.M.; Xhanari, E.; Covani, U.; Canullo, L. Definitive Abutments Placed at Implant Insertion and Never Removed: Is It an Effective Approach? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Oral Maxillofac. Surg. 2018, 76, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, S.; Matthys, C.; Nassar, R.; Christiaens, V.; Cosyn, J.; De Bruyn, H. Adapting the vertical position of implants with a conical connection in relation to soft tissue thickness prevents early implant surface exposure: A 2-year prospective intra-subject comparison. J. Clin. Periodontol. 2018, 45, 605–612. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Lindhe, J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J. Clin. Periodontol. 1997, 24, 568–572. [Google Scholar] [CrossRef]
- Koutouzis, T.; Gholami, F.; Reynolds, J.; Lundgren, T.; Kotsakis, G.A. Abutment Disconnection/Reconnection Affects Peri-implant Marginal Bone Levels: A Meta-Analysis. Int. J. Oral Maxillofac. Implants 2017, 32, 575–581. [Google Scholar] [CrossRef]
- Becker, K.; Mihatovic, I.; Golubovic, V.; Schwarz, F. Impact of abutment material and dis-/re-connection on soft and hard tissue changes at implants with platform-switching. J. Clin. Periodontol. 2012, 39, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Iglhaut, G.; Becker, K.; Golubovic, V.; Schliephake, H.; Mihatovic, I. The impact of dis-/reconnection of laser microgrooved and machined implant abutments on soft- and hard-tissue healing. Clin. Oral Implants Res. 2013, 24, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Praça, L.F.G.; Teixeira, R.C.; Rego, R.O. Influence of abutment disconnection on peri-implant marginal bone loss: A randomized clinical trial. Clin. Oral Implants Res. 2020, 31, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Bignozzi, I.; Cocchetto, R.; Cristalli, M.P.; Iannello, G. Immediate positioning of a definitive abutment versus repeated abutment replacements in post-extractive implants: 3-year follow-up of a randomised multicentre clinical trial. Eur. J. Oral Implantol. 2010, 3, 285–296. [Google Scholar]
- Degidi, M.; Nardi, D.; Daprile, G.; Piattelli, A. Nonremoval of Immediate Abutments in Cases Involving Subcrestally Placed Postextractive Tapered Single Implants: A Randomized Controlled Clinical Study. Clin. Implant Dent. Relat. Res. 2014, 16, 794–805. [Google Scholar] [CrossRef]
- Grandi, T.; Guazzi, P.; Samarani, R.; Garuti, G. Immediate positioning of definitive abutments versus repeated abutment replacements in immediately loaded implants: Effects on bone healing at the 1-year follow-up of a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2012, 5, 9–16. [Google Scholar]
- Koutouzis, T.; Koutouzis, G.; Gadalla, H.; Neiva, R. The Effect of Healing Abutment Reconnection and Disconnection on Soft and Hard Peri-implant Tissues: A Short-Term Randomized Controlled Clinical Trial. Int. J. Oral Maxillofac. Implants 2013, 28, 807–814. [Google Scholar] [CrossRef]
- Siebert, C.; Rieder, D.; Eggert, J.; Wichmann, M.G.; Heckmann, S.M. Long-Term Esthetic Outcome of Tissue-Level and Bone-Level Implants in the Anterior Maxilla. Int. J. Oral Maxillofac. Implants 2018, 33, 905–912. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Gonzalez-Martin, O.; Veltri, M.; Moraguez, O.; Belser, U.C. Quantitative three-dimensional methodology to assess volumetric and profilometric outcome of subepithelial connective tissue grafting at pontic sites: A prospective pilot study. Int. J. Periodontics Restor. Dent. 2014, 34, 673–679. [Google Scholar] [CrossRef] [PubMed]
- van Steenberghe, D. Outcomes and their measurement in clinical trials of endosseous oral implants. Ann. Periodontol. 1997, 2, 291–298. [Google Scholar] [CrossRef]
- Belser, U.; Buser, D.; Higginbottom, F. Consensus statements and recommended clinical procedures regarding esthetics in implant dentistry. Int. J. Oral Maxillofac. Implants 2004, 19, 73–74. [Google Scholar]
- Brennan, D.S.; Spencer, A.J. Dimensions of oral health related quality of life measured by EQ-5D+ and OHIP-14. Health Qual. Life Outcomes 2004, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.; Blomqvist, F.; Wennerberg, A.; Johansson, A. A retrospective analysis of early and delayed loading of full-arch mandibular prostheses using three different implant systems: Clinical results with up to 5 years of loading. Clin. Implant Dent. Relat. Res. 2009, 11, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Martinez-de Fuentes, R.; Goldstein, M.; Arnhart, C.; Barlattani, A.; Jackowski, J.; Knauf, M.; Lorenzoni, M.; Maiorana, C.; Mericske-Stern, R.; et al. Randomized controlled trial comparing a variable-thread novel tapered and a standard tapered implant: Interim one-year results. J. Prosthet. Dent. 2009, 101, 293–305. [Google Scholar] [CrossRef]
- Lekholm, U.; Grondahl, K.; Jemt, T. Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin. Implant Dent. Relat. Res. 2006, 8, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, I. Clinical and radiological results of patients treated with two loading protocols for mandibular overdentures on Branemark implants. J. Clin. Periodontol. 2006, 33, 233–238. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Avci, M.; Kuran, S.; Ozbek, E.N. A 4-year prospective clinical and radiological study of maxillary dental implants supporting single-tooth crowns using early and delayed loading protocols. Clin. Implant Dent. Relat. Res. 2007, 9, 222–227. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Sennerby, L.; Tumer, C.; Yenigul, M.; Avci, M. Stability and marginal bone level measurements of unsplinted implants used for mandibular overdentures: A 1-year randomized prospective clinical study comparing early and conventional loading protocols. Clin. Oral Implants Res. 2006, 17, 501–505. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Tozum, T.F.; Tumer, C.; Ozbek, E.N. A 2-year clinical report of patients treated with two loading protocols for mandibular overdentures: Early versus conventional loading. J. Periodontol. 2006, 77, 1998–2004. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Tumer, C. Early versus late loading of unsplinted TiUnite surface implants supporting mandibular overdentures: A 2-year report from a prospective study. J. Oral Rehabil. 2007, 34, 773–780. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Tumer, C.; Avci, M.; Hersek, N.; Celik-Bagci, E. A short-term clinical trial on selected outcomes for immediately loaded implant-supported mandibular overdentures. Int. J. Prosthodont. 2006, 19, 515–519. [Google Scholar]
- Zembic, A.; Glauser, R.; Khraisat, A.; Hammerle, C.H. Immediate vs. early loading of dental implants: 3-year results of a randomized controlled clinical trial. Clin. Oral Implants Res. 2010, 21, 481–489. [Google Scholar] [CrossRef]
- Babbush, C.A.; Kanawati, A.; Kotsakis, G.A. Marginal Bone Stability Around Tapered, Platform-Shifted Implants Placed with an Immediately Loaded Four-Implant-Supported Fixed Prosthetic Concept: A Cohort Study. Int. J. Oral Maxillofac. Implants 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Sanz-Sanchez, I.; Sanz-Martin, I.; Figuero, E.; Sanz, M. Clinical efficacy of immediate implant loading protocols compared to conventional loading depending on the type of the restoration: A systematic review. Clin. Oral Implants Res. 2015, 26, 964–982. [Google Scholar] [CrossRef]
- Lambrechts, T.; Doornewaard, R.; De Bruyckere, T.; Matthijs, L.; Deschepper, E.; Cosyn, J. A multicenter cohort study on the association of the one-abutment one-time concept with marginal bone loss around bone level implants. Clin. Oral Implants Res. 2020. [Google Scholar] [CrossRef]
- Atieh, M.A.; Tawse-Smith, A.; Alsabeeha, N.H.M.; Ma, S.; Duncan, W.J. The One Abutment-One Time Protocol: A Systematic Review and Meta-Analysis. J. Periodontol. 2017, 88, 1173–1185. [Google Scholar] [CrossRef]
- Linkevicius, T.; Linkevicius, R.; Alkimavicius, J.; Linkeviciene, L.; Andrijauskas, P.; Puisys, A. Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular-shaped implants: A prospective clinical trial. Clin. Oral Implants Res. 2018, 29, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Pollaris, L.; Van der Linden, F.; De Bruyn, H. Minimally Invasive Single Implant Treatment (M.I.S.I.T.) based on ridge preservation and contour augmentation in patients with a high aesthetic risk profile: One-year results. J. Clin. Periodontol. 2015, 42, 398–405. [Google Scholar] [CrossRef] [PubMed]
- De Bruyckere, T.; Eeckhout, C.; Eghbali, A.; Younes, F.; Vandekerckhove, P.; Cleymaet, R.; Cosyn, J. A randomized controlled study comparing guided bone regeneration with connective tissue graft to re-establish convexity at the buccal aspect of single implants: A one-year CBCT analysis. J. Clin. Periodontol. 2018, 45, 1375–1387. [Google Scholar] [CrossRef]
- Esposito, M.; Zucchelli, G.; Cannizzaro, G.; Checchi, L.; Barausse, C.; Trullenque-Eriksson, A.; Felice, P. Immediate, immediate-delayed (6 weeks) and delayed (4 months) post-extractive single implants: 1-year post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 11–26. [Google Scholar]
- Gultekin, B.A.; Gultekin, P.; Leblebicioglu, B.; Basegmez, C.; Yalcin, S. Clinical evaluation of marginal bone loss and stability in two types of submerged dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Agliardi, E.; Tallarico, M.; Barlattani, A. Clinical and Radiological Outcomes of Two Implants with Different Prosthetic Interfaces and Neck Configurations: Randomized, Controlled, Split-Mouth Clinical Trial. Clin. Implant Dent. Relat. Res. 2014, 16, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Slagter, K.W.; Meijer, H.J.; Bakker, N.A.; Vissink, A.; Raghoebar, G.M. Immediate Single-Tooth Implant Placement in Bony Defects in the Esthetic Zone: A 1-Year Randomized Controlled Trial. J. Periodontol. 2016, 87, 619–629. [Google Scholar] [CrossRef]
- Wessing, B.; Emmerich, M.; Bozkurt, A. Horizontal Ridge Augmentation with a Novel Resorbable Collagen Membrane: A Retrospective Analysis of 36 Consecutive Patients. Int. J. Periodontics Restor. Dent. 2016, 36, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kroll, P.; Hou, L.; Radaideh, H.; Sharifi, N.; Han, P.P.; Mulligan, R.; Enciso, R. Oral Health-Related Outcomes in Edentulous Patients Treated With Mandibular Implant-Retained Dentures Versus Complete Dentures: Systematic Review With Meta-Analyses. J. Oral Implantol. 2018, 44, 313–324. [Google Scholar] [CrossRef] [PubMed]



| Patient Characteristics | n (%) | |
| Gender | Female | 31 (43%) |
| Male | 41 (57%) | |
| Age (mean ± SD, range) (years) | 53.2 ± 12.7 (28–76) | |
| Smoking status | Non-smoking | 64 (88.9%) |
| Smoking, ≤5 per day | 3 (4.2%) | |
| Smoking, 6–10 per day | 5 (7.0%) | |
| History of periodontitis | Yes | 12 (16.7%) |
| No | 60 (83.3%) | |
| Implants per patient | 1 | 49 (68.1%) |
| 2 | 17 (23.6%) | |
| 3 | 3 (4.2%) | |
| 4 | 1 (1.4%) | |
| 5 | 2 (2.8%) | |
| Implant Characteristics | n (%) | |
| Implant diameter (mm) | 3.5 | 19 (17.9%) |
| 4.3 | 58 (54.7%) | |
| 5.0 | 20 (18.9%) | |
| 5.5 | 9 (8.5%) | |
| Implant length (mm) | 8.5 | 15 (14.2%) |
| 10.0 | 55 (51.9%) | |
| 11.5 | 32 (30.2%) | |
| 13.0 | 4 (3.8%) | |
| Implant Site/Surgical Characteristics | n (%) | |
| Location | Maxilla | 37 (34.9%) |
| Premolars | 20 (18.9%) | |
| Molars | 17 (16.0%)) | |
| Mandible | 69 (65.1%) | |
| Premolars | 13 (12.3%) | |
| Molars | 56 (52.8%) | |
| Bone quality | Very dense bone | 10 (9.4%) |
| Dense bone | 40 (37.7%) | |
| Soft bone | 45 (42.5%) | |
| Very soft bone | 11 (10.4%) | |
| Bone quantity | A | 43 (40.6%) |
| B | 46 (43.4%) | |
| C | 17 (16.0%) | |
| D, E | 0 | |
| Bone mill use | Yes | 57 (53.8%) |
| No | 49 (46.2%) | |
| Soft tissue grafting | Yes 1 | 8 (7.5%) |
| Advanced platelet-rich fibrin | 3 (2.8%) | |
| Free gingival graft | 5 (4.7%) | |
| No | 98 (92.5%) | |
| Insertion torque (mean ± SD, range) (Ncm) | 49.6 ± 11.8 (35–70) | |
| Parameter | Implant Insertion | Prosthesis Placement | 3-Month Follow-Up | 6-Month Follow-Up | 12-Month Follow-Up |
|---|---|---|---|---|---|
| n | 106 | 98 | 95 | 94 | 95 |
| Keratinized mucosa status | |||||
| No keratinized mucosa around the implant | - | - | 1 | - | 1 |
| Mucosa surrounding the implant is partially keratinized | 7 | 3 | 5 | 3 | 2 |
| The entire mucosa surrounding the implant is keratinized | 99 | 95 | 89 | 91 | 92 |
| Keratinized mucosa height | |||||
| Mean ± SD (mm) | 3.5 ± 1.1 | 2.9 ± 1.2 | 3.1 ± 1.3 | 3.3 ± 1.4 | 3.2 ± 1.3 |
| Modified Plaque Index | |||||
| No detectable plaque | not assessed | not assessed | 66 | 56 | 44 |
| Plaque only recognized by running a probe across the marginal surface of the implant | 28 | 35 | 45 | ||
| Plaque can be seen with the naked eye | 1 | 3 | 6 | ||
| Abundance of soft matter | - | - | - | ||
| Modified Bleeding Index | |||||
| No bleeding when a periodontal probe is passed along the gingival margin adjacent to the implant | not assessed | not assessed | 68 | 71 | 76 |
| Isolated bleeding spots visible | 25 | 17 | 15 | ||
| Blood forms a confluent red line on the margin | - | 6 | 4 | ||
| Heavy or profuse bleeding | 2 | - | - | ||
| Gingival Index | |||||
| Normal gingiva surrounding crown/control tooth | not assessed | not assessed | 78 | 70 | 70 |
| Mild inflammation—slight color change, slight edema | 15 | 21 | 22 | ||
| Moderate inflammation—redness, edema, and glazing | 2 | 3 | 3 | ||
| Severe inflammation—marked redness and edema, tendency to spontaneous bleeding | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, G.; Staas, T.; Linkevicius, T.; Valantiejiene, V.; González-Martin, O.; Rompen, E. Clinical Performance of a Novel Two-Piece Abutment Concept: Results from a Prospective Study with a 1-Year Follow-Up. J. Clin. Med. 2021, 10, 1594. https://doi.org/10.3390/jcm10081594
Fabbri G, Staas T, Linkevicius T, Valantiejiene V, González-Martin O, Rompen E. Clinical Performance of a Novel Two-Piece Abutment Concept: Results from a Prospective Study with a 1-Year Follow-Up. Journal of Clinical Medicine. 2021; 10(8):1594. https://doi.org/10.3390/jcm10081594
Chicago/Turabian StyleFabbri, Giacomo, Tristan Staas, Tomas Linkevicius, Valda Valantiejiene, Oscar González-Martin, and Eric Rompen. 2021. "Clinical Performance of a Novel Two-Piece Abutment Concept: Results from a Prospective Study with a 1-Year Follow-Up" Journal of Clinical Medicine 10, no. 8: 1594. https://doi.org/10.3390/jcm10081594
APA StyleFabbri, G., Staas, T., Linkevicius, T., Valantiejiene, V., González-Martin, O., & Rompen, E. (2021). Clinical Performance of a Novel Two-Piece Abutment Concept: Results from a Prospective Study with a 1-Year Follow-Up. Journal of Clinical Medicine, 10(8), 1594. https://doi.org/10.3390/jcm10081594






