Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
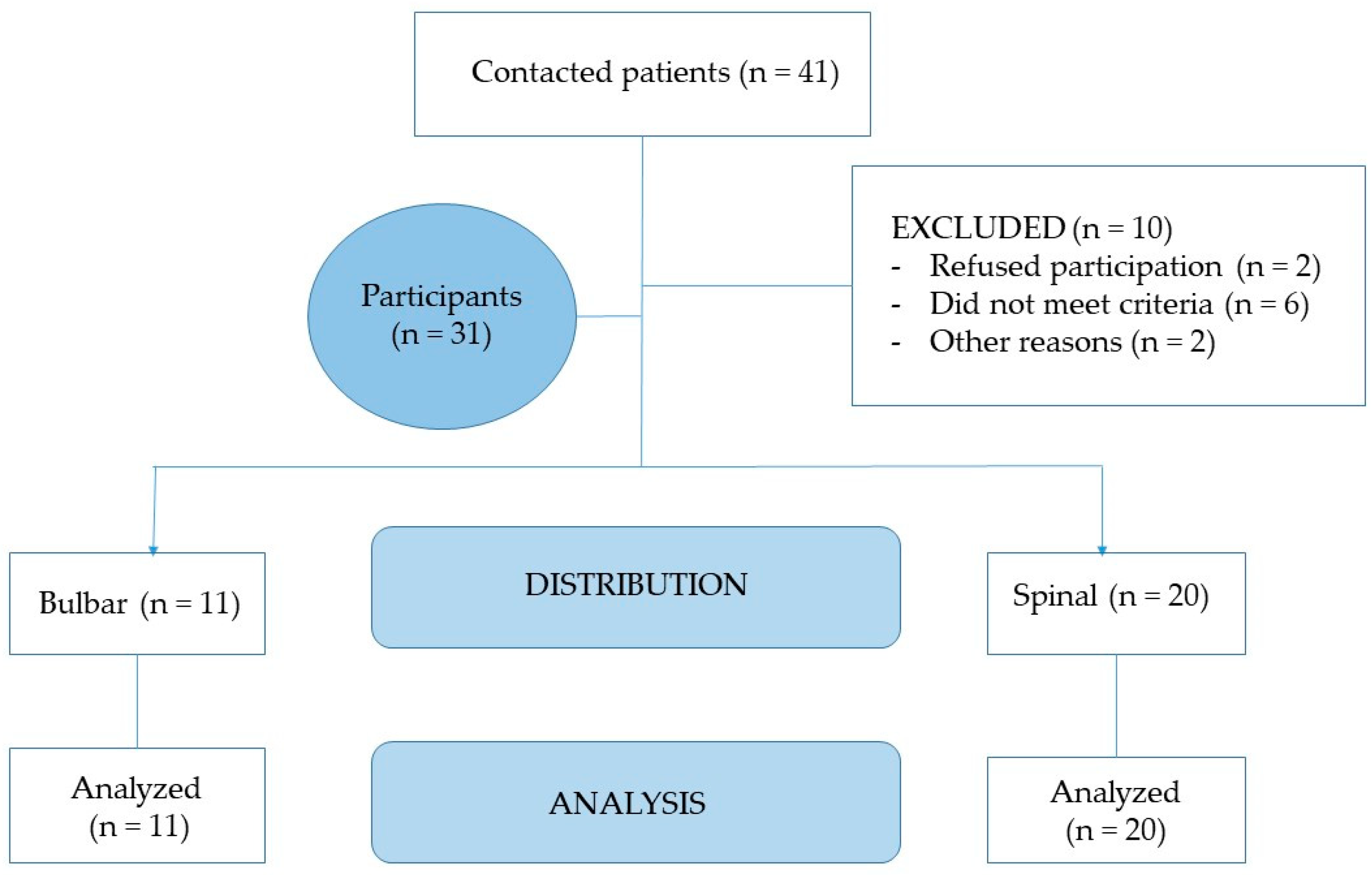
2.2. Study Population
2.3. Methods, Devices, and Procedures
2.4. Ethical Aspects
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marin, B.; Boumédiene, F.; Logroscino, G.; Couratier, P.; Babron, M.C.; Leutenegger, A.L.; Copetti, M.; Preux, P.M.; Beghi, E. Variation in world wide incidence of amyotrophic lateral sclerosis: A meta-analysis. Int. J. Epidemiol. 2017, 46, 57–74. [Google Scholar]
- Gordon, P.H. Amyotrophic Lateral Sclerosis: An Update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Int. Soc. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.; Gonzalo, I.; Ruiz-Soto, M.; Berciano, J. Why Do Motor Neurons Degenerate? Actualization in the Pathogenesis of Amyotrophic Lateral Sclerosis. Span. Soc. Neurol. 2019, 34, 27–37. [Google Scholar]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Ari, C.; Poff, A.M.; Held, H.E.; Landon, C.S.; Goldhagen, C.R.; Mavromates, N.; D’Agostino, D.P. Metabolic Therapy with Deanna Protocol Supplementation Delays Disease Progression and Extends Survival in Amyotrophic Lateral Sclerosis (ALS) Mouse Model. PLoS ONE 2014, 9, e103526. [Google Scholar] [CrossRef]
- Hamasaki, H.; Takeuchi, Y.; Masui, Y.; Ohta, Y.; Abe, K.; Yoshino, H.; Yanai, H. Development of diabetes in a familial amyotrophic lateral sclerosis patient carrying the I113T SOD1 mutation. Case Report—PubMed. Neuroendocrinol. Lett. 2015, 5, 414–416. [Google Scholar]
- Sun, Y.; Lu, C.J.; Chen, R.C.; Hou, W.H.; Li, C.Y. Risk of amyotrophic lateral sclerosis in patients with diabetes: A nationwide population-based cohort study. J. Epidemiol. 2015, 25, 445–451. [Google Scholar] [CrossRef]
- Wannarong, T.; Ungprasert, P. Diabetes mellitus is associated with a lower risk of amyotrophic lateral sclerosis: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2020, 199, 106248. [Google Scholar] [CrossRef]
- Tefera, T.W.; Borges, K. Neuronal glucose metabolism is impaired while astrocytic TCA cycling is unaffected at symptomatic stages in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. J. Cereb. Blood Flow Metab. 2019, 39, 1710–1724. [Google Scholar] [CrossRef]
- Cistaro, A.; Valentini, M.C.; Chiò, A.; Nobili, F.; Calvo, A.; Moglia, C.; Montuschi, A.; Morbelli, S.; Salmaso, D.; Fania, P.; et al. Brain hypermetabolism in amyotrophic lateral sclerosis: A FDG PET study in ALS of spinal and bulbar onset. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 251–259. [Google Scholar] [CrossRef]
- Pagani, M.; Chiò, A.; Valentini, M.C.; Öberg, J.; Nobili, F.; Calvo, A.; Moglia, C.; Bertuzzo, D.; Morbelli, S.; De Carli, F.; et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 2014, 83, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Q.; Chen, Y.; Cao, B.; Ou, R.W.; Zhang, L.; Hou, Y.; Gao, X.; Shang, H. Blood Hemoglobin A1c Levels and Amyotrophic Lateral Sclerosis Survival. Mol. Neurodegener. 2017. [Google Scholar] [CrossRef]
- Mariosa, D.; Hammar, N.; Malmström, H.; Ingre, C.; Jungner, I.; Ye, W.; Fang, F.; Walldius, G. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann. Neurol. 2017, 81, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Pradat, P.F.; Bruneteau, G.; Gordon, P.H.; Dupuis, L.; Bonnefont-Rousselot, D.; Simon, D. Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 166–171. [Google Scholar] [CrossRef]
- Sardiwal, S.; Magnusson, P.; Goldsmith, D.J.; Lamb, E.J. Bone alkaline phosphatase in CKD-mineral Bone Disorder. W.B. Saunders 2013. [Google Scholar] [CrossRef]
- Ramaiah, S.K. A toxicologist Guide to the Diagnostic Interpretation of Hepatic Biochemical Parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef]
- Bouhajja, H.; Abdelhedi, R.; Amouri, A.; Kacem, F.H.; Marrakchi, R.; Safi, W.; Mrabet, H.; Chtourou, L.; Charfi, N.; Fourati, M.; et al. Potential role of liver enzyme levels as predictive markers of glucose metabolism disorders in a Tunisian population. Can. J. Physiol. Pharmacol. 2018, 96, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-C.; Tsai, S.P.; Jhao, J.-Y.; Jiang, W.-K.; Tsao, C.K.; Chang, L.-Y. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci. Rep. 2017, 7, 4649. [Google Scholar] [CrossRef]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “Mediator“ of Systemic Metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare. Dis. 2009, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kasarskis, E.J.; Berryman, S.; English, T.; Nyland, J.; Vanderleest, J.G.; Schneider, A.; Berger, R.; McClain, C. The use of upper extremity anthropometrics in the clinical assessment of patients with amyotrophic lateral sclerosis. Muscle Nerve 1997, 20, 330–335. [Google Scholar] [CrossRef]
- O’Reilly, É.J.; Wang, H.; Weisskopf, M.G.; Fitzgerald, K.C.; Falcone, G.; McCullough, M.L.; Thun, M.; Park, Y.; Kolonel, L.N.; Ascherio, A. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2012, 14, 205–211. [Google Scholar] [CrossRef]
- Paganoni, S.; Deng, J.; Jaffa, M.; Cudkowicz, M.E.; Wills, A.-M. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011, 44, 20–24. [Google Scholar] [CrossRef]
- Calvo, A.; Moglia, C.; Lunetta, C.; Marinou, K.; Ticozzi, N.; Ferrante, G.D.; Scialo, C.; Sorarù, G.; Trojsi, F.; Conte, A.; et al. Factors predicting survival in ALS: A multicenter Italian study. J. Neurol. 2017, 264, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Pradat, P.-F.; Ludolph, A.C.; Loeffler, J.-P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011, 10, 75–82. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Borja-Magno, A.I.; Leal-Escobar, G.; Verdugo-Hernández, S. Validación de ecuaciones de estimación de peso y talla con circunferencias corporales en adultos mayores mexicanos. Nutr. Hosp. 2015, 32, 2898–2902. [Google Scholar]
- Ramón Alvero Cruz, J.; Cabañas Armesilla, D.; de Lucas, A.H.; Martinez Riaza, L.; Pascual Moreno, C.; Manzañido Porta, J.; Quintana Sillero, M.; Belando Sirvent, J.E. Body composition assessment in sports medicine. statement of spanish group of kinanthropometry of spanish federation of sports medicine. Arch. Med. Deporte 2009, 26, 166–179. [Google Scholar]
- Lirola, E.M.L.; Ibabe, M.C.I.; Herreros, J.M.P. La circunferencia de la pantorrilla como marcador rápido y fiable de desnutrición en el anciano que ingresa en el hospital. Relación con la edad y sexo del paciente. Nutr. Hosp. 2016, 33, 565–571. [Google Scholar]
- Camacho, A.; Esteban, J.; Paradas, C. Report by the Spanish Foundation for the Brain on the social impact of amyotrophic lateral sclerosis and other neuromuscular disorders. Neurología 2018, 33, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Frisancho, A.R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Kollewe, K.; Mauss, U.; Krampfl, K.; Petri, S.; Dengler, R.; Mohammadi, B. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J. Neurol. Sci. 2008, 275, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- De Wel, B.; Goosens, V.; Sobota, A.; Van Camp, E.; Geukens, E.; Van Kerschaver, G.; Jagut, M.; Claes, K.; Claeys, K.G. Nusinersen Treatment Significantly Improves Hand Grip Strength, Hand Motor Function and MRC Sum Scores in Adult Patients with Spinal Muscular Atrophy Types 3 and 4. J. Neurol. 2020, 268, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Balendra, R.; Jones, A.; Jivraj, N.; Steen, I.N.; Young, C.A.; Shaw, P.J.; Turner, M.R.; Leigh, P.N.; Al-Chalabi, A. Use of clinical staging in amyotrophic lateral sclerosis for phase 3 clinical trials. J. Neurol. Neurosurg. Psychiatry 2014, 86, 45–49. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013, 10, 2191–2194.
- Povedano, M.; Saez, M.; Martínez-Matos, J.-A.; Barceló, M.A. Spatial Assessment of the Association between Long-Term Exposure to Environmental Factors and the Occurrence of Amyotrophic Lateral Sclerosis in Catalonia, Spain: A Population-Based Nested Case-Control Study. Neuroepidemiology 2018, 51, 33–49. [Google Scholar] [CrossRef]
- Browne, S.E.; Yang, L.; DiMauro, J.-P.; Fuller, S.W.; Licata, S.C.; Beal, M.F. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol. Dis. 2006, 22, 599–610. [Google Scholar] [CrossRef]
- Palamiuc, L.; Schlagowski, A.; Ngo, S.T.; Vernay, A.; Dirrig-Grosch, S.; Henriques, A.; Boutillier, A.L.; Zoll, J.; Echaniz-Laguna, A.; Loeffler, J.P.; et al. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol. Med. 2015, 7, 526–546. [Google Scholar] [CrossRef]
- Dupuis, L.; Di Scala, F.; Rene, F.; de Tapia, M.; Oudart, H.; Pradat, P.-F.; Meininger, V.; Loeffler, J.-P. Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis. FASEB J. 2003, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- So, E.; Mitchell, J.C.; Memmi, C.; Chennell, G.; Vizcay-Barrena, G.; Allison, L.; Shaw, C.E.; Vance, C. Mitochondrial abnormalities and disruption of the neuromuscular junction precede the clinical phenotype and motor neuron loss in hFUSWT transgenic mice. Hum. Mol. Genet. 2017, 27, 463–474. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Hatazawa, J.; Brooks, R.A.; Di Chiro, G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann. Neurol. 1987, 22, 580–586. [Google Scholar] [CrossRef]
- Cheng, Y.; Shang, H. Aberrations of Biochemical Indicators in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Transl. Neurodegener. 2021, 3. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolny, G.; Lepore, E.; Martini, M.; Barberi, L.; Nunn, A.; Scicchitano, B.M.; Musarò, A. Metabolic Changes Associated With Muscle Expression of SOD1G93A. Front. Physiol. 2018, 9, 831. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Kahn, B.B. Glucose Transporters and Insulin Action—Implications for Insulin Resistance and Diabetes Mellitus. New Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.H.; Kang, L.; Ayala, J.E.; Fueger, P.T.; Lee-Young, R.S. The physiological regulation of glucose flux into muscle in vivo. J. Exp. Biol. 2010, 214, 254–262. [Google Scholar] [CrossRef]
- Salvioni, C.C.D.S.; Stanich, P.; Oliveira, A.S.B.; Orsini, M. Anthropometry of Arm: Nutritional Risk Indicator in Amyotrophic Lateral Sclerosis. Neurol. Int. 2015, 7, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Regulation of skeletal muscle fat oxidation during exercise in humans. Med. Sci. Sports Exerc. 2002, 34, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Fat as a fuel: Emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. 2010, 199, 509–518. [Google Scholar] [CrossRef]
- Fan, W.; Atkins, A.R.; Yu, R.T.; Downes, M.; Evans, R.M. Road to exercise mimetics: Targeting nuclear receptors in skeletal muscle. J. Mol. Endocrinol. 2013, 51, T87–T100. [Google Scholar] [CrossRef]
- Muoio, D.M.; Way, J.M.; Tanner, C.J.; Winegar, D.A.; Kliewer, S.A.; Houmard, J.A.; Kraus, W.E.; Dohm, G.L. Peroxisome Proliferator-Activated Receptor- Regulates Fatty Acid Utilization in Primary Human Skeletal Muscle Cells. Diabetes 2002, 51, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Hedström, M.; Cristea, A.; Dalén, N.; Larsson, L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol. 2007, 190, 229–241. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Pellegrino, M.A.; Carlizzi, C.N.; Bottinelli, R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 100, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Russ, D.W.; Grandy, J.S.; Toma, K.; Ward, C.W. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol. 2010, 201, 391–403. [Google Scholar] [CrossRef]
- Manini, T. Development of Physical Disability in Older Adults. Curr. Aging Sci. 2011, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, But Not Muscle Mass, Is Associated With Mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar]

| Bulbar Onset ALS N = 11 | Spinal Onset ALS N = 20 | Bulbar Onset ALS N = 11 | Healthy Control N = 29 | Spinal Onset ALS N = 20 | Healthy Control N = 29 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (%) | Frequency (%) | Chi-Square (p-Value a) | Frequency (%) | Frequency (%) | Chi-Square (p-Value a) | Frequency (%) | Frequency (%) | Chi-Square (p-Value a) | ||
| Sex | Female | 4 (36.36%) | 7 (35%) | 0.040 (0.84) | 4 (36.36%) | 10 (34.48%) | 0.012(0.91) | 7 (35%) | 10 (34.48%) | 0.001 (0.97) |
| Male | 7 (63.64) | 13 (65%) | 7 (63.64) | 19 (65.51%) | 13 (65%) | 19 (65.51%) | ||||
| King’s ALS clinical staging | 1 | 6 (54.5%) | 11 (45.5%) | 0.021 (0.886) | ||||||
| 2 | 4 (36.3%) | 11 (63.7%) | ||||||||
| Mean (SD) | Mean (SD) | Z (p-Value b) | Mean (SD) | Mean (SD) | Z (p-value b) | Mean (SD) | Mean (SD) | Z (p-Value b) | ||
| Age(years) | 60.80 (11.62) | 58.99 (9.46) | −0.269 (0.79) | 60.80 (11.62) | 59.8 (8.71) | 0.789 (0.423) | 58.99 (9.46) | 59.8 (8.71) | 1.856 (0,06) | |
| Weight(kg) | 68.0 (10.5) | 69.5 (8.6) | −0.421 (0.674) | 68.0 (10.5) | 82.93 (13.88) | 2.584 (0.009 **) | 69.5 (8.6) | 82.93 (13.88) | 3.023 (0.002 **) | |
| BMI | 23.9 (2.5) | 24.7 (2.8) | −0.774 (0.439) | 23.9 (2.5) | 28.62 (3.76) | 1.53 (0.126) | 24.7 (2.8) | 28.62 (3.76) | 0.834 (0.41) | |
| Time since symptom onset (months) | 43.87 (22.95) | 39.63 (15.98) | −0.398 (0.699) | |||||||
| ALSFRS-R | 36.7 (6.5) | 33.9 (9.9) | −0.405 (0.687) | |||||||
| ALS Patients ALSFRS-R Score <40 N = 18 | ALS Patients ALSFRS-R Score >40 N = 13 | Mann-Whitney U TEST | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | p-Value | |
| Glucose (mg/dL) | 98.8 | 10.1 | 93.6 | 8.48 | 1.279 | 0.200 |
| AP (UI/L) | 79.5 | 34.8 | 75.5 | 24.2 | −0.310 | 0.756 |
| Bulbar Onset ALS N = 11 | Spinal Onset ALS N = 20 | Mann-Whitney U Test | ||||
| Mean | SD | Mean | SD | Z | p-Value | |
| Glucose (mg/dL) | 92.82 | 8.6 | 98.60 | 10.5 | −1.122 | 0.287 |
| AP (UI/L) | 66.93 | 14.2 | 84.35 | 34.6 | −0.994 | 0.329 |
| Healthy Control N = 29 | Bulbar Onset ALS N = 11 | |||||
| Mean | SD | Mean | SD | Z | p-Value | |
| Glucose (mg/dL) | 93.03 | 7.15 | 92.82 | 8.6 | −0.093 | 0.926 |
| AP (UI/L) | 66.17 | 6.49 | 66.93 | 14.2 | −0.088 | 0.930 |
| Healthy Control N = 29 | Spinal Onset ALS N = 20 | |||||
| Mean | SD | Mean | SD | Z | p-Value | |
| Glucose (mg/dL) | 93.03 | 7.15 | 98.60 | 10.5 | −0.870 | 0.384 |
| AP (UI/L) | 66.17 | 9.49 | 84.35 | 34.6 | −2.028 | 0.243 |
| Whole Sample ALS N = 31 Mean ± SD (95% CI) | Bulbar Onset ALS N = 11 Mean ± SD (95% CI) | Spinal Onset ALS N = 20 Mean ± SD (95% CI) | Mann-Whitney U Test | ||
|---|---|---|---|---|---|
| Z | p | ||||
| Barthel index | 75.3 ± 22.9 (63.8–86.7) | 94.0 ± 13.4 (77.3–110.6) | 68.1 ± 22.0 (54.7–81.3) | −2.265 | 0.026 * |
| Fat % | 18.2 ± 3.8 (16.4–19.9) | 15.1 ± 3.2 (11.7–18.4) | 19.4 ± 3.5 (17.4–21.3) | −2.180 | 0.029 * |
| Muscle % | 36.8 ± 3.1 (32.2–40.6) | 39.9 ± 2.2 (37.6–42.2) | 35.6 ± 2.5 (34.2–37.1) | −2.725 | 0.006 ** |
| MRC | 58.2 ± 16.4 (50.7–65.7) | 73.0 ± 7.1 (65.5–80.4) | 52.3 ± 15.3 (43.8–60.8) | −2.618 | 0.009 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rubia Ortí, J.E.; Armero, J.L.P.; Sanchis-Sanchis, C.E.; Sancho-Castillo, S.; Salazar, A.; Caplliure-Llopis, J.; Navarro-Illana, E.; Barrios, C.; Escribá-Alepuz, J.; Benlloch, M. Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose? J. Clin. Med. 2021, 10, 1582. https://doi.org/10.3390/jcm10081582
de la Rubia Ortí JE, Armero JLP, Sanchis-Sanchis CE, Sancho-Castillo S, Salazar A, Caplliure-Llopis J, Navarro-Illana E, Barrios C, Escribá-Alepuz J, Benlloch M. Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose? Journal of Clinical Medicine. 2021; 10(8):1582. https://doi.org/10.3390/jcm10081582
Chicago/Turabian Stylede la Rubia Ortí, Jose Enrique, Jose Luis Platero Armero, Claudia Emmanuela Sanchis-Sanchis, Sandra Sancho-Castillo, Alejandro Salazar, Jordi Caplliure-Llopis, Esther Navarro-Illana, Carlos Barrios, Jesús Escribá-Alepuz, and María Benlloch. 2021. "Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose?" Journal of Clinical Medicine 10, no. 8: 1582. https://doi.org/10.3390/jcm10081582
APA Stylede la Rubia Ortí, J. E., Armero, J. L. P., Sanchis-Sanchis, C. E., Sancho-Castillo, S., Salazar, A., Caplliure-Llopis, J., Navarro-Illana, E., Barrios, C., Escribá-Alepuz, J., & Benlloch, M. (2021). Muscle Function Differences between Patients with Bulbar and Spinal Onset Amyotrophic Lateral Sclerosis. Does It Depend on Peripheral Glucose? Journal of Clinical Medicine, 10(8), 1582. https://doi.org/10.3390/jcm10081582








