The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Patients and Study Design
2.2. Workup, Chemotherapy, and Surgical Procedure
2.3. Primary and Secondary Outcome Parameters
2.4. Staging and Tumour Assessments
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Treatment Parameters
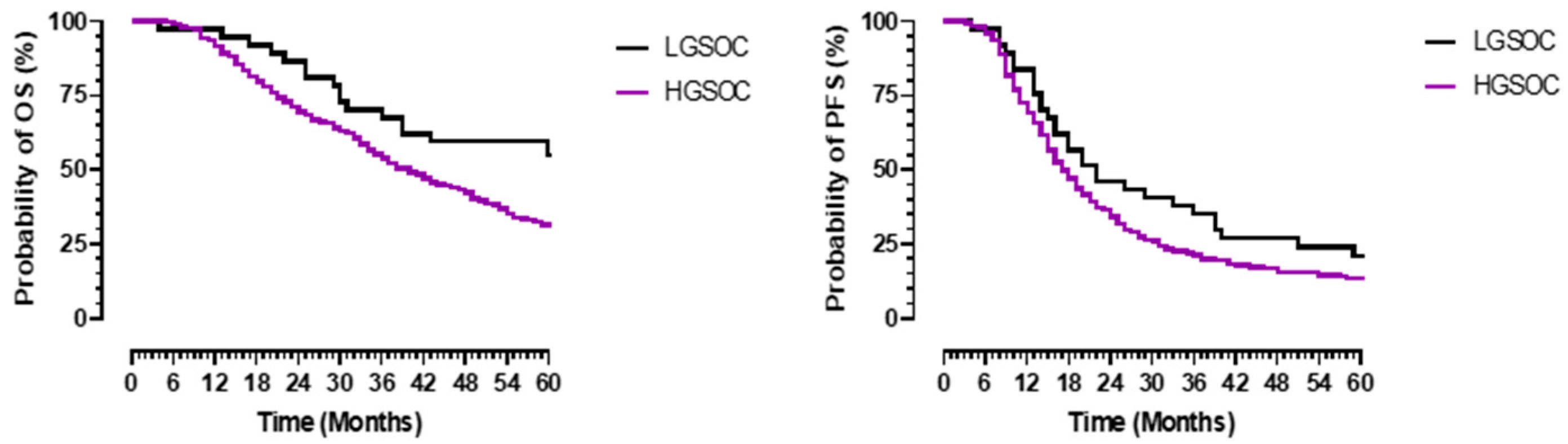
3.3. Survival Outcomes
3.4. Impact of Surgical Approach on Survival Outcomes
3.5. Multivariate Analysis of Surgical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harries, M.; Gore, M. Part I: Chemotherapy for epithelial ovarian cancer-treatment at first diagnosis. Lancet Oncol. 2002, 3, 529–536. [Google Scholar] [CrossRef]
- Bell, R.; Petticrew, M.; Sheldon, T. The performance of screening tests for ovarian cancer: Results of a systematic review. Br. J. Obstet. Gynaecol. 1998, 105, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Jacobs, I.J. Ovarian cancer screening in the general population: Current status. Int. J. Gynecol. Cancer 2001, 11 (Suppl. 1), 3–6. [Google Scholar] [CrossRef]
- Kaku, T.; Ogawa, S.; Kawano, Y.; Ohishi, Y.; Kobayashi, H.; Hirakawa, T.; Nakano, H. Histological classification of ovarian cancer. Med. Electron. Microsc. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R.F.; et al. Reclassification of serous ovarian carcinoma by a 2-tier system: A Gynecologic Oncology Group Study. Cancer 2012, 118, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih, I.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Shih, I. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Schmeler, K.M.; Sun, C.C.; Bodurka, D.C.; Deavers, M.T.; Malpica, A.; Coleman, R.L.; Ramirez, P.T.; Gershenson, D.M. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2008, 108, 510–514. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Bodurka, D.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Deavers, M.; Malpica, A.L.; Kavanagh, J.J. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009, 114, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Sun, C.C.; Lu, K.H.; Coleman, R.L.; Sood, A.K.; Malpica, A.; Deavers, M.T.; Silva, E.G.; Bodurka, D.C. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet. Gynecol. 2006, 108, 361–368. [Google Scholar] [CrossRef]
- Makar, A.P.; Tropé, C.G.; Tummers, P.; Denys, H.; Vandecasteele, K. Advanced Ovarian Cancer: Primary or Interval Debulking? Five Categories of Patients in View of the Results of Randomized Trials and Tumor Biology: Primary Debulking Surgery and Interval Debulking Surgery for Advanced Ovarian Cancer. Oncologist 2016, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzelmann-Schwarz, V.A.; Kurzeder, C.; Schmid, S.; Gabriel, N.; Mueller, A.; Fehr, M.K.; Marth, C.; Du Bois, A.; Klar, M. ENGOT-ov54/Swiss-GO-2/MATAO including LOGOS (Low-Grade Ovarian cancer Sub-study): Maintenance Therapy with Aromatase inhibitor in epithelial Ovarian cancer—A randomized, double-blinded, placebo-controlled, multicenter phase III Trial. J. Clin. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Fader, A.; Gien, L.; Miller, A.; Covens, A.; Gershenson, D. A randomized phase III, two-arm trial of paclitaxel, carboplatin, and maintenance letrozole versus letrozole monotherapy in patients with stage II-IV, primary low-grade serous carcinoma of the ovary or peritoneum. J. Clin. Oncol. 2021, 39, TPS5601. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, E.R. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Eggink, F.A.; Koopmans, C.M.; Nijman, H.W. Surgery for patients with newly diagnosed advanced ovarian cancer: Which patient, when and extent? Curr. Opin. Oncol. 2017, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.T.; Chambers, S.K.; Voynick, I.M.; Schwartz, P.E. Neoadjuvant chemotherapy in stage X ovarian carcinoma. Gynecol. Oncol. 1990, 37, 327–331. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.; Kitchener, H.; Lopes, A.D.B.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.R.; Hermans, R.H.M.; De Hingh, I.H.J.T.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Newsham, A.C.; Johnston, C.; Hall, G.; Leahy, M.G.; Smith, A.B.; Vikram, A.; Donnelly, A.M.; Velikova, G.; Selby, P.J.; Fisher, S.E. Development of an advanced database for clinical trials integrated with an electronic patient record system. Comput. Biol. Med. 2011, 41, 575–586. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 359–374. [Google Scholar]
- Höckel, M. Laterally extended endopelvic resection (LEER)—Principles and practice. Gynecol. Oncol. 2008, 111, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Luna-Pérez, P. Composite pelvic exenteration: Is it worthwhile? Ann. Surg. Oncol. 2004, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.; Santillan, A.; Eisenhauer, E.L.; Hu, J.; Podratz, K.C.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. A new frontier for quality of care in gynecologic oncology surgery: Multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol. Oncol. 2007, 107, 99–106. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Plaxe, S.C. Epidemiology of low-grade serous ovarian cancer. Am. J. Obstet. Gynecol. 2008, 198, 459.e1–459.e9. [Google Scholar] [CrossRef]
- Braicu, E.I.; Sehouli, J.; Richter, R.; Pietzner, K.; Denkert, C.; Fotopoulou, C. Role of histological type on surgical outcome and survival following radical primary tumour debulking of epithelial ovarian, fallopian tube and peritoneal cancers. Br. J. Cancer 2011, 105, 1818–1824. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer, Including Fallopian Tube Cancer and Primary Peritoneal Cancer; version 1.2021. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 13 April 2021).
- Gershenson, D.M.; Sun, C.C.; Wong, K.K. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br. J. Cancer 2015, 113, 1254–1258. [Google Scholar] [CrossRef] [Green Version]
- Arjona-Sanchez, A.; Rufian-Peña, S.; Artiles, M.; Sánchez-Hidalgo, J.M.; Casado-Adam, Á.; Cosano, A.; Thoelecke, H.; Ramnarine, S.; Garcilazo, D.; Briceño-Delgado, J. Residual tumour less than 0.25 centimetres and positive lymph nodes are risk factors for early relapse in recurrent ovarian peritoneal carcinomatosis treated with cytoreductive surgery, HIPEC and systemic chemotherapy. Int. J. Hyperth. 2018, 34, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, J.P.; Harter, P.; Heitz, F.; Pujade-Lauraine, E.; Reuss, A.; Kristensen, G.; Ray-Coquard, I.; Heitz, J.; Traut, A.; Pfisterer, J.; et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol. Oncol. 2016, 140, 457–462. [Google Scholar] [CrossRef]
- Laios, A.; Gryparis, A.; DeJong, D.; Hutson, R.; Theophilou, G.; Leach, C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J. Ovarian Res. 2020, 13, 117. [Google Scholar] [CrossRef]
- Fader, A.N.; Java, J.; Krivak, T.C.; Bristow, R.E.; Tergas, A.I.; Bookman, M.A.; Armstrong, D.K.; Tanner, E.; Gershenson, D.M. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2014, 132, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Burkill, G.J.; Allen, S.D.; A’hern, R.P.; Gore, M.E.; King, D.M. Significance of tumour calcification in ovarian carcinoma. Br. J. Radiol. 2009, 82, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Oldt, R., III; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef] [Green Version]
- Girolimetti, G.; Perrone, A.M.; Santini, D.; Barbieri, E.; Guerra, F.; Ferrari, S.; Zamagni, C.; DE Iaco, P.; Gasparre, G.; Turchetti, D. BRCA-associated ovarian cancer: From molecular genetics to risk management. Biomed. Res. Int. 2014, 2014, 787143. [Google Scholar] [CrossRef]
- Wong, K.K.; Lu, K.H.; Malpica, A.; Bodurka, D.C.; Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Bodurka, D.C.; Coleman, R.L.; Lu, K.H.; Malpica, A.; Sun, C.C. Hormonal Maintenance Therapy for Women with Low-Grade Serous Cancer of the Ovary or Peritoneum. J. Clin. Oncol. 2017, 35, 1103–1111. [Google Scholar] [CrossRef]
- Llueca, A.; Escrig, J.; Serra-Rubert, A.; Gomez-Quiles, L.; Rivadulla, I.; Játiva-Porcar, R.; Moreno-Clarí, E.; Montañés-Pauls, B.; Granel-Villach, L.; Villegas-Cánovas, C.; et al. Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur. J. Surg. Oncol. 2018, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef] [PubMed]
- Despierre, E.; Yesilyurt, B.T.; Lambrechts, S.; Johnson, N.; Verheijen, R.; van der Burg, M.; Casado, A.; Rustin, G.; Berns, E.; Leunen, K.; et al. Epithelial ovarian cancer: Rationale for changing the one-fits-all standard treatment regimen to subtype-specific treatment. Int. J. Gynecol. Cancer 2014, 24, 468–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
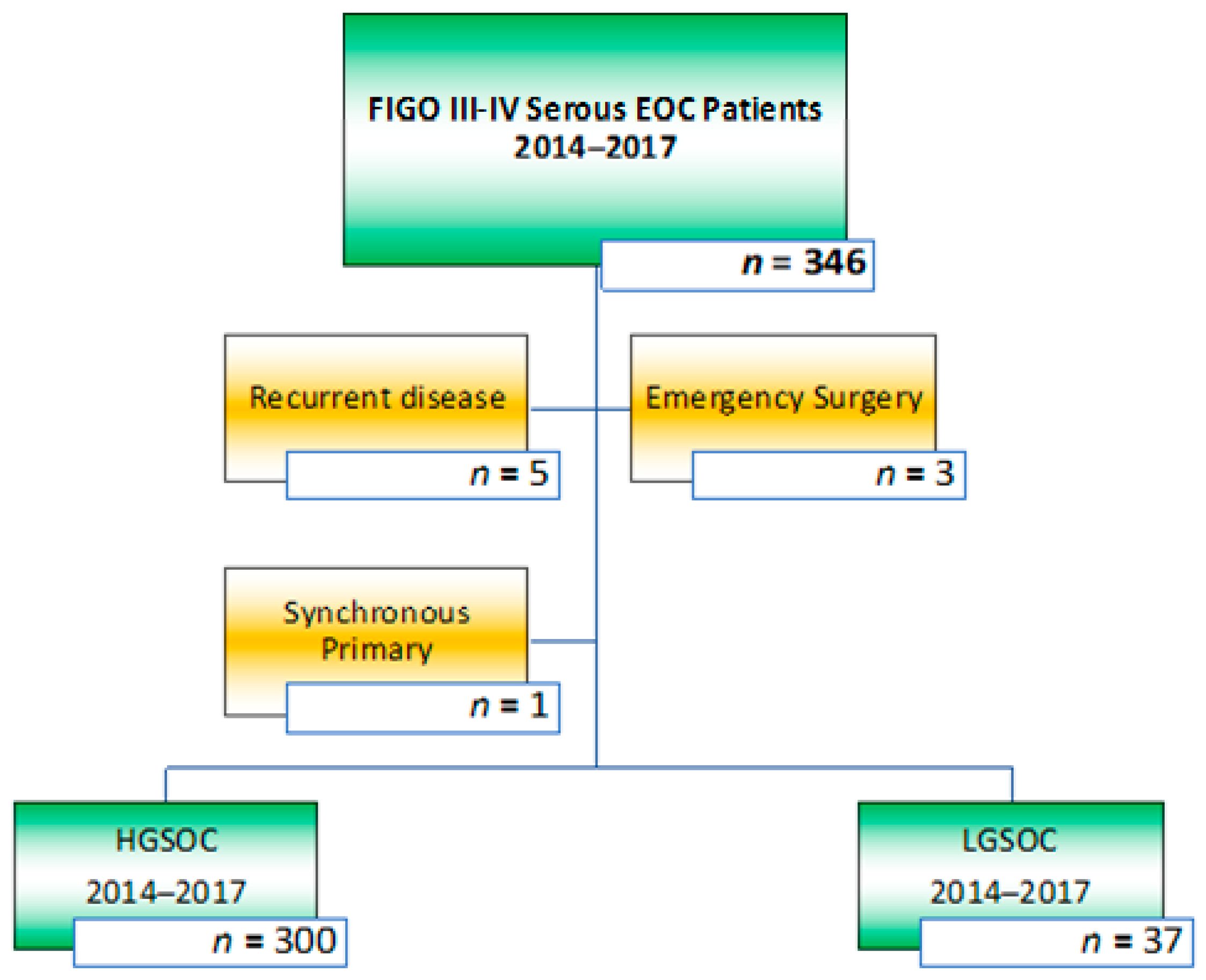



| Low Grade Serous EOC 2014–2017 | High Grade Serous EOC 2014–2017 | p-Value | |
|---|---|---|---|
| Patients | n = 37 | n = 300 | |
| Age (yrs) (Mean, SD) | 61.3 ± 10.9 | 63.9 ± 10.2 | 0.164 |
| Performance status (PS) | 0.419 | ||
| PS 0 | 20 (54.1%) | 124 (41.3%) | |
| PS 1 | 12 (32.4%) | 122 (40.7%) | |
| PS 2 | 3 (8.1%) | 42 (14.0%) | |
| PS 3/4 | 2 (5.4%) | 12 (4.0%) | |
| Pre-treatment CA125 (U/mL) (Median, Range) | 122 (25–9657) | 875 (13–28,600) | <0.0001 |
| Pre-treatment Cytology/Histology | |||
| Cytology | 0 (0%) | 2 (0.7%) | |
| Biopsy | 37 (100%) | 298 (99.3%) | |
| Pre-Treatment CT (Chest/Abdomen/Pelvis) | <0.0001 | ||
| Calcified Deposits Present | 29 (78.4%) | 17 (5.7%) | |
| Absent Calcifications | 8 (21.6%) | 283 (94.3%) | |
| FIGO Stage | 0.478 | ||
| III A-B | 7 (11.9%) | 23 (13.9%) | |
| III C | 24 (62.1%) | 189 (58.8%) | |
| IV A-B | 6 (26.0%) | 88 (27.3%) | |
| Low-Grade Serous EOC 2014–2017 | High-Grade Serous EOC 2014–2017 | p-Value | |
|---|---|---|---|
| Patients | n = 37 | n = 300 | |
| Initial Treatment | < 0.0001 | ||
| Interval debulking surgery | 13 (35.1%) | 240 (80.0%) | |
| Primary debulking surgery | 24 (64.9%) | 60 (20.0%) | |
| Peritoneal cancer index (PCI) (Median, Range) | 8 (2–21) | 5 (1–24) | 0.0216 |
| Surgical Cytoreduction | 0.8329 | ||
| Complete (CC 0–1) | 17 (73%) | 264 (88.0%) | |
| Incomplete (CC ≥ 2) | 10 (27%) | 36 (12%) | |
| Surgical Complexity Score (SCS) | <0.0001 | ||
| Low (1–3) | 14 (37.8%) | 213 (71.0%) | |
| Intermediate (4–7) | 20 (54%) | 74 (24.6%) | |
| High (8–18) | 3 (8.1%) | 13 (4.4%) | |
| Operative time (minutes) (Mean, SD) | 207 ± 93 | 150 ± 63 | <0.0001 |
| Intra-operative Blood Loss (cc) (Mean, SD) | 632 ± 329 | 478 ± 323 | 0.0019 |
| Post-operative Destination | <0.0001 | ||
| Regular Ward | 23 (62.2%) | 251 (83.7%) | 0.0015 |
| HDU/ICU | 9 (4.9%) | 72 (37.1%) | |
| Length of Hospital Stay (days) (Median, Range) | 9 (4–30) | 7 (3–68) | 0.0002 |
| Peri-operative Morbidity (Clavien–Dindo) | <0.0001 | ||
| 0–2 | 27 (73.0%) | 280 (93.4%) | |
| 3–4 | 10 (27.0%) | 19 (6.3%) | |
| 5 | 0 (0.0%) | 1 (0.3%) | |
| Adjuvant Treatment | <0.0001 | ||
| Platinum-based chemotherapy | 10 (27 %) | 297 (99%) | |
| Other (Chemo-)Therapy | 0 (0.0%) | 2 (0.7%) | |
| No Adjuvant Treatment | 27 (73%) | 1 (0.3%) | |
| End of Treatment CA125 (U/mL) (Median, Range) | 17 (5–139) | 13 (3–4019) | 0.2152 |
| Multivariate Analysis OS LGSOC | Multivariate Analysis OS HGSOC | |||||
|---|---|---|---|---|---|---|
| Covariates | HR | p | 95% CI | HR | p | 95% CI |
| FIGO stage | ||||||
| III A-B | 0.0001 | 0.9 | 0.0–1.1 | 0.9 | 0.83 | 0.38–2.2 |
| III C | 0.125 | 0.16 | 0.07–2.3 | 0.74 | 0.59 | 0.25–2.2 |
| IV A | 0.26 | 0.158 | 0.0–4.1 | 0.218 | 0.162 | 0.5–1.1 |
| IV B | 0.061 | 0.18 | 0.01–3.7 | 1.1 | 0.54 | 0.7–1.9 |
| Cytoreduction | ||||||
| Complete (CC 0–1) | 62.3 | <0.001 | 6.8–567.9 | 4.0 | <0.001 | 2.4–6.6 |
| Surgical complexity score | ||||||
| >4 | 5.3 | 0.024 | 1.2–22.8 | 0.88 | 0.56 | 0.6–1.3 |
| Surgical setting | ||||||
| Primary debulking | 3.2 | 0.16 | 0.6–16.6 | 1.8 | 0.017 | 1.1–2.9 |
| Clavien–dindo | ||||||
| 0/1 | 0.0001 | 0.93 | 0–0.01–0.089 | 0.84 | 0.78 | 0.26–2.7 |
| 2 | 0.02 | 0.21 | 0.002–0.5 | 0.86 | 0.646 | 0.27–2.8 |
| 3 | 0.09 | 0.18 | 0.003–3 | 1.2 | 0.727 | 0.35–4.5 |
| 4 | 0.013 | 0.04 | 0.001–0.082 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, R.L.; Laios, A.; Jackson, D.; Nugent, D.; Orsi, N.M.; Theophilou, G.; Thangavelu, A.; de Jong, D. The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction. J. Clin. Med. 2021, 10, 5927. https://doi.org/10.3390/jcm10245927
Johnson RL, Laios A, Jackson D, Nugent D, Orsi NM, Theophilou G, Thangavelu A, de Jong D. The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction. Journal of Clinical Medicine. 2021; 10(24):5927. https://doi.org/10.3390/jcm10245927
Chicago/Turabian StyleJohnson, Racheal Louise, Alexandros Laios, David Jackson, David Nugent, Nicolas Michel Orsi, Georgios Theophilou, Amudha Thangavelu, and Diederick de Jong. 2021. "The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction" Journal of Clinical Medicine 10, no. 24: 5927. https://doi.org/10.3390/jcm10245927
APA StyleJohnson, R. L., Laios, A., Jackson, D., Nugent, D., Orsi, N. M., Theophilou, G., Thangavelu, A., & de Jong, D. (2021). The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction. Journal of Clinical Medicine, 10(24), 5927. https://doi.org/10.3390/jcm10245927








