Headache Worsening after COVID-19 Vaccination: An Online Questionnaire-Based Study on 841 Patients with Migraine
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Statistical Analysis
3. Results
3.1. Population
3.2. Headache Symptoms after COVID-19 Vaccine in Migraine Patients
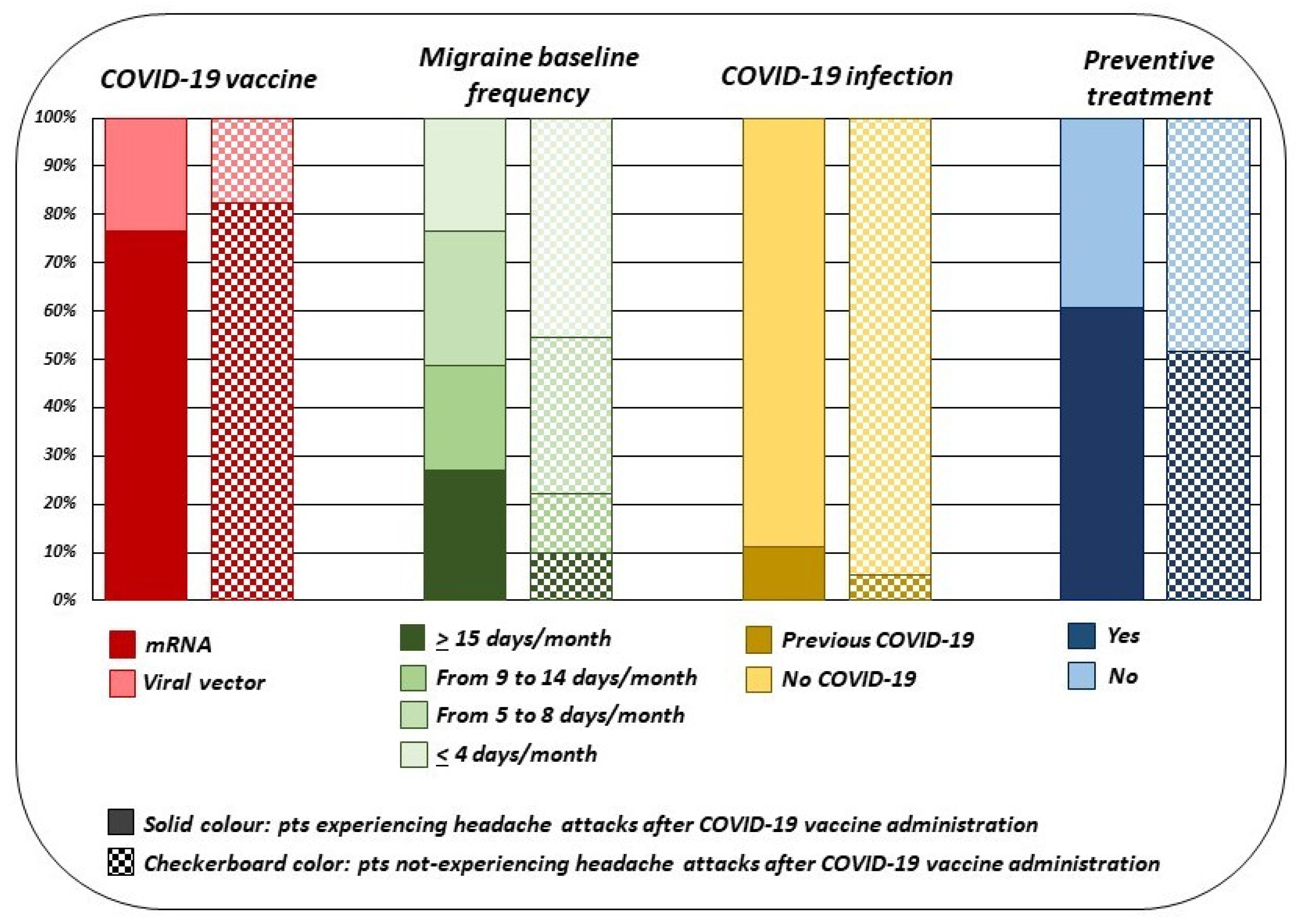
3.3. Post-Hoc Sub Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Vaccines. European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 1 July 2021).
- Gringeri, M.; Mosini, G.; Battini, V.; Cammarata, G.; Guarnieri, G.; Carnovale, C.; Clementi, E.; Radice, S. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): New insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum. Vaccines Immunother. 2021, 17, 1–3. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Göbel, C.H.; Heinze, A.; Karstedt, S.; Morscheck, M.; Tashiro, L.; Cirkel, A.; Hamid, Q.; Halwani, R.; Temsah, M.H.; Ziemann, M.; et al. Headache Attributed to Vaccination Against COVID-19 (Coronavirus SARS-CoV-2) with the ChAdOx1 nCoV-19 (AZD1222) Vaccine: A Multicenter Observational Cohort Study. Pain Ther. 2021, 10, 1309–1330. [Google Scholar] [CrossRef] [PubMed]
- Göbel, C.H.; Heinze, A.; Karstedt, S.; Morscheck, M.; Tashiro, L.; Cirkel, A.; Hamid, Q.; Halwani, R.; Temsah, M.H.; Ziemann, M.; et al. Clinical characteristics of headache after vaccination against COVID-19 (coronavirus SARS-CoV-2) with the BNT162b2 mRNA vaccine: A multicentre observational cohort study. Brain Commun. 2021, 3, fcab169. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted managements and vaccine development. Cytokine Growth Factor Rev. 2021, 58, 16–29. [Google Scholar] [CrossRef]
- Collignon, C.; Bol, V.; Chalon, A.; Surendran, N.; Morel, S.; van den Berg, R.A.; Capone, S.; Bechtold, V.; Temmerman, S.T. Innate Immune Responses to Chimpanzee Adenovirus Vector 155 Vaccination in Mice and Monkeys. Front. Immunol. 2020, 11, 579872. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; La Mantia, L.; Rigamonti, A.; Usai, S.; Mascoli, N.; Milanese, C.; Bussone, G.; Besta, C. Prevalence of primary headaches in people with multiple sclerosis. Cephalalgia 2004, 24, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Prosperini, L.; Ciuffoli, A.; Pizzolato, R.; Salvetti, M.; Pozzilli, C.; Sette, G. Primary headache and multiple sclerosis: Preliminary results of a prospective study. Neurol. Sci. 2008, 29, S146–S148. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.; Marano, E.; Morra, V.B.; Lanzillo, R.; De Vito, M.; Parente, E.; Orefice, G. Multiple sclerosis and headache co-morbidity. A case-control study. Neurol. Sci. 2007, 28, 133–135. [Google Scholar] [CrossRef]
- La Mantia, L.; D’Amico, D.; Rigamonti, A.; Mascoli, N.; Bussone, G.; Milanese, C. Interferon treatment may trigger primary headaches in multiple sclerosis patients. Mult. Scler. 2006, 12, 476–480. [Google Scholar] [CrossRef]
- Villani, V.; Prosperini, L.; De Giglio, L.; Pozzilli, C.; Salvetti, M.; Sette, G. The impact of interferon beta and natalizumab on comorbid migraine in multiple sclerosis. Headache 2012, 52, 1130–1135. [Google Scholar] [CrossRef]
- Yang, C.H.; Murti, A.; Pfeffer, S.R.; Basu, L.; Kim, J.G.; Pfeffer, L.M. IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc. Natl. Acad. Sci. USA 2000, 97, 13631–13636. [Google Scholar] [CrossRef]
- Hadjilambreva, G.; Mix, E.; Rolfs, A.; Müller, J.; Strauss, U. Neuromodulation by a cytokine: Interferon-beta differentially augments neocortical neuronal activity and excitability. J. Neurophysiol. 2005, 93, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, Q.; Ren, Z.; Li, F.; Chen, W.; Lin, X.; Zhang, H.; Tai, G. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol. Sci. 2015, 36, 535–540. [Google Scholar] [CrossRef]
- Ramezani, M.; Komaki, A.; Eftekharian, M.M.; Mazdeh, M.; Ghafouri-Fard, S. Over-expression of IL-6 coding gene in the peripheral blood of migraine with aura patients. Hum. Antibodies. 2021, 29, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Becerra, L.; Borsook, D. Migraine: Maladaptive brain responses to stress. Headache 2012, 52, 102–106. [Google Scholar] [CrossRef]
- Borsook, D.; Maleki, N.; Becerra, L.; McEwen, B. Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 2012, 73, 219–234. [Google Scholar] [CrossRef]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.M.J.M.; Karatas, H. Migraine and neuroinflammation: The inflammasome perspective. J. Headache Pain. 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef]
- Levy, D.; Burstein, R.; Strassman, A.M. Mast cell involvement in the pathophysiology of migraine headache: A hypothesis. Headache 2006, 46, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Hasselmark, L.; Alecci, M.; Cananzi, A.; Perini, F.; Welch, K.M. Platelet secretion from dense and alpha-granules in vitro in migraine with or without aura. J. Neurol. Neurosurg. Psychiatry 1994, 57, 557–561. [Google Scholar] [CrossRef][Green Version]
- D’Andrea, G.; Toldo, M.; Cortelazzo, S.; Milone, F.F. Platelet activity in migraine. Headache 1982, 22, 207–212. [Google Scholar]
- D’Andrea, G.; Toldo, M.; Cananzi, A.; Ferro-Milone, F. Study of platelet activation in migraine: Control by low doses of aspirin. Stroke 1984, 15, 271–275. [Google Scholar] [CrossRef]
- Yager, E.J.; Ahmed, M.; Lanzer, K.; Randall, T.D.; Woodland, D.L.; Blackman, M.A. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008, 205, 711–723. [Google Scholar] [CrossRef]
- Naylor, K.; Li, G.; Vallejo, A.N.; Lee, W.W.; Koetz, K.; Bryl, E.; Witkowski, J.; Fulbright, J.; Weyand, C.M.; Goronzy, J.J. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005, 174, 7446–7452. [Google Scholar] [CrossRef]
- Messaoudi, I.; Lemaoult, J.; Guevara-Patino, J.A.; Metzner, B.M.; Nikolich-Zugich, J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J. Exp. Med. 2004, 200, 1347–1358. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J. T cell aging: Naive but not young. J. Exp. Med. 2005, 201, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Yeh, W.Z.; Blizzard, L.; Taylor, B.V. What is the actual prevalence of migraine? Brain Behav. 2018, 8, e00950. [Google Scholar] [CrossRef] [PubMed]
| Baseline % (n) | |
|---|---|
| Age (mean years ± SD) | 44.94 ± 12.13 |
| Disease history (mean years ± SD) | 25.45 ± 13.93 |
| Diagnosis | |
| Migraine with aura | 26.04% (219) |
| Migraine without aura | 65.76% (553) |
| Both migraine with aura and migraine without aura | 8.20% (69) |
| Baseline headache days/month | |
| <4 | 22.95% (193) |
| 5–8 | 33.29% (280) |
| 9–14 | 20.10% (169) |
| >15 | 23.66% (199) |
| Pts taking preventive treatment | 57.67% (485) |
| Painkillers | |
| Triptans | 24.26% (204) |
| Acetaminophen | 4.99% (42) |
| NSAIDs | 28.18% (237) |
| Triptans or NSAIDs | 24.38% (205) |
| Combination drugs | 14.27% (120) |
| Others | ≈1% |
| Nothing | ≈1% |
| Pts with previous COVID-19 | 9.16% (77) |
| Vaccine administered | |
| Comirnaty | 67.30% (566) |
| Vaxzervria | 19.50% (164) |
| mRNA-1273 | 11.06% (93) |
| Janssen | 2.14% (18) |
| Pts completed both vaccine doses | 46.85% (394) |
| After the First Dose (841 pts) % (n) | After the Second Dose (394 pts) % (n) | |
|---|---|---|
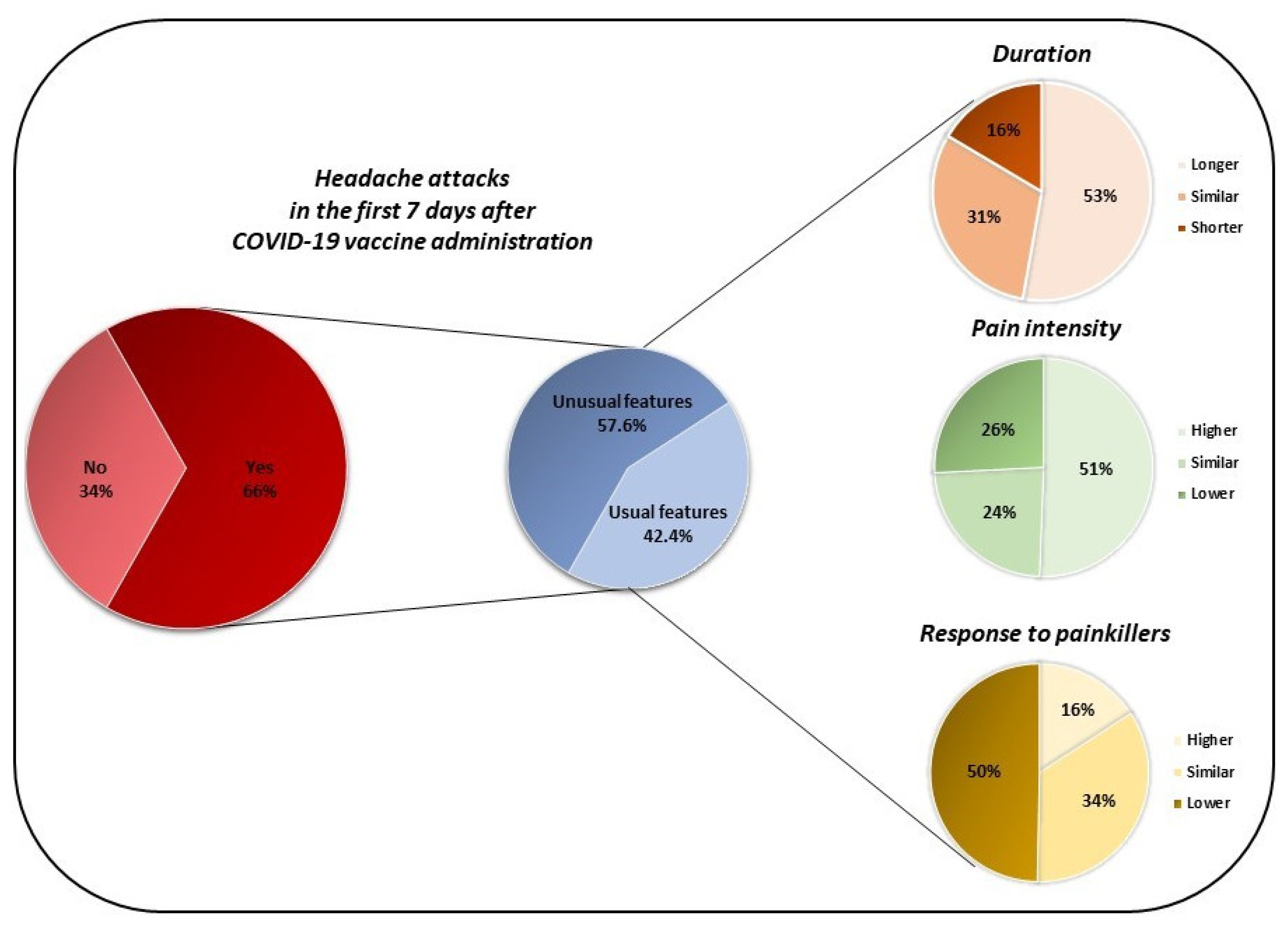
| Pts with headache after vaccination | 66.47% (559) | 60.15% (237) |
| From 1 to 24 h | 48.48% (271) | 51.9% (123) |
| From 24 h to 3 days | 29.52% (165) | 32.07% (76) |
| From 3 to 7 days | 22% (123) | 16.03% (38) |
| Headache with different features | 57.6% (322) | 54.85% (130) |
| (compared to baseline) | ||
| Attack duration | ||
| Greater | 52.80% (170) | 50.77% (66) |
| Similar | 30.75% (99) | 30% (29) |
| Lower | 16.46% (53) | 19.23% (25) |
| Pain intensity | ||
| Greater | 50.62% (163) | 56.92% (74) |
| Similar | 23.6% (76) | 21.54% (28) |
| Lower | 25.78% (83) | 21.54% (28) |
| Response to painkillers | ||
| Greater | 15.84% (51) | 13.85% (18) |
| Similar | 34.47% (111) | 33.08% (43) |
| Lower | 49.69% (160) | 53.08% (69) |
| Pts with other adverse reactions | 74.55% (627) | 73,60% (290) |
| Fever | 18.79% (158) | 27.66% (109) |
| Pain at the injection site | 58.15% (489) | 51.52% (203) |
| Fatigue | 31.63% (266) | 40.86% (161) |
| Diarrhoea | 4.76% (40) | 7.10% (28) |
| Myalgia | 32.10% (270) | 45.94% (181) |
| Others | 7.85% (66) | 8.12% (32) |
| Onset of side effects after vaccination | ||
| From 1 to 24 h | 57.58% (361) | 59.31% (172) |
| From 24 h to 3 days | 33.01% (207) | 33.10% (96) |
| From 3 to 7 days | 9.41% (59) | 7.59% (22) |
| Comirnaty | mRNA-1273 | Vaxzevria | Janssen | Migraine Patients | |
|---|---|---|---|---|---|
| Injection site pain (after the first dose) | 83% (16–55) 71% (>55) | 84.2% | 16% (18–55) 3% (56–69) 6% (>70) | 48.6% | 58.26% |
| Injection site pain (after the second dose) | 78% (16–55) 66% (>55) | 88.6% | 14% (18–55) 3% (56–69) 0% (>70) | 51.52% | |
| Headache (after the first dose) | 42% (16–55) 25% (>55) | 32.7% | 65% (18–55) 50% (56–69) 13% (>70) | 38.9% | 66.47% |
| Headache (after the second dose) | 52% (16–55) 39% (>55) | 58.6% | 31% (18–55) 34% (56–69) 9% (>70) | 60.15% | |
| Fatigue (after the first dose) | 47% (16–55) 34% (>55) | 37.2% | 76% (18–55) 50% (56–69) 30% (>70) | 38.2% | 31.75% |
| Fatigue (after the second dose) | 59% (16–55) 51% (>55) | 65.3% | 55% (18–55) 41% (56–69) 20% (>70) | 40.96% | |
| Myalgia (after the first dose) | 21% (16–55) 14% (>55) | 22.7% | 53% (18–55) 37% (56–69) 11% (>70) | 33.2% | 31.27% |
| Myalgia (after the second dose) | 37% (16–55) 29% (>55) | 58% | 35% (18–55) 24% (56–69) 11% (>70) | 45.18% | |
| Fever (after the first dose) | 4% (16–55) 1% (>55) | 0.8% | 24% (18–55) 0% (56–69) 4% (>70) | 9% | 18.79% |
| Fever (after the second dose) | 16% (16–55) 11% (>55) | 15.5% | 0% (18–55) 0% (56–69) 0% (>70) | 27.66% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, M.; Tessitore, A.; Orologio, I.; Sozio, P.; Napolitano, G.; Siciliano, M.; Tedeschi, G.; Russo, A. Headache Worsening after COVID-19 Vaccination: An Online Questionnaire-Based Study on 841 Patients with Migraine. J. Clin. Med. 2021, 10, 5914. https://doi.org/10.3390/jcm10245914
Silvestro M, Tessitore A, Orologio I, Sozio P, Napolitano G, Siciliano M, Tedeschi G, Russo A. Headache Worsening after COVID-19 Vaccination: An Online Questionnaire-Based Study on 841 Patients with Migraine. Journal of Clinical Medicine. 2021; 10(24):5914. https://doi.org/10.3390/jcm10245914
Chicago/Turabian StyleSilvestro, Marcello, Alessandro Tessitore, Ilaria Orologio, Pasquale Sozio, Giuseppe Napolitano, Mattia Siciliano, Gioacchino Tedeschi, and Antonio Russo. 2021. "Headache Worsening after COVID-19 Vaccination: An Online Questionnaire-Based Study on 841 Patients with Migraine" Journal of Clinical Medicine 10, no. 24: 5914. https://doi.org/10.3390/jcm10245914
APA StyleSilvestro, M., Tessitore, A., Orologio, I., Sozio, P., Napolitano, G., Siciliano, M., Tedeschi, G., & Russo, A. (2021). Headache Worsening after COVID-19 Vaccination: An Online Questionnaire-Based Study on 841 Patients with Migraine. Journal of Clinical Medicine, 10(24), 5914. https://doi.org/10.3390/jcm10245914







