Impact of Psoriasis and Hidradenitis Suppurativa in Pregnancy, a Systematic Review
Abstract
:1. Introduction
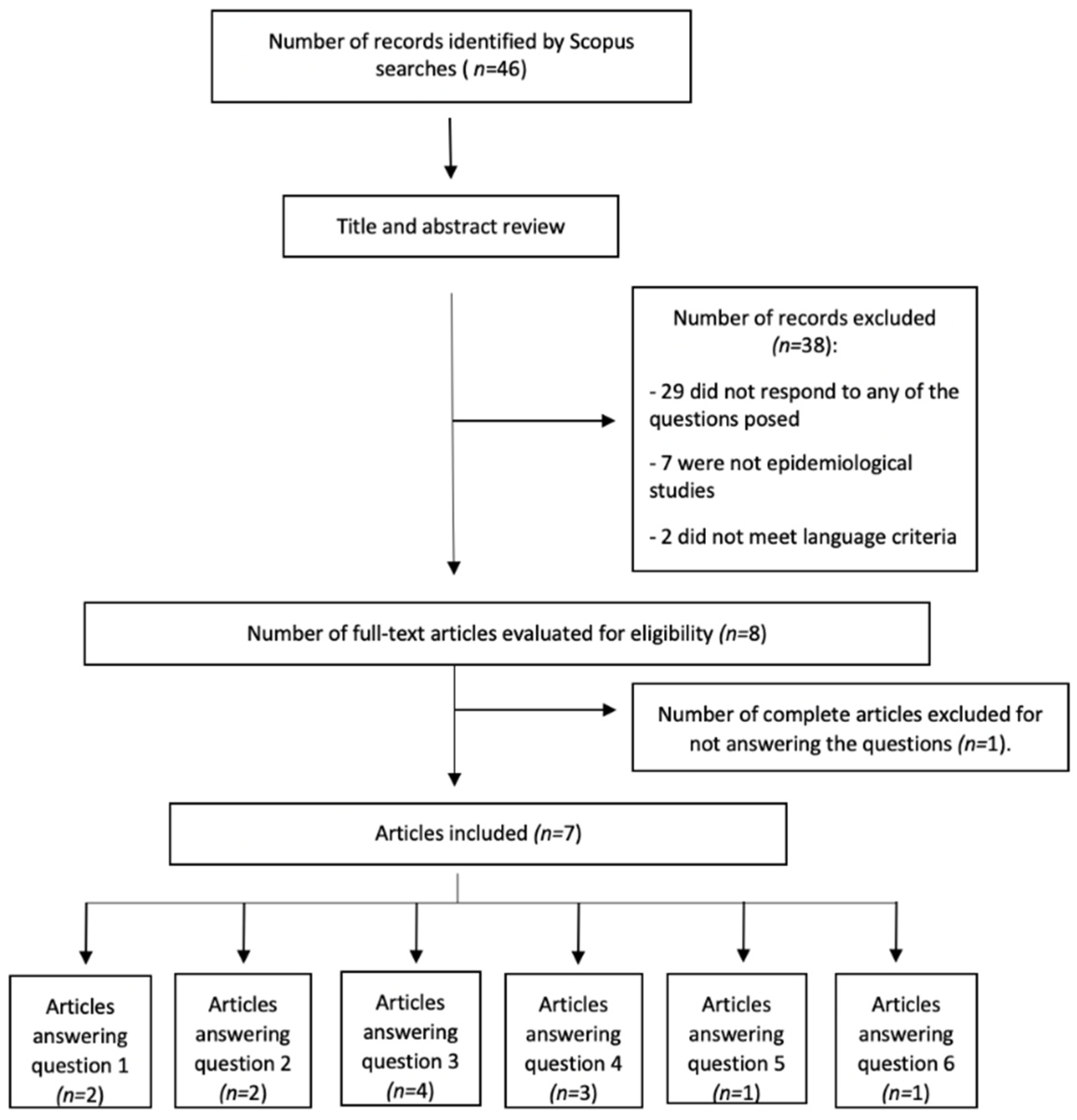
2. Materials and Methods
2.1. Objectives and Research Questions
2.2. Inclusion and Exclusion Criteria
2.3. Bibliographic Search and Data Extraction
2.4. Variables
2.5. Risk of Bias Assessment
3. Results
3.1. Hidradenitis Suppurativa
3.1.1. Does HS Affect Gestational Desire?
3.1.2. Does HS Imply a Decrease in Fertility?
3.1.3. Does HS Negatively Affect Pregnancy Outcome?
3.1.4. How Does HS Behave during Pregnancy?
3.1.5. Does Treatment of HS Affect Pregnancy?
3.1.6. Does Pregnancy Mean a Change in The Treatment of HS?
3.2. Psoriasis
3.2.1. Does Psoriasis Affect Gestational Desire?
3.2.2. Does Psoriasis Lead to Decreased Fertility?
3.2.3. Does Psoriasis Affect the Course of Pregnancy?
3.2.4. How Does Psoriasis Behave during Pregnancy?
3.2.5. Does Psoriasis Treatment Affect Pregnancy?
3.2.6. Does Pregnancy Change the Treatment of Psoriasis?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clebak, K.T.; Helm, L.; Helm, M.F.; Seiverling, E.V. The many variants of psoriasis. J. Fam. Pract. 2020, 69, 192–200. [Google Scholar] [PubMed]
- Mazlin, M.B.; Chang, C.C.; Baba, R. Comorbidities associated with psoriasis—Data from the Malaysian psoriasis registry. Med. J. Malays. 2012, 67, 518–521. [Google Scholar]
- Wu, J.J.; Penfold, R.B.; Primatesta, P.; Fox, T.K.; Stewart, C.; Reddy, S.P.; Egeberg, A.; Liu, J.; Simon, G. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Ross, J.; Seminog, O.; Hawton, K.; Goldacre, M.J. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: Comparisons between disorders using English national record linkage. J. R. Soc. Med. 2014, 107, 194–204. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Carrascosa, J.M.; Toro, M. Prevalencia de la psoriasis en España en la era de los agentes biológicos. Actas Dermosifiliogr. 2014, 105, 504–509. [Google Scholar] [CrossRef]
- Von Laffert, M.; Stadie, V.; Wohlrab, J.; Marsch, W.C. Hidradenitis suppurativa/acne inversa: Bilocated epithelial hyperplasia with very different sequelae. Br. J. Dermatol. 2011, 164, 367–371. [Google Scholar] [CrossRef]
- Von Der Werth, J.M.; Williams, H.C. The natural history of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 389–392. [Google Scholar] [CrossRef]
- Jemec, G.B.E.; Hansen, U. Histology of hidradenitis suppurativa. J. Am. Acad. Dermatol. 1996, 34, 994–999. [Google Scholar] [CrossRef]
- Kouris, A.; Platsidaki, E.; Christodoulou, C.; Efstathiou, V.; Dessinioti, C.; Tzanetakou, V.; Korkoliakou, P.; Zisimou, C.; Antoniou, C.; Kontochristopoulos, G. Quality of Life and Psychosocial Implications in Patients with Hidradenitis Suppurativa. Dermatology 2017, 232, 687–691. [Google Scholar] [CrossRef]
- Molina-Leyva, A.; Cuenca-Barrales, C. Pruritus and Malodour in Patients with Hidradenitis Suppurativa: Impact on Quality of Life and Clinical Features Associated with Symptom Severity. Dermatology 2020, 236, 59–65. [Google Scholar] [CrossRef]
- Hung, C.T.; Chiang, C.P.; Chung, C.H.; Tsao, C.H.; Chien, W.C.; Wang, W.M. Increased risk of cardiovascular comorbidities in hidradenitis suppurativa: A nationwide, population-based, cohort study in Taiwan. J. Dermatol. 2019, 46, 867–873. [Google Scholar] [CrossRef]
- Cosmatos, I.; Matcho, A.; Weinstein, R.; Montgomery, M.O.; Stang, P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J. Am. Acad. Dermatol. 2013, 68, 412–419. [Google Scholar] [CrossRef]
- Adelekun, A.A.; Villa, N.M.; Hsiao, J.L.; Micheletti, R.G. Pregnancy in Hidradenitis Suppurativa—Patient Perspectives and Practice Gaps. JAMA Dermatol. 2020, 157, 227. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Salvador-Rodriguez, L.; Rodriguez-Tejero, A.; Sanchez-Diaz, M.; Arias-Santiago, S.; Molina-Leyva, A. Reproductive potential and outcomes in patients with hidradenitis suppurativa: Clinical profile and therapeutic implications. Life 2021, 11, 277. [Google Scholar] [CrossRef]
- Bitan, D.T.; Kridin, K.; Hodak, E.; Cohen, A.; Sherman, S. The association between hidradenitis suppurativa and male and female infertility: A population-based study. Australas. J. Dermatol. 2021, 62, 13529. [Google Scholar]
- Lyons, A.B.; Peacock, A.; McKenzie, S.A.; Jacobsen, G.; Naik, H.B.; Shi, V.Y.; Hamzavi, I.; Hsiao, J. Retrospective cohort study of pregnancy outcomes in hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 945–947. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Hendricks, A.J.; Thompson, A.M.; Mata, E.M.; Collier, E.K.; Grogan, T.R.; Shi, V.Y.; Hsiao, J.L. Menses, pregnancy, delivery, and menopause in hidradenitis suppurativa: A patient survey. Int. J. Women’s Dermatol. 2020, 6, 368–371. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; van Straalen, K.R.; Prens, E.P.; van der Zee, H.H. Menses and pregnancy affect symptoms in hidradenitis suppurativa: A cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Lyons, A.B.; Peacock, A.; McKenzie, S.A.; Jacobsen, G.; Naik, H.B.; Shi, V.Y.; Hamzavi, I.H.; Hsiao, J.L. Evaluation of Hidradenitis Suppurativa Disease Course During Pregnancy and Postpartum. JAMA Dermatol. 2020, 156, 681. [Google Scholar] [CrossRef]
- Filippi, F.; Odorici, G.; Conti, A.; Di Lernia, V.; Di Nuzzo, S.; Chessa, M.A.; Corazza, M.; Patrizi, A.; Bardazzi, F. Biological therapy in psoriatic patients whishing fatherhood: A multi-centre Italian experience in real life. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e468–e470. [Google Scholar] [CrossRef]
- Caldarola, G.; Milardi, D.; Grande, G.; Quercia, A.; Baroni, S.; Morelli, R.; Marana, R.; Pontecorvi, A.; De Simone, C.; Peris, K. Untreated Psoriasis Impairs Male Fertility: A Case-Control Study. Dermatology 2017, 233, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Lambe, M.; Bergstrom, A.V.; Johansson, A.L.V.; Weibull, C.E. Reproductive patterns and maternal and pregnancy outcomes in women with psoriasis—A population-based study. J. Am. Acad. Dermatol. 2020, 82, 1109–1116. [Google Scholar] [CrossRef]
- Tuğrul Ayanoğlu, B.; Özdemir, E.D.; Türkoğlu, O.; Alhan, A. Diminished ovarian reserve in patients with psoriasis. Taiwan. J. Obstet. Gynecol. 2018, 57, 227–230. [Google Scholar] [CrossRef]
- Heppt, F.; Colsman, A.; Maronna, A.; Uslu, U.; Heppt, M.V.; Kiesewetter, F.; Sticherling, M. Influence of TNF-alpha inhibitors and fumaric acid esters on male fertility in psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1860–1866. [Google Scholar] [CrossRef]
- Gonzalez-Cantero, A.; Carretero, G.; Rivera, R.; Ferrándiz, C.; Daudén, E.; Cueva, P.; Gómez-García, F.J.; Belinchón, I.; Herrera-Ceballos, E.; Ruiz-Genao, D.; et al. Women with moderate-to-severe psoriasis in Spain (BIOBADADERM registry) show more than a 50% reduction in age-adjusted fertility rate when compared with the general population. Br. J. Dermatol. 2019, 181, 1085–1087. [Google Scholar] [CrossRef]
- Bandoli, G.; Chambers, C.D. Autoimmune conditions and comorbid depression in pregnancy: Examining the risk of preterm birth and preeclampsia. J. Perinatol. 2017, 37, 1082–1087. [Google Scholar] [CrossRef] [Green Version]
- Maccari, F.; Fougerousse, A.C.; Reguiai, Z.; Taieb, C. Contraception, Sexuality and Pregnancy in Women with Psoriasis: Real-Life Experience of 235 Women. Clin. Cosmet. Investig. Dermatol. 2020, 13, 817–823. [Google Scholar] [CrossRef]
- Jin, H.; Cho, H.-H.; Kim, W.-J.; Mun, J.-H.; Song, M.; Kim, H.-S.; Ko, H.C.; Kim, M.B.; Kim, H.; Kim, B.S. Clinical features and course of generalized pustular psoriasis in Korea. J. Dermatol. 2015, 42, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Singh, N.; Strouse, J.; Baer, R.J.; Donovan, B.M.; Feuer, S.K.; Nidey, N.; Ryckman, K.K.; Jelliffe-Pawlowski, L.L.; Chambers, C.D. Mediation of Adverse Pregnancy Outcomes in Autoimmune Conditions by Pregnancy Complications: A Mediation Analysis of Autoimmune Conditions and Adverse Pregnancy Outcomes. Arthritis Care Res. 2020, 72, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yee Ng, C.; Chiou, M.; Kuo, C. Fetal–neonatal and maternal outcomes in women with psoriasis vulgaris: A nationwide population-based registry linkage study in Taiwan. J. Dermatol. 2021, 48, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bröms, G.; Haerskjold, A.; Granath, F.; Kieler, H.; Pedersen, L.; Berglind, I.A. Effect of Maternal Psoriasis on Pregnancy and Birth Outcomes: A Population-based Cohort Study from Denmark and Sweden. Acta Derm. Venereol. 2018, 98, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Kimball, A.B.; Guenther, L.; Kalia, S.; de Jong, E.M.G.J.; Lafferty, K.P.; Chen, D.Y.; Langholff, W.; Shear, N.H. Pregnancy Outcomes in Women with Moderate-to-Severe Psoriasis from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2021, 157, 301. [Google Scholar] [CrossRef]
- Boggs, J.M.E.; Griffin, L.; Ahmad, K.; Hackett, C.; Ramsay, B.; Lynch, M. A retrospective review of pregnancies on biologics for the treatment of dermatological conditions. Clin. Exp. Dermatol. 2020, 45, 880–883. [Google Scholar] [CrossRef]
- Echeverría-García, B.; Nuño-González, A.; Dauden, E.; Vanaclocha, F.; Torrado, R.; Belinchón, I.; Pérez-Zafrilla, B. Serie de casos de pacientes psoriásicas expuestas a terapia biológica durante el embarazo. Registro BIOBADADERM y revisión de la literatura. Actas Dermosifiliogr. 2017, 108, 168–170. [Google Scholar] [CrossRef]
- Lau, B.-W.; Lim, D.-Z.; Capon, F.; Barker, J.N.; Choon, S.-E. Juvenile generalized pustular psoriasis is a chronic recalcitrant disease: An analysis of 27 patients seen in a tertiary hospital in Johor, Malaysia. Int. J. Dermatol. 2017, 56, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Odorici, G.; Di Lernia, V.; Bardazzi, F.; Magnano, M.; Di Nuzzo, S.; Cortelazzi, C.; Lasagni, C.; Bigi, L.; Corazza, M.; Pellacani, G.; et al. Psoriasis and pregnancy outcomes in biological therapies: A real-life, multi-centre experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e374–e377. [Google Scholar] [CrossRef]
- Galluzzo, M.; D’Adamio, S.; Bianchi, L.; Talamonti, M. Psoriasis in pregnancy: Case series and literature review of data concerning exposure during pregnancy to ustekinumab. J. Dermatolog. Treat. 2019, 30, 40–44. [Google Scholar] [CrossRef]
- Haycraft, K.; DiRuggiero, D.; Rozzo, S.J.; Mendelsohn, A.M.; Bhutani, T. Outcomes of pregnancies from the tildrakizumab phase I–III clinical development programme. Br. J. Dermatol. 2020, 183, 184–186. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Feldman, S.R.; Isaacs, J.D.; Kimball, A.B.; Strand, V.; Warren, R.B.; Xibillé, D.; Chen, Y.; Frazier, D.; Geier, J.; et al. Pregnancy Outcomes in the Tofacitinib Safety Databases for Rheumatoid Arthritis and Psoriasis. Drug Saf. 2016, 39, 755–762. [Google Scholar] [CrossRef]
- Babuna Kobaner, G.; Polat Ekinci, A. Use of biologic therapies for psoriasis during pregnancy and long-term outcomes of exposed children: A 14-year real-life experience at a tertiary center in Turkey and review of the literature. Dermatol. Ther. 2020, 33, e14420. [Google Scholar] [CrossRef]
- Russo, R.; Gasparini, G.; Cozzani, E.; Burlando, M.; Parodi, A. Considerations on inhibition of IL-23 in psoriatic women of childbearing potential. Dermatol. Ther. 2021, 34, e14931. [Google Scholar] [CrossRef]
- Kawai, Y.; Tsuchiya, T.; Aoki, S. Pregnancy Outcomes of Patients Exposed to Adalimumab in Japan. Dig. Dis. 2019, 37, 123–130. [Google Scholar] [CrossRef]
- Carman, W.J.; Accortt, N.A.; Anthony, M.S.; Iles, J.; Enger, C. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol. Drug Saf. 2017, 26, 1109–1118. [Google Scholar] [CrossRef]
- Watson, N.; Wu, K.; Farr, P.; Reynolds, N.J.; Hampton, P.J. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: A case series of 10 pregnancies. Br. J. Dermatol. 2019, 180, 195–196. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.; Thomsen, S.F. Use of TNF-inhibitors and ustekinumab for psoriasis during pregnancy: A patient series. Dermatol. Ther. 2017, 30, e12454. [Google Scholar] [CrossRef]
- Babuna Kobaner, G.; Polat Ekinci, A. Infliximab for the treatment of recalcitrant generalized pustular psoriasis of pregnancy: Report of a challenging case. Dermatol. Ther. 2020, 33, e13571. [Google Scholar] [CrossRef]
- Belleudi, V.; Poggi, F.R.; Perna, S.; Naldi, L.; Bortolus, R.; Rosa, A.C.; Kirchmayer, U.; Davoli, M.; Addis, A. Drug discontinuation in pregnant women with psoriasis: The PSO–MOTHER cohort study. Pharmacoepidemiol. Drug Saf. 2020, 29, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, Z.U.; Salek, M.S.; Finlay, A.Y. Chronic diseases influence major life changing decisions: A new domain in quality of life research. J. R. Soc. Med. 2012, 105, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomjous, B.S.; de Vries Johanna, I.P.; Zijlstra, E.; Cramer, K.; Voskuyl, A.E.; Bultink, A.I. Desire to have children and preferences regarding to pre-pregnancy counselling in women with SLE. Rheumatology 2020, 60, 1–8. [Google Scholar]
- Andreoli, L.; Lazzaroni, M.G.; Carini, C.; Dall’Ara, F.; Nalli, C.; Reggia, R.; Rodrigues, M.; Benigno, C.; Baldissera, E.; Bartoloni-Bocci, E.; et al. “Disease knowledge index” and perspectives on reproductive issues: A nationwide study on 398 women with autoimmune rheumatic diseases. Jt. Bone Spine 2019, 86, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sbaragli, C.; Morgante, G.; Goracci, A.; Hofkens, T.; De Leo, V.; Castrogiovanni, P. Infertility and psychiatric morbidity. Fertil. Steril. 2008, 90, 2107–2111. [Google Scholar] [CrossRef]
- Molina-Leyva, A.; Salvador-Rodriguez, L.; Martinez-Lopez, A.; Ruiz-Carrascosa, J.C.; Arias-Santiago, S. Association between Psoriasis and Sexual and Erectile Dysfunction in Epidemiologic Studies: A Systematic Review. JAMA Dermatol. 2019, 155, 98–106. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Montero-Vílchez, T.; Szepietowski, J.C.; Matusiak, L.; Molina-Leyva, A. Sexual impairment in patients with hidradenitis suppurativa: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 345–352. [Google Scholar] [CrossRef]
- Killasli, H.; Sartorius, K.; Emtestam, L.; Svensson, Å. Hidradenitis Suppurativa in Sweden: A Registry-Based Cross-Sectional Study of 13,538 Patients. Dermatology 2020, 236, 281–288. [Google Scholar] [CrossRef]
- Yang, C.S.; Teeple, M.; Muglia, J.; Robinson-Bostom, L. Inflammatory and glandular skin disease in pregnancy. Clin. Dermatol. 2016, 34, 335–343. [Google Scholar] [CrossRef]
- Desimone, C.; Caldarola, G.; Moretta, G.; Piscitelli, L.; Ricceri, F.; Prignano, F. Moderate-to-severe psoriasis and pregnancy: Impact on fertility, pregnancy outcome and treatment perspectives. G. Ital. Dermatol. Venereol. 2019, 154, 305–314. [Google Scholar]
- Perng, P.; Zampella, J.G.; Okoye, G.A. Considering the impact of pregnancy on the natural history of hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, e13–e14. [Google Scholar] [CrossRef]
- Preda-Naumescu, A.; Ahmed, H.N.; Mayo, T.T.; Yusuf, N. Hidradenitis suppurativa: Pathogenesis, clinical presentation, epidemiology, and comorbid associations. Int. J. Dermatol. 2021, 60, e449–e458. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Dickinson-Blok, J.L.; Gulliver, W.; Horvath, B.; Hughes, R.; Kimball, A.; Kirby, B.; Martorell, A.; Podda, M.; et al. Hidradenitis suppurativa/acne inversa: A practical framework for treatment optimization—Systematic review and recommendations from the HS ALLIANCE working group. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 19–31. [Google Scholar] [CrossRef]
- Martínez López, J.A.; García Vivar, M.L.; Cáliz, R.; Freire, M.; Galindo, M.; Hernández, M.V.; Longo, F.J.L.; Taboada, V.M.; Reigosa, J.M.P.; Rubio, E.; et al. Recommendations for the evaluation and management of patients with rheumatic autoimmune and inflammatory diseases during the reproductive age, pregnancy, postpartum and breastfeeding. Reum. Clin. 2017, 13, 264–281. [Google Scholar] [CrossRef]
- Leachman, S.A.; Reed, B.R. The Use of Dermatologic Drugs in Pregnancy and Lactation. Dermatol. Clin. 2006, 24, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Paredes, J.; Sandoval Paz, C. Uso de fármacos durante el embarazo. Horiz. Médico 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Hoffman, M.B.; Farhangian, M.; Feldman, S.R. Psoriasis during pregnancy: Characteristics and important management recommendations. Expert Rev. Clin. Immunol. 2015, 11, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, L.L.; Luna, P.C.; Echeverria, C.; Larralde, M. Psoriasis and pregnancy in the biologic era, a feared scenario. What do we do now? Dermatol. Ther. 2019, 32, e13137. [Google Scholar] [CrossRef]
- Renton, C. Late-onset psoriasis: Diagnosis, assessment and management. Br. J. Community Nurs. 2018, 23, 58–63. [Google Scholar] [CrossRef]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The role of tnf-α and anti-tnf-α agents during preconception, pregnancy, and breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R. Treatment with immunosuppressive and biologic drugs of pregnant women with systemic rheumatic or autoimmune disease. Med. Clín. 2016, 147, 352–360. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Hunger, R.E.; Schlapbach, C. Hidradenitis Suppurativa: Current Understanding of Pathogenic Mechanisms and Suggestion for Treatment Algorithm. Front. Med. 2020, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Yee, D.; Collier, E.K.; Atluri, S.; Jaros, J.; Shi, V.Y.; Hsiao, J.L. Gender differences in sexual health impairment in hidradenitis suppurativa: A systematic review. Int. J. Women’s Dermatol. 2020, 10, 259–264. [Google Scholar] [CrossRef]


| Study | Study Design | Number of Patients | Control Population a | Question Which Is Answered | CEBM |
|---|---|---|---|---|---|
| Hidradenitis Suppurativa | |||||
| Adelekun A. A. et al. [13], 2020, United States | Cross-sectional | 59 | - | 1, 2, 3 | 4 |
| Montero-Vilchez et al. [14], 2021; Spain | Case series | 104 | - | 1, 6 | 4 |
| D. Tzur Bitan et al. [15], 2021; Israel | Cross-sectional | 4191 | 20,941 | 2 | 4 |
| Vossen, A. R. J. V et al. [18], 2017; Netherlands | Cross-sectional | 96 | - | 4 | 4 |
| Fernández, J. M. et al. [17], 2021; United States | Cross-sectional | 279 | - | 3, 4 | 4 |
| Lyons A. B. et al. [16], 2020; United States | Case series | 127 | - | 3 | 4 |
| Lyons A. B. et al. [19], 2020; United States | Case series | 127 | - | 4, 6 | 4 |
| Psoriasis | |||||
| González-Cantero et al. [25], 2019; Spain | Prospective cohort study | 732 | - | 2 | 2b |
| Lambe et al. 1 [22], 2020; Sweden | Cross-sectional | 33,488 | 1,634,095 | 2 | 4 |
| Lambe et al. 2 [22], 2020; Sweden | Population cohort study | 15,975 | 1,464,517 | 3 | 2b |
| Filippi et al. [20], 2020; Italy | Case series | 32 | - | 2, 5 | 4 |
| Tuğrul Ayanoğlu et al. [23], 2018; Turkey and United States | Prospective case-control study. | 14 | 35 | 2, 3 | 3b |
| Caldarola et al. [21], 2017; Italy | Case-control study. | 50 | 50 | 2 | 3b |
| Heppt et al. [24], 2017; Germany | Case series | 27 | - | 2 | 4 |
| Bandoli & Chambers [26], 2017; United States | Case-control study. | 330 | 1703 | 3 | 3b |
| G. Bröms et al. [31], 2018; Denmark and Sweden | Population cohort study | 8097 | 943,846 | 3 | 2b |
| Bandoli et al. [29], 2020; United States | Population cohort study | 1255 | 2,962,633 | 3 | 2b |
| Y. Huang et al. [30], 2021; Taiwn | Population cohort study | 4058 | 2,346,281 | 3 | 2b |
| Kimball et al. [32], 2021; United States | Cohort study | 220 | - | 3, 5 | 2b |
| Maccari et al. [27], 2020; France | Cross-sectional | 149 | - | 3, 4, 6 | 4 |
| H. Jin et al. [28], 2015; Corea | Case series | 2 | - | 3, 4 | 4 |
| Lau et al. [35], 2017; Malaysia | Case series | 27 | - | 4 | 4 |
| J. M. E. Boggs et al. [33], 2020; Ireland | Case series | 8 | - | 4, 5, 6 | 4 |
| Echeverría-García et al. [34], 2017; Spain | Case series | 7 | - | 4, 5, 6 | 4 |
| Galluzzo et al. [37], 2019; Italy | Case series | 3 | - | 4, 5, 6 | 4 |
| Odorici et al. [36], 2019; Italy | Case series | 12 | - | 4, 5, 6 | 4 |
| Kawai et al. [42], 2019; Japan | Case series | 73 | - | 5, 6 | 4 |
| Watson et al. [44], 2019; United Kingdom | Case series | 7 | - | 5, 6 | 4 |
| Lund & Thomsen [45], 2017; Denmark | Case series | 7 | - | 5, 6 | 4 |
| Haycraft et al. [38], 2020; United States | Case series | 14 | - | 5, 6 | 4 |
| M. E. B. Clowse et al. [39], 2016; different countries | Case series | 16 | - | 5, 6 | 4 |
| Babuna Kobaner and Polat Ekinci [40], 2020; Turkey | Case series | 6 | - | 5, 6 | 4 |
| Russo et al. [41], 2021; Italy | Case series | 4 | - | 5, 6 | 4 |
| Carman et al. [43], 2017; United States b | Retrospective cohort study | 81 | 1754 | 5 | 2b |
| Belleudi et al. [47], 2020; Italy c | Retrospective cohort study | 525 | 293 | 6 | 2b |
| Lambe et al. [22], 2020 a | Bandoli & Chambers [26], 2017 | G. Bröms et al. [31], 2018 b | Bandoli et al. [29], 2020 | Y. Huang et al. [30], 2021 c | Kimball et al. [32], 2021 | Maccari et al. [27], 2020 | |
|---|---|---|---|---|---|---|---|
| Preterm delivery | OR = 1.07 (0.99–1.15) | 14.2% (47/330) d | OR = 0.97 (0.87–1.08) | 10.8% (135/1255) | OR = 1.13 (1.02–1.25) | 9.1% (22/243) | - |
| Gestation at term | - | - | - | - | - | 90.9% (221/243) | 97.3% (145/149) |
| Preeclampsia | OR = 1.09 (0.99–1.19) | 6.4% (21/330) e | OR = 1.15 (1.01–1.30) | - | OR = 1.57 (1.31–1.89) | - | - |
| Gestational hypertension | OR = 1.37 (1.19–1.58) | OR = 1.17 (1.02–1.35) | - | OR = 1.42 (1.12–1.80 | - | - | |
| Gestational diabetes | - | 10% (33/330) | OR = 1.20 (1.02–1.40) | - | OR = 1.13 (1.00–1.27) | - | - |
| Newborn small for gestational age | OR = 1.00 (0.90–1.11) | - | OR = 0.95 (0.84–1.09) | 7.8% (98/1255) g | OR = 1.12 (1.02–1.23) | - | - |
| Newborn large for gestational age | OR = 1.11 (1.01–1.21) | - | - | - | OR = 0.97 (0.88–1.07) | - | - |
| premature rupture of membranes | OR= 1.15; (1.04–1.27) | - | - | - | - | - | - |
| Congenital malformations | Cleft palate ((OR = 1.69); 1.07–2.66) and non-specified malformations (OR, 1.08; 1.01–1.16) | - | OR = 1.01 (0.90–1.13) | - | OR = 0.87 (0.75–1.00) | 0.8% (2/244) | - |
| Cesarean section | - | - | OR = 1.11 (1.02–1.20) f | 42.1% (528/1255) | OR = 1.06 (1.01–1.12) | - | - |
| Antepartum hemorrhage | - | - | OR = 1.04 (0.90–1.19) | - | OR = 0.98 (0.84–1.13) | - | - |
| Postpartum hemorrhage | - | - | - | - | Uterine atony: OR 1.41 (1.01–1.98) Severe hemorrhage: OR 1.57 (1.36–1.82) | - | - |
| Newborn stillborn | OR = 1.23 (0.96–1.57) | - | OR = 0.74 (0.46–1.19) | - | OR = 1.48 (1.11–1.96) g | 0.4% (1/244) | - |
| Study | Treatment | Number of Pregnancies | Treatment Discontinuation a | Spontaneous Abortion | Elective Abortion | Healthy Newborn b | Complications b |
|---|---|---|---|---|---|---|---|
| Filippi et al. [20] | Adalimumab | 11 | - | 18.2% (2/11) | 0% (0/11) | 100% (9/9) | - |
| Ustekinumab | 6 | - | 0% (0/6) | 0% (0/6) | 100% (6/6) | - | |
| Etanercept | 9 | - | 0% (0/9) | 11.1% (1/9) | 100% (8/8) | - | |
| Infliximab | 15 | - | 0% (0/15) | 20% (3/15) | 100% (12/12) | - | |
| Secukinumab | 3 | - | 0% (0/3) | 0% (0/3) | 100% (3/3) | - | |
| Kimball et al. [32] | All biologic treatments | 168 | - | 16.7% (28/168) | 5.4% (9/168) | 93.1% (122/131) | 0.8% (1/131) Newborn stillborn 0.8% (1/131) Congenital malformations |
| Ustekinumab | 70 | - | 14.3% (10/70) | 5.7% (4/70) | 94.6% (53/56) | 1.8% (1/56) Congenital malformations | |
| Infliximab o golimumab | 14 | - | 7.1% (1/14) | 0% (0/14) | 92.9% (13/14) | - | |
| Other biologic treatments c | 84 | - | 20.2% (17/84) | 6% (5/84) | 90.3% (56/62) | 1.6% (1/62) Newborn stillborn | |
| J. M. E. Boggs et al. [33] | Adalimumab | 9 | 55.6% (5/9) | 11.1% (1/9) | 0% (0/9) | 100% (8/8) | - |
| Infliximab | 1 | 0% (0/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | - | |
| Ustekinumab | 1 | 0% (0/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | - | |
| Etanercept | 1 | 100% (1/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | - | |
| Secukinumab | 2 | 50% (1/2) | 100% (2/2) | 0% (0/2) | 0% | - | |
| Echeverría-García et al. [34] | Etanercept | 5 | 100% (5/5) | 0% (0/5) | 20% (1/5) | 100% (4/4) | 25% (1/4) Psoriasis worsening 25% (1/4) Erythema nodosum and vaginal infection 25% (1/4) Amniotic sac detachment |
| Adalimumab | 3 | 100% (3/3) | 0% (0/3) | 0% (0/3) | 100% (3/3) | 33.3% (1/3) High blood pressure 33.3% (1/3) respiratory distress in the newborn | |
| Ustekinumab | 1 | 100% (1/1) | 0% (1/1) | 0% (0/1) | 100% (1/1) | - | |
| Galluzzo et al. [37] | Ustekinumab | 4 | 100% (4/4) | - | - | 100% (4/4) | 25% (1/4) psoriasis worsening |
| Odorici et al. [36] | Adalimumab | 1 | 100% (1/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | 100% (1/1) psoriasis worsening |
| Secukinumab | 2 | 100% (2/2) | 0% (0/2) | 0% (0/2) | 100% (2/2) | 100% (2/2) psoriasis worsening | |
| Etanercept | 1 | 100% (1/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | - | |
| Ustekinumab | 6 | 66.7% (4/6) | 16.7% (1/6) | 16.7% (1/6) | 75% (3/4) f | 75% (3/4) psoriasis worsening | |
| Infliximab | 4 | 75% (3/4) g | 0% (0/4) | 25% (1/4) | 100% (3/3) | 100% (3/3) psoriasis worsening | |
| Kawai et al. [42] | Adalimumab | 5 | 60% (3/5) | 20% (1/5) | - | 80% (4/5) | 20% (1/5) Gestational hypertension and herpes simplex |
| Watson et al. [44] | Ustekinumab | 10 | 100% (10/10) | 20% (2/10) | 0% (0/10) | 100% (8/8) | 12.5% (1/8) gestational diabetes |
| Lund & Thomsen [45] | Infliximab | 6 | 50% (3/6) | 0% (0/6) | 0% (0/6) | 100% (6/6) | 16.7% (1/6) gestational diabetes |
| Ustekinumab | 4 f | 100% (4/4) | 0% (0/4) | 0% (0/4) | 75% (3/4) | - | |
| Adalimumab | 2 | 0% (0/2) | 0% (0/2) | 0% (0/2) | 100% (2/2) | - | |
| Haycraft et al. [38] | Tildrakizumab | 14 | 71.4% (10/14) e | 14.3% (2/14) | 28.6% (4/14) | 87.5% (7/8) | 12.5% (1/8) preterm labor |
| M. E. B. Clowse et al. [39] | Tofacitinib | 16 d | 100% (16/16) | 6.3% (1/16) | 25% (4/16) | 100% (9/9) | |
| Babuna Kobaner and Polat Ekinci [40] | Adalimumab | 2 | 50% (1/2) | 50% (1/2) | 100% (1/1) | ||
| Etanecept | 1 | 100% (1/1) | 100% (1/1) | ||||
| Infliximab | 3 | 66.67% (2/3) | 100% (3/3) | ||||
| Secukinumab | 1 | 0% (0/1) | 100% (1/1) | ||||
| Ustekinumab | 2 | 100% (2/2) | 50% (1/2) | 50% (1/2) ectopic pregnancy | |||
| Russo et al. [41] | Adalimumab | 2 | 100% (2/2) | 100% (2/2) | |||
| Ustekinumab | 1 | 100% (1/1) | 100% (1/1) | ||||
| Guselkumab | 1 | 100% (1/1) | 100% (1/1) | ||||
| Carman et al. [43] | Etanercept | 81 | 16% (13/81) | 8.6% (7/81) | 100% (61/61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Alcala, M.-A.; Sánchez-Díaz, M.; Arias-Santiago, S.; Molina-Leyva, A. Impact of Psoriasis and Hidradenitis Suppurativa in Pregnancy, a Systematic Review. J. Clin. Med. 2021, 10, 5894. https://doi.org/10.3390/jcm10245894
Ferrer-Alcala M-A, Sánchez-Díaz M, Arias-Santiago S, Molina-Leyva A. Impact of Psoriasis and Hidradenitis Suppurativa in Pregnancy, a Systematic Review. Journal of Clinical Medicine. 2021; 10(24):5894. https://doi.org/10.3390/jcm10245894
Chicago/Turabian StyleFerrer-Alcala, Maria-Angeles, Manuel Sánchez-Díaz, Salvador Arias-Santiago, and Alejandro Molina-Leyva. 2021. "Impact of Psoriasis and Hidradenitis Suppurativa in Pregnancy, a Systematic Review" Journal of Clinical Medicine 10, no. 24: 5894. https://doi.org/10.3390/jcm10245894
APA StyleFerrer-Alcala, M.-A., Sánchez-Díaz, M., Arias-Santiago, S., & Molina-Leyva, A. (2021). Impact of Psoriasis and Hidradenitis Suppurativa in Pregnancy, a Systematic Review. Journal of Clinical Medicine, 10(24), 5894. https://doi.org/10.3390/jcm10245894









