Is It Possible to Predict Clonal Thrombocytosis in Triple-Negative Patients with Isolated Thrombocytosis Based Only on Clinical or Blood Findings?
Abstract
1. Introduction
2. Methods
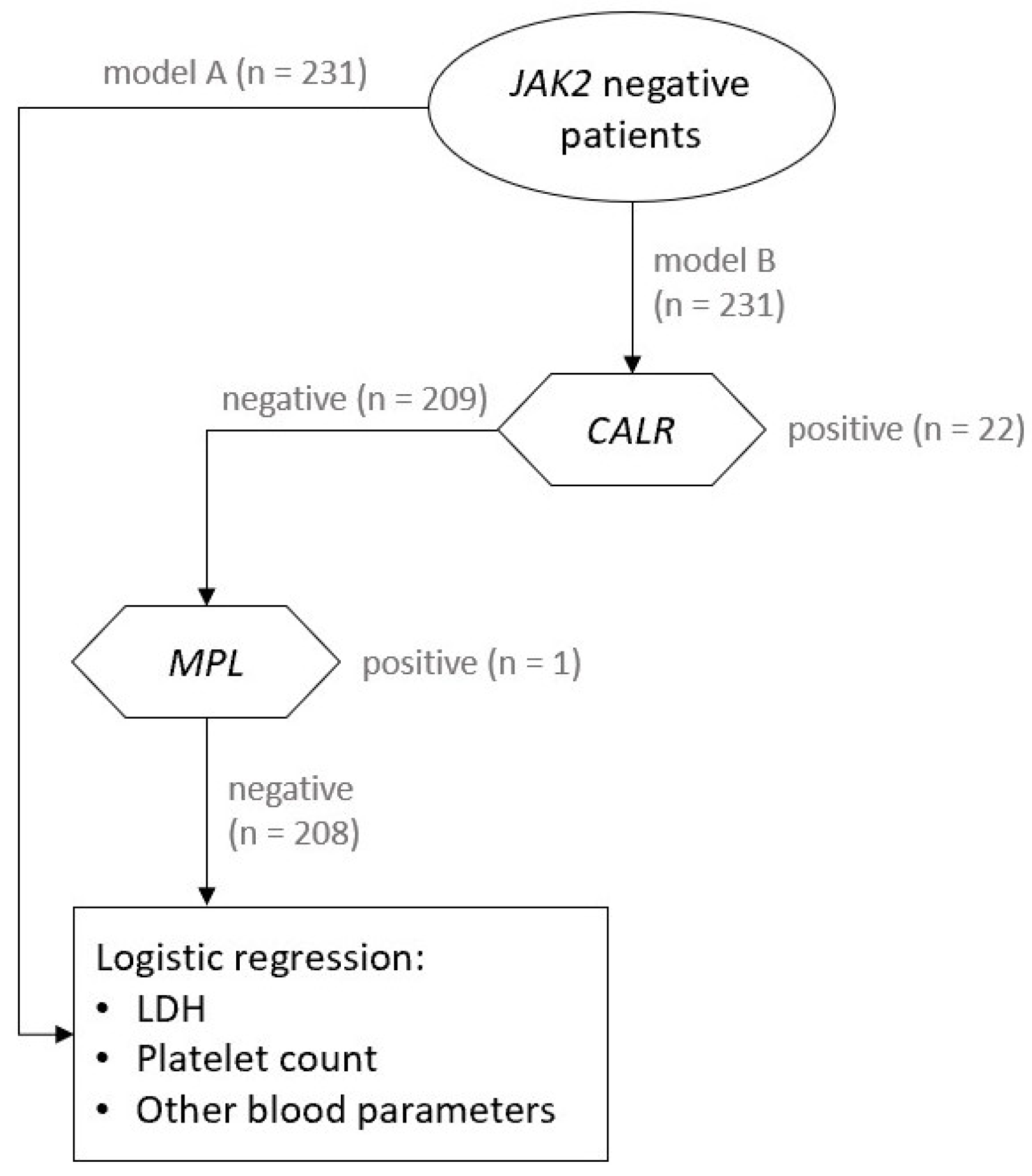
2.1. Study Design and Study Population
2.2. Blood Count and Biochemical Analysis
2.3. Molecular-Genetic Testing
2.3.1. CALR Mutation Detection
2.3.2. MPL Mutation Detection
2.4. Patient Evaluation
2.5. Statistical Analysis
2.5.1. Descriptive Statistics
2.5.2. Predictive Value of Blood Parameters and Gene Expression
3. Results
3.1. Molecular-Genetic Testing and Haematological Diagnosis
3.2. Prediction of Clonal Thrombocytosis Based on Blood Parameters
3.2.1. Model A
3.2.2. Model B
3.3. Diagnostic Value of Platelet Count
3.4. The Value of LDH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CALR | calreticulin |
| CRP | C-reactive protein |
| ET | Essential thrombocythaemia |
| HRM | High resolution melting |
| IL-6 | Interleukin-6 |
| IL-11 | Interleukin-11 |
| JAK-2 | Janus kinase-2 |
| LDH | Lactate dehydrogenase |
| MCV | Mean corpuscular volume |
| MPL | Thrombopoetin receptor |
| MPN | Myeloproliferative neoplasm |
| NGS | Next-generation sequencing |
| OR | Odds ratio |
| PMF | Primary myelofibrosis |
| PV | Polycythaemia vera |
| Q1–Q3 | Interquartile range |
| qPCR | Quantitative polymerase chain reaction |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| TIBC | Total iron binding capacity |
| WBC | White blood count |
| WHO | World Health Organisation |
References
- Mitus, A.J.; Schafer, A.I. Thrombocytosis and thrombocythemia. Hematol. Oncol. Clin. N. Am. 1990, 4, 157–178. [Google Scholar] [CrossRef]
- Harrison, C.N.; Bareford, D.; Butt, N.; Campbell, P.; Conneally, E.; Drummond, M.; Erber, W.; Everington, T.; Green, A.R.; Hall, G.W.; et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br. J. Haematol. 2010, 149, 352–375. [Google Scholar] [CrossRef] [PubMed]
- Griesshammer, M.; Bangerter, M.; Sauer, T.; Wennauer, R.; Bergmann, L.; Heimpel, H. Aetiology and clinical significance of thrombocytosis: Analysis of 732 patients with an elevated platelet count. J. Intern. Med. 1999, 245, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Elliott, M. Thrombosis in myeloproliferative disorders: Prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin. Thromb. Hemost. 2007, 33, 313–320. [Google Scholar] [CrossRef]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef]
- Abdulkarim, K.; Samuelsson, J.; Johansson, P.; Andreasson, B. Risk factors for vascular complications and treatment patterns at diagnosis of 2389 PV and ET patients: Real-world data from the Swedish MPN Registry. Eur. J. Haematol. 2017, 98, 577–583. [Google Scholar] [CrossRef]
- Schafer, A.I. Thrombocytosis. N. Engl. J. Med. 2004, 350, 1211–1219. [Google Scholar] [CrossRef]
- Tefferi, A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010, 24, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef]
- James, C.; Ugo, V.; Le Couédic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garçon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xing, S.; Li, Z.; Fu, X.; Li, Q.; Krantz, S.B.; Zhao, Z.J. Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 2005, 280, 22788–22792. [Google Scholar] [CrossRef] [PubMed]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.A.; Campbell, P.J.; Scott, L.M.; Bench, A.J.; Erber, W.N.; Bareford, D.; Wilkins, B.S.; Reilly, J.T.; Hasselbalch, H.C.; Bowman, R.; et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood 2008, 112, 141–149. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Wang, X.; Zhang, Z.; Yeh, C.H.; Uyeji, J.; Albitar, M. MPL mutation profile in JAK2 mutation-negative patients with myeloproliferative disorders. Diagn. Mol. Pathol. 2011, 20, 34–39. [Google Scholar] [CrossRef]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Langabeer, S.E. Chasing down the triple-negative myeloproliferative neoplasms: Implications for molecular diagnostics. Jak-Stat 2016, 5, e1248011. [Google Scholar] [CrossRef]
- Belcic Mikic, T.; Pajic, T.; Sever, M. CALR mutations in a cohort of JAK2 V617F negative patients with suspected myeloproliferative neoplasms. Sci. Rep. 2019, 9, 19838. [Google Scholar] [CrossRef] [PubMed]
- Pajič, T.; Belčič Mikič, T.; Podgornik, H.; Klun, J.; Šućurović, S.; Zver, S.; Sever, M. Genetic Variant Detection in the CALR gene using High Resolution Melting Analysis. J. Vis. Exp. JoVE 2020, 162, e61642. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Tischer, A.; Wassie, E.A.; Finke, C.M.; Belachew, A.A.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.D. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood 2014, 124, 2465–2466. [Google Scholar] [CrossRef] [PubMed]
- Munoz, V.; Serrano, L. Development of the multiple sequence approximation within the AGADIR model of alpha-helix formation: Comparison with Zimm-Bragg and Lifson-Roig formalisms. Biopolymers 1997, 41, 495–509. [Google Scholar] [CrossRef]
- Harrell, J.F.E. Regression Modeling Strategies, with Applications to Linear Models, Logistic Regression, and Survival Analysis, 2nd ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Tefferi, A.; Ho, T.C.; Ahmann, G.J.; Katzmann, J.A.; Greipp, P.R. Plasma interleukin-6 and C-reactive protein levels in reactive versus clonal thrombocytosis. Am. J. Med. 1994, 97, 374–378. [Google Scholar] [CrossRef]
- Kadikoylu, G.; Yavasoglu, I.; Bolaman, Z.; Senturk, T. Platelet parameters in women with iron deficiency anemia. J. Natl. Med. Assoc. 2006, 98, 398–402. [Google Scholar]
- Appleby, N.; Angelov, D. Clinical and laboratory assessment of a patient with thrombocytosis. Br. J. Hosp. Med. (Lond. Engl. 2005) 2017, 78, 558–564. [Google Scholar] [CrossRef]
- Mazzotta, S.; Guerranti, R.; Gozzetti, A.; Bucalossi, A.; Bocchia, M.; Sammassimo, S.; Petralia, S.; Ogueli, G.I.; Lauria, F. Increased serum lactate dehydrogenase isoenzymes in Ph-negative chronic myeloproliferative diseases: A metabolic adaptation? Hematology 2006, 11, 239–244. [Google Scholar] [CrossRef]
- Cárcamo, C.; Pallarés, E.; Rubí, J.; Cesar, J.M. Lactate dehydrogenase isoenzymes in patients with essential thrombocythemia. Thromb. Res. 1993, 70, 111–116. [Google Scholar] [CrossRef]
- Mudireddy, M.; Barraco, D.; Hanson, C.A.; Pardanani, A.; Gangat, N.; Tefferi, A. The prognostic relevance of serum lactate dehydrogenase and mild bone marrow reticulin fibrosis in essential thrombocythemia. Am. J. Hematol. 2017, 92, 454–459. [Google Scholar] [CrossRef]
- Santhosh-Kumar, C.R.; Yohannan, M.D.; Higgy, K.E.; al-Mashhadani, S.A. Thrombocytosis in adults: Analysis of 777 patients. J. Intern. Med. 1991, 229, 493–495. [Google Scholar] [CrossRef]
- Rose, S.R.; Petersen, N.J.; Gardner, T.J.; Hamill, R.J.; Trautner, B.W. Etiology of thrombocytosis in a general medicine population: Analysis of 801 cases with emphasis on infectious causes. J. Clin. Med. Res. 2012, 4, 415–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robbins, G.; Barnard, D.L. Thrombocytosis and microthrombocytosis: A clinical evaluation of 372 cases. Acta Haematol. 1983, 70, 175–182. [Google Scholar] [CrossRef]
- Aydogan, T.; Kanbay, M.; Alici, O.; Kosar, A. Incidence and etiology of thrombocytosis in an adult Turkish population. Platelets 2006, 17, 328–331. [Google Scholar] [CrossRef]
- Bergmann, K.; Bergmann, O.J. An unusual case of extreme thrombocytosis caused by iron deficiency. BMJ Case Rep. 2020, 13, e231833. [Google Scholar] [CrossRef] [PubMed]
- Holbro, A.; Volken, T.; Buser, A.; Sigle, J.P.; Halter, J.P.; Passweg, J.R.; Tichelli, A.; Infanti, L. Iron deficiency and thrombocytosis. Vox Sang. 2017, 112, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Park, P.W.; Seo, Y.H.; Kim, K.H.; Park, S.H.; Jeong, J.H.; Ahn, J.Y. The relationship between iron parameters and platelet parameters in women with iron deficiency anemia and thrombocytosis. Platelets 2013, 24, 348–351. [Google Scholar] [CrossRef]
- Kuku, I.; Kaya, E.; Yologlu, S.; Gokdeniz, R.; Baydin, A. Platelet counts in adults with iron deficiency anemia. Platelets 2009, 20, 401–405. [Google Scholar] [CrossRef]
- Eder, A.F.; Yau, Y.Y.; West, K. The effect of iron balance on platelet counts in blood donors. Transfusion 2017, 57, 304–312. [Google Scholar] [CrossRef]
- Taskapan, H.; Bahceci, F.; Taskapan, C.; Sahin, I.; Kaya, E.; Aydogdu, I. Transient severe thrombocytopenia in a patient on CAPD after intravenous iron administration. Perit. Dial. Int. 2003, 23, 408–409. [Google Scholar] [CrossRef]
- Choi, S.I.; Simone, J.V.; Jackson, C.W. Megakaryocytopoiesis in experimental iron deficiency anemia. Blood 1974, 43, 111–120. [Google Scholar] [CrossRef]
- Araneda, M.; Krishnan, V.; Hall, K.; Kalbfleisch, J.; Krishnaswamy, G.; Krishnan, K. Reactive and clonal thrombocytosis: Proinflammatory and hematopoietic cytokines and acute phase proteins. South. Med. J. 2001, 94, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ertenli, I.; Kiraz, S.; Ozturk, M.A.; Haznedaroglu, I.; Celik, I.; Calguneri, M. Pathologic thrombopoiesis of rheumatoid arthritis. Rheumatol. Int. 2003, 23, 49–60. [Google Scholar] [CrossRef]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Takashima, S.; Inokuchi, T.; Takahara, M.; Sugihara, Y.; Harada, K.; Okada, H. An Elevated Platelet Count Increases the Risk of Relapse in Ulcerative Colitis Patients with Mucosal Healing. Gut Liver 2018, 12, 420–425. [Google Scholar] [CrossRef]
- Prina, E.; Ferrer, M.; Ranzani, O.T.; Polverino, E.; Cilloniz, C.; Moreno, E.; Mensa, J.; Montull, B.; Menendez, R.; Cosentini, R.; et al. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest 2013, 143, 767–775. [Google Scholar] [CrossRef]
- Choe, E.I.; Kasabian, A.K.; Kolker, A.R.; Karp, N.S.; Zhang, L.; Bass, L.S.; Nardi, M.; Josephson, G.; Karpatkin, M. Thrombocytosis after major lower extremity trauma: Mechanism and possible role in free flap failure. Ann. Plast. Surg. 1996, 36, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Boxer, M.A.; Braun, J.; Ellman, L. Thromboembolic risk of postsplenectomy thrombocytosis. Arch. Surg. 1978, 113, 808–809. [Google Scholar] [CrossRef]
- Aster, R.H. Pooling of platelets in the spleen: Role in the pathogenesis of “hypersplenic” thrombocytopenia. J. Clin. Investig. 1966, 45, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Mohren, M.; Markmann, I.; Dworschak, U.; Franke, A.; Maas, C.; Mewes, S.; Weiss, G.; Jentsch-Ullrich, K. Thromboembolic complications after splenectomy for hematologic diseases. Am. J. Hematol. 2004, 76, 143–147. [Google Scholar] [CrossRef]
- Winslow, E.R.; Brunt, L.M.; Drebin, J.A.; Soper, N.J.; Klingensmith, M.E. Portal vein thrombosis after splenectomy. Am. J. Surg. 2002, 184, 631–635, discussion 635–636. [Google Scholar] [CrossRef]
- Fenaux, P.; Simon, M.; Caulier, M.T.; Lai, J.L.; Goudemand, J.; Bauters, F. Clinical course of essential thrombocythemia in 147 cases. Cancer 1990, 66, 549–556. [Google Scholar] [CrossRef]
- Randi, M.L.; Bertozzi, I.; Rumi, E.; Elena, C.; Finazzi, G.; Vianelli, N.; Polverelli, N.; Ruggeri, M.; Vannucchi, A.M.; Antonioli, E.; et al. Pregnancy complications predict thrombotic events in young women with essential thrombocythemia. Am. J. Hematol. 2014, 89, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Griesshammer, M.; Struve, S.; Harrison, C.M. Essential thrombocythemia/polycythemia vera and pregnancy: The need for an observational study in Europe. Semin. Thromb. Hemost. 2006, 32, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Alimam, S.; Bewley, S.; Chappell, L.C.; Knight, M.; Seed, P.; Gray, G.; Harrison, C.; Robinson, S. Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br. J. Haematol. 2016, 175, 31–36. [Google Scholar] [CrossRef]
- Palumbo, G.A.; Stella, S.; Pennisi, M.S.; Pirosa, C.; Fermo, E.; Fabris, S.; Cattaneo, D.; Iurlo, A. The Role of New Technologies in Myeloproliferative Neoplasms. Front. Oncol. 2019, 9, 321. [Google Scholar] [CrossRef]
- Pardanani, A.; Lasho, T.; Finke, C.; Oh, S.T.; Gotlib, J.; Tefferi, A. LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 2010, 24, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, H.C.; Chiang, Y.H.; Chen, C.G.; Huang, L.; Wang, W.T.; Cheng, C.C.; Lin, J.; Chang, Y.F.; Chang, M.C.; et al. Targeted next-generation sequencing identified novel mutations in triple-negative myeloproliferative neoplasms. Med. Oncol. 2017, 34, 83. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Baxter, E.J.; Wedge, D.C.; Angelopoulos, N.; Cantrill, R.; Godfrey, A.L.; Papaemmanuil, E.; Gundem, G.; MacLean, C.; et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N. Engl. J. Med. 2018, 379, 1416–1430. [Google Scholar] [CrossRef]
- Gardner, J.A.; Peterson, J.D.; Turner, S.A.; Soares, B.L.; Lancor, C.R.; Dos Santos, L.L.; Kaur, P.; Ornstein, D.L.; Tsongalis, G.J.; de Abreu, F.B. Detection of CALR Mutation in Clonal and Nonclonal Hematologic Diseases Using Fragment Analysis and Next-Generation Sequencing. Am. J. Clin. Pathol. 2016, 146, 448–455. [Google Scholar] [CrossRef]

| Characteristics | Clonal Thrombocytosis (n = 51) | Reactive Thrombocytosis (n = 180) |
|---|---|---|
| Female, n (%) | 34 (67) | 139 (77) |
| Age at onset, mean (SD), years | 57.5 (18.5) | 48.9 (16.3) |
| Erythrocyte count, median (Q1–Q3), ×1012/L | 4.38 (4.17–4.66) | 4.51 (4.3–4.8) |
| Haemoglobin, median (Q1–Q3), g/L | 130 (112.5–139) | 130 (122–140.2) |
| Haematocrit, median (Q1–Q3) | 0.39 (0.36–0.42) | 0.39 (0.37–0.41) |
| MCV, median (Q1–Q3), fl | 88.4 (85.3–91.7) | 86.4 (83.4–89.4) |
| Platelet count, median (Q1–Q3), ×109/L | 605 (473–726) | 403 (361–444) |
| WBC count, median (Q1–Q3), ×109/L | 9.2 (7.9–11.1) | 8.61 (6.97–10.66) |
| Neutrophil count, median (Q1–Q3), ×109/L | 6 (4.62–7.21) | 5.19 (4.15–7.14) |
| Ferritin, median (Q1–Q3), µg/L | 102 (32.5–189.5) | 36.5 (12.2–90.5) |
| Saturation of transferrin, median (Q1–Q3), % | 23 (14.5–32) | 19.3 (11.9–31) |
| Iron, median (Q1–Q3), µmol/L | 13.1 (9.5–17.35) | 12 (7.45–18.2) |
| TIBC, median (Q1–Q3), µmol/L | 54.6 (50.3–66.5) | 61.5 (55.1–68.5) |
| LDH, median (Q1–Q3), µkat/L | 3.22 (2.82–4.25) | 2.81 (2.5–3.13) |
| C-reactive protein ≥ 5, mg/L | 17 (34) | 62 (35) |
| Splenomegaly, n (%) | 3 (6) | 2 (1) |
| CALR-positive patients, n (%) | 22 (43) | - |
| MPL-positive patients, n (%) | 1 (1.96) | - |
| Triple negative patients, n (%) | 28 (54.9) | - |
| Predictor | Coefficient | SE | p | OR |
|---|---|---|---|---|
| Model A | ||||
| Platelet count | / | / | <0.001 ** | / |
| Linear effect | 0.025 | 0.007 | <0.001 ** | 1.025 |
| Non-linear effect | −0.028 | 0.01 | 0.006 * | 0.973 |
| LDH | 0.518 | 0.17 | 0.002 * | 1.679 |
| Model B | ||||
| Platelet count | / | / | <0.001 ** | / |
| Linear effect | 0.033 | 0.011 | 0.003 * | 1.034 |
| Non-linear effect | −0.03 | 0.012 | 0.01 * | 0.97 |
| LDH | 0.017 | 0.231 | 0.941 | 1.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belčič Mikič, T.; Vratanar, B.; Pajič, T.; Anžej Doma, S.; Debeljak, N.; Preložnik Zupan, I.; Sever, M.; Zver, S. Is It Possible to Predict Clonal Thrombocytosis in Triple-Negative Patients with Isolated Thrombocytosis Based Only on Clinical or Blood Findings? J. Clin. Med. 2021, 10, 5803. https://doi.org/10.3390/jcm10245803
Belčič Mikič T, Vratanar B, Pajič T, Anžej Doma S, Debeljak N, Preložnik Zupan I, Sever M, Zver S. Is It Possible to Predict Clonal Thrombocytosis in Triple-Negative Patients with Isolated Thrombocytosis Based Only on Clinical or Blood Findings? Journal of Clinical Medicine. 2021; 10(24):5803. https://doi.org/10.3390/jcm10245803
Chicago/Turabian StyleBelčič Mikič, Tanja, Bor Vratanar, Tadej Pajič, Saša Anžej Doma, Nataša Debeljak, Irena Preložnik Zupan, Matjaž Sever, and Samo Zver. 2021. "Is It Possible to Predict Clonal Thrombocytosis in Triple-Negative Patients with Isolated Thrombocytosis Based Only on Clinical or Blood Findings?" Journal of Clinical Medicine 10, no. 24: 5803. https://doi.org/10.3390/jcm10245803
APA StyleBelčič Mikič, T., Vratanar, B., Pajič, T., Anžej Doma, S., Debeljak, N., Preložnik Zupan, I., Sever, M., & Zver, S. (2021). Is It Possible to Predict Clonal Thrombocytosis in Triple-Negative Patients with Isolated Thrombocytosis Based Only on Clinical or Blood Findings? Journal of Clinical Medicine, 10(24), 5803. https://doi.org/10.3390/jcm10245803








