Early Childhood Pneumonia Is Associated with Reduced Lung Function and Asthma in First Nations Australian Children and Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Data Collection
2.3. Definitions
2.4. Spirometry
2.5. Participant Selection for the Current Analysis
2.6. Statistical Analyses
3. Results
3.1. Study Population
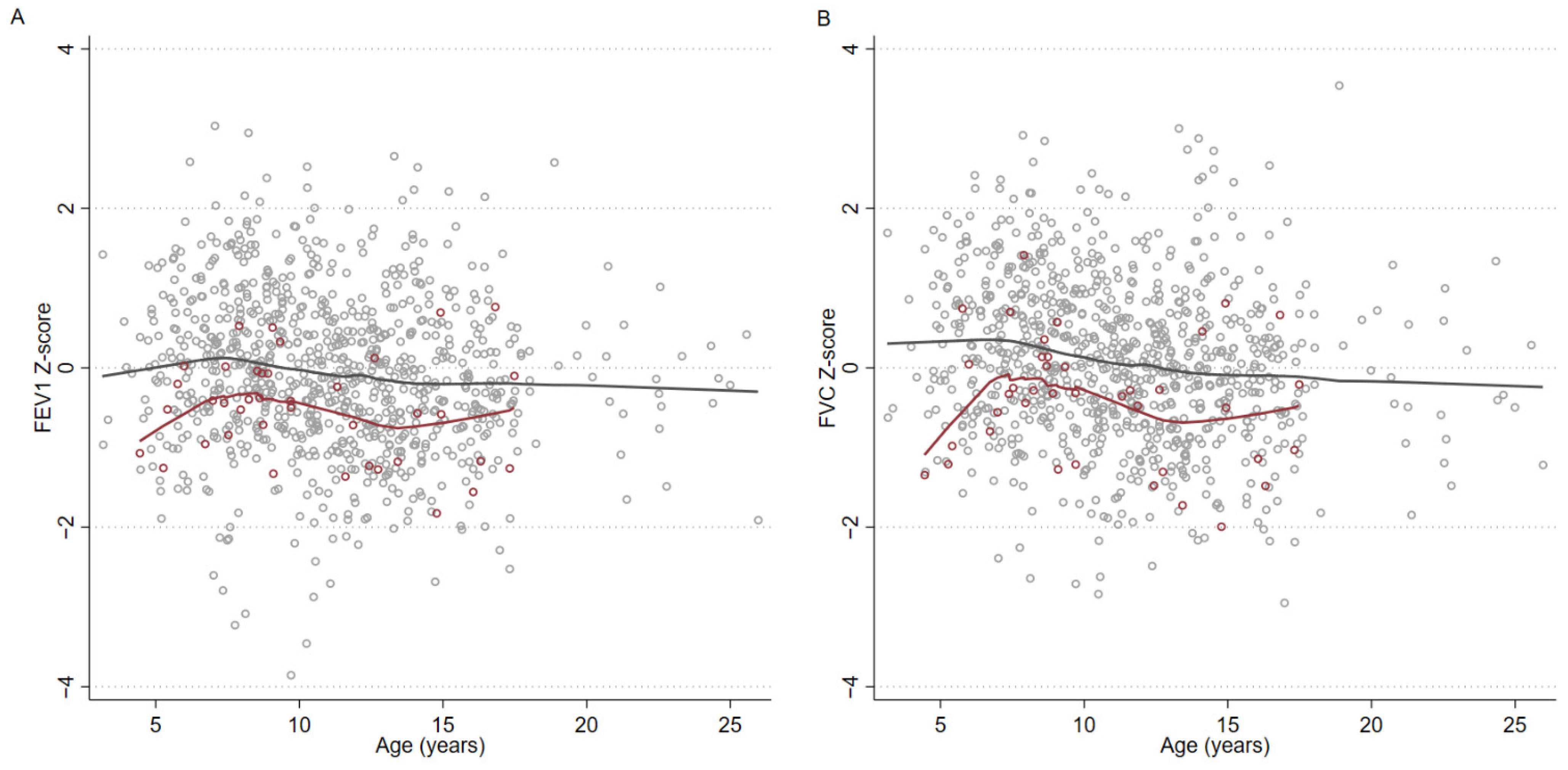
3.2. Spirometry
3.3. Associations with Subsequent Diagnosis of Asthma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, H.; Simões, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.F.; Feikin, D.R.; Mackenzie, G.A.; Moiïsi, J.C.; Roca, A.; et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Rudan, I.; Boschi-Pinto, C.; Biloglav, Z.; Mulholland, K.; Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 2008, 86, 408–416B. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Basnayake, T.L.; Morgan, L.C.; Chang, A.B. The global burden of respiratory infections in indigenous children and adults: A review. Respirology 2017, 22, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Johns, D.P.; Thompson, B.R.; Bui, D.S.; Gurrin, L.C.; Matheson, M.C.; McDonald, C.F.; Wood-Baker, R.; et al. Childhood pneumonia, pleurisy and lung function: A cohort study from the first to sixth decade of life. Thorax 2019, 75, 28–37. [Google Scholar] [CrossRef] [PubMed]
- van Meel, E.R.; den Dekker, H.T.; Elbert, N.J.; Jansen, P.W.; Moll, H.A.; Reiss, I.K.; de Jongste, J.C.; Jaddoe, V.W.V.; Duijts, L. A population-based prospective cohort study examining the influence of early-life respiratory tract infections on school-age lung function and asthma. Thorax 2018, 73, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Gray, D.M.; Turkovic, L.; Willemse, L.; Visagie, A.; Vanker, A.; Stein, D.; Sly, P.; Hall, G.; Zar, H.J. Lung Function in African Infants in the Drakenstein Child Health Study. Impact of Lower Respiratory Tract Illness. Am. J. Respir. Crit. Care Med. 2017, 195, 212–220. [Google Scholar] [CrossRef]
- Chan, J.Y.; Stern, D.A.; Guerra, S.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Pneumonia in Childhood and Impaired Lung Function in Adults: A Longitudinal Study. Pediatrics 2015, 135, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusel, M.M.H.; Kebadze, T.; Johnston, S.L.; Holt, P.; Sly, P.D. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur. Respir. J. 2012, 39, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Holberg, C.J.; Wright, A.L.; Halonen, M.; Taussig, L.M.; Morgan, W.J.; Martinez, F.D. Association of radiologically ascer-tained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: A prospective study. Am. J. Respir. Crit. Care Med. 1999, 159, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.A.; Upton, M.N.; Henderson, A.J.; Dedman, D.; McCarthy, A.; Davey Smith, G.; Yoav, B.-S. Lower respiratory tract infection in the first year of life is associated with worse lung function in adult life: Prospective results from the Barry Caerphilly Growth study. Ann. Epidemiol. 2013, 23, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Svanes, C.; Sunyer, J.; Plana, E.; Dharmage, S.; Heinrich, J.; Jarvis, D.; De Marco, R.; Norbäck, D.; Raherison, C.; Villani, S.; et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2009, 65, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Dharmage, S.C.; Erbas, B.; Jarvis, D.; Wjst, M.; Raherison, C.; Norback, D.; Heinrich, J.; Sunyer, C.; Svanes, C. Do childhood respiratory infections continue to in-fluence adult respiratory morbidity? Eur. Respir. J. 2009, 33, 237–244. [Google Scholar] [CrossRef]
- Grimwood, K.; Chang, A.B. Long-term effects of pneumonia in young children. Pneumonia 2015, 6, 101. [Google Scholar] [CrossRef] [Green Version]
- Agusti, A.; Faner, R. Lung function trajectories in health and disease. Lancet Respir. Med. 2019, 7, 358–364. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Belgrave, D.C.M.; Granell, R.; Turner, S.W.; Curtin, J.A.; Buchan, I.E.; Le Souëf, P.N.; Simpson, A.; Henderson, A.J.; Custovic, A. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: A retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med. 2018, 6, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Agustí, A.; Noell, G.; Brugada, J.; Faner, R. Lung function in early adulthood and health in later life: A transgenerational cohort analysis. Lancet Respir. Med. 2017, 5, 935–945. [Google Scholar] [CrossRef]
- Duong, M.; Islam, S.; Rangarajan, S.; Leong, D.; Kurmi, O.; Teo, K.; Killian, K.; Dagenais, G.; Lear, S.; Wielgosz, A.; et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): An international, community-based cohort study. Lancet Glob. Health 2019, 7, e613–e623. [Google Scholar] [CrossRef] [Green Version]
- Collaro, A.J.; Chang, A.B.; Marchant, J.M.; Chatfield, M.D.; Dent, A.; Blake, T.; Mawn, P.; Fong, K.; McElrea, M.S. Associations between lung function and future cardiovascular morbidity and overall mortality in a predominantly First Nations population: A cohort study. Lancet Reg. Health West. Pac. 2021, 13, 100188. [Google Scholar] [CrossRef]
- Blake, T.L.; Chang, A.B.; Chatfield, M.D.; Marchant, J.M.; McElrea, M.S. Global Lung Function Initiative-2012 ‘other/mixed’ spirometry reference equation provides the best overall fit for Australian Aboriginal and/or Torres Strait Islander children and young adults. Respirology 2020, 25, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H.; et al. An official American Thoracic Society/European Respiratory Society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.N.; Faoagali, J.L.; Buda, P.J.; Sheridan, J.W. Further observations on the immune response to recombinant hepatitis B vaccine after administration to Aboriginal and Torres Strait Island children. J. Paediatr. Child. Health 1997, 33, 67–70. [Google Scholar] [CrossRef]
- Moberley, S.; Licciardi, P.V.; Balloch, A.; Andrews, R.; Leach, A.J.; Kirkwood, M.; Binks, P.; Mulholland, K.; Carapetis, J.; Tang, M.L.; et al. Repeat pneumococcal polysaccharide vaccine in Indigenous Australian adults is associated with decreased immune responsiveness. Vaccine 2017, 35, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Higbee, D.H.; Granell, R.; Smith, G.D.; Dodd, J.W. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: A UK Biobank cohort analysis. Lancet Respir. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Australian Health Ministers’ Advisory Council. Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report; Australian Government: Canberra, Australia, 2017.
- Collaro, A.J.; Chang, A.B.; Marchant, J.M.; Chatfield, M.D.; Dent, A.; Blake, T.; Mawn, P.; Fong, K.; McElrea, M.S. Determinants and Follow-up of Lung Function Data from a Predominantly First Nations Cohort of Adults Referred to Specialist Respiratory Outreach Clinics in Regional and Remote Queensland. Lung 2021, 199, 417–425. [Google Scholar] [CrossRef] [PubMed]
| All Subjects (n = 909) | Never-Pneumonia (n = 861) | Ever-Pneumonia (n = 48) | Pneumonia Occurred When <3 Years of Age (n = 26) | Pneumonia Occurred When 3–5 Years of Age (n = 13) | First pneumonia When >5 Years of Age (n = 9) | ||
|---|---|---|---|---|---|---|---|
| Median (IQR) or n (%) | |||||||
| Age when lung function undertaken | 11.0 (8.2, 13.8) | 11.0 (8.2, 13.8) | 10.1 (8.1, 14.1) | 9.1 (8.0, 14.1) | 9.1 (7.4, 11.9) | 13.4 (11.6, 16.7) | |
| Female | 464 (51%) | 442 (51%) | 22 (46%) | 9 (35%) | 7 (54%) | 6 (67%) | |
| Household smoking | 130 (14%) | 126 (15%) | 4 (8%) | 2 (8%) | 1 (8%) | 1 (11%) | |
| Gestation age (weeks) | >36 | 464 (51%) | 438 (51%) | 26 (54%) | 15 (58%) | 7 (54%) | 4 (44%) |
| 34–36 | 45 (5%) | 42 (5%) | 3 (8%) | 1 (4%) | 1 (8%) | 1 (11%) | |
| 30–33 | 10 (1%) | 10 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| <30 | 6 (1%) | 6 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 385 (42%) | 365 (42%) | 20 (42%) | 10 (38%) | 5 (38%) | 4 (44%) | |
| Eczema | 48 (5%) | 45 (5%) | 3 (6%) | 2 (8%) | 0 (0%) | 1 (11%) | |
| Hayfever | 59 (6%) | 53 (6%) | 6 (12%) | 4 (15%) | 0 (0%) | 0 (0%) | |
| Rash | 44 (5%) | 43 (5%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (11%) | |
| Wheeze | 115 (13%) | 104 (12%) | 11 (23%) | 6 (23%) | 1 (8%) | 2 (22%) | |
| FEV1 | Litres (L) | 2.02 (1.51, 2.77) | 2.03 (1.52, 2.77) | 1.97 (1.38, 2.75) | 1.77 (1.32, 2.76) | 1.74 (1.35, 2.12) | 2.73 (2.13, 2.99) |
| Z–score | −0.13 (−0.71, 0.54) | −0.11 (−0.70, 0.58) | −0.42 (−1.01, 0.12) | −0.51 (−1.07, −0.04) | −0.44 (−1.17, −0.24) | 0.01 (−0.43, 0.42) | |
| % Predicted | 98.5 (91.5, 106.4) | 98.7 (91.6, 106.8) | 94.9 (87.0, 100.2) | 94.0 (86.0, 99.6) | 94.7 (85.8, 97.2) | 100.1 (95.1, 104.9) | |
| FVC | Litres (L) | 2.33 (1.74, 3.18) | 2.34 (1.75, 3.18) | 2.26 (1.60, 3.22) | 1.99 (1.54, 3.39) | 1.99 (1.55, 2.38) | 3.03 (2.61, 3.44) |
| Z–score | 0.04 (−0.58, 0.72) | 0.06 (−0.58, 0.73) | −0.30 (−1.00, 0.21) | −0.28 (−1.14, 0.14) | −0.47 (−1.03, −0.31) | 0.05 (−0.34, 0.81) | |
| % Predicted | 100.4 (93.3, 108.3) | 100.6 (93.3, 108.6) | 96.6 (88.2, 102.5) | 97.0 (87.6, 101.6) | 94.7 (88.8, 96.5) | 100.5 (96.0, 109.2) | |
| Univariable (n = 909) | Multivariable (n = 909) | ||||||
|---|---|---|---|---|---|---|---|
| Model A | Model B | ||||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | ||
| Age when lung function undertaken (per 1 year increase) | −0.03 (−0.05, −0.01) | <0.01 | −0.03 (−0.04, −0.01) | <0.01 | −0.03 (−0.05, −0.01) | <0.01 | |
| Sex (Female) (n = 465) | 0.01 (−0.12, 0.13) | 0.93 | |||||
| Household smoking (n = 130) | −0.19 (−0.37, −0.01) | 0.04 | −0.19 (−0.37, −0.01) | 0.04 | −0.19 (−0.37, −0.01) | 0.03 | |
| Gestational age (weeks) | >36 (n = 464) | Reference | - | Reference | - | Reference | - |
| 34–36 (n = 45) | −0.01 (−0.30, 0.29) | 0.97 | −0.00 (−0.29, 0.29) | 1.00 | −0.01 (−0.30, 0.29) | 0.96 | |
| 30–34 (n = 10) | −0.46 (−1.07, 0.14) | 0.13 | −0.40 (−1.01, 0.20) | 0.19 | −0.40 (−1.00, 0.21) | 0.20 | |
| <30 (n = 6) | −1.06 (−1.84, −0.27) | <0.01 | −0.95 (−1.73, −0.17) | 0.02 | −0.95 (−1.72, −0.17) | 0.02 | |
| Unknown (n = 385) | −0.14 (−0.27, −0.01) | 0.03 | −0.15 (−0.28, −0.02) | 0.03 | −0.15 (−0.28, −0.01) | 0.03 | |
| Eczema (n = 48) | −0.16 (−0.45, 0.12) | 0.26 | |||||
| Hay fever (n = 59) | −0.18 (−0.43, 0.08) | 0.18 | −0.07 (−0.34, 0.19) | 0.58 | −0.06 (−0.33, 0.20) | 0.64 | |
| Rash (n = 44) | −0.15 (−0.45, 0.15) | 0.32 | |||||
| Wheeze (n = 115) | −0.29 (−0.48, −0.09) | <0.01 | −0.22 (−0.42, −0.02) | 0.03 | −0.23 (−0.43, −0.03) | 0.02 | |
| Ever-pneumonia (n = 49) | −0.34 (−0.62, −0.06) | 0.02 | −0.35 (−0.63, −0.07) | 0.01 | |||
| Age (years) of first pneumonia | Never (n = 464) | Reference | - | Reference | - | ||
| 0–2 (n = 27) | −0.40 (−0.78, −0.02) | 0.04 | −0.42 (−0.79, −0.04) | 0.03 | |||
| 3–5 (n = 13) | −0.54 (−1.07, −0.00) | 0.05 | −0.62 (−1.14, −0.09) | 0.02 | |||
| >5 (n = 9) | 0.13 (−0.51, 0.77) | 0.70 | 0.23 (−0.40, 0.86) | 0.48 | |||
| Univariable (n = 909) | Multivariable (n = 909) | ||||||
|---|---|---|---|---|---|---|---|
| Model A | Model B | ||||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | ||
| Age when lung function undertaken (per 1 year increase) | −0.04 (−0.06, −0.03) | <0.01 | −0.04 (−0.06, −0.03) | <0.01 | −0.04 (−0.06, −0.03) | <0.01 | |
| Sex (Female) (n = 465) | 0.06 (−0.07, 0.19) | 0.33 | |||||
| Household smoking (n = 130) | −0.15 (−0.33, 0.04) | 0.12 | −0.16 (−0.34, 0.03) | 0.09 | −0.16 (−0.34, 0.02) | 0.09 | |
| Gestational age (weeks) | >36 (n = 464) | Reference | - | Reference | - | Reference | - |
| 34–36 (n = 45) | 0.07 (−0.23, 0.37) | 0.66 | 0.07 (−0.22, 0.37) | 0.63 | 0.06 (−0.23, 0.36) | 0.66 | |
| 30–34 (n = 10) | −0.33 (−0.95, 0.30) | 0.30 | −0.32 (−0.93, 0.29) | 0.30 | −0.32 (−0.93, 0.29) | 0.30 | |
| <30 (n = 6) | −1.23 (−2.03, −0.43) | <0.01 | −1.23 (−2.01, −0.44) | <0.01 | −1.23 (−2.01, −0.45) | <0.01 | |
| Unknown (n = 385) | −0.14 (−0.27, −0.00) | 0.05 | −0.11 (−0.24, 0.03) | 0.12 | −0.10 (−0.23, 0.03) | 0.14 | |
| Eczema | −0.11 (−0.40, 0.18) | 0.47 | |||||
| Hay fever | 0.02 (−0.24, 0.28) | 0.88 | |||||
| Rash | −0.13 (−0.43, 0.18) | 0.41 | |||||
| Ever-wheeze (n = 115) | −0.04 (−0.24, 0.15) | 0.67 | |||||
| Ever-pneumonia (n = 49) | −0.37 (−0.66, −0.09) | 0.01 | −0.40 (−0.68, −0.12) | <0.01 | |||
| Age (years) of first pneumonia | Never (n = 464) | Reference | - | Reference | - | ||
| 0–2 (n = 27) | −0.45 (−0.83, −0.06) | 0.02 | −0.50 (−0.88, −0.12) | 0.01 | |||
| 3–5 (n = 13) | −0.56 (−1.10, −0.01) | 0.05 | −0.63 (−1.17, −0.10) | 0.02 | |||
| >5 (n = 9) | 0.10 (−0.56, 0.75) | 0.77 | 0.22 (−0.42, 0.87) | 0.49 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collaro, A.J.; Chang, A.B.; Marchant, J.M.; Chatfield, M.D.; Vicendese, D.; Blake, T.L.; McElrea, M.S.; Dharmage, S.C. Early Childhood Pneumonia Is Associated with Reduced Lung Function and Asthma in First Nations Australian Children and Young Adults. J. Clin. Med. 2021, 10, 5727. https://doi.org/10.3390/jcm10245727
Collaro AJ, Chang AB, Marchant JM, Chatfield MD, Vicendese D, Blake TL, McElrea MS, Dharmage SC. Early Childhood Pneumonia Is Associated with Reduced Lung Function and Asthma in First Nations Australian Children and Young Adults. Journal of Clinical Medicine. 2021; 10(24):5727. https://doi.org/10.3390/jcm10245727
Chicago/Turabian StyleCollaro, Andrew J., Anne B. Chang, Julie M. Marchant, Mark D. Chatfield, Don Vicendese, Tamara L. Blake, Margaret S. McElrea, and Shyamali C. Dharmage. 2021. "Early Childhood Pneumonia Is Associated with Reduced Lung Function and Asthma in First Nations Australian Children and Young Adults" Journal of Clinical Medicine 10, no. 24: 5727. https://doi.org/10.3390/jcm10245727
APA StyleCollaro, A. J., Chang, A. B., Marchant, J. M., Chatfield, M. D., Vicendese, D., Blake, T. L., McElrea, M. S., & Dharmage, S. C. (2021). Early Childhood Pneumonia Is Associated with Reduced Lung Function and Asthma in First Nations Australian Children and Young Adults. Journal of Clinical Medicine, 10(24), 5727. https://doi.org/10.3390/jcm10245727









