Preventing Breast Cancer-Related Lymphedema: Feasibility of Axillary Reverse Mapping Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Study
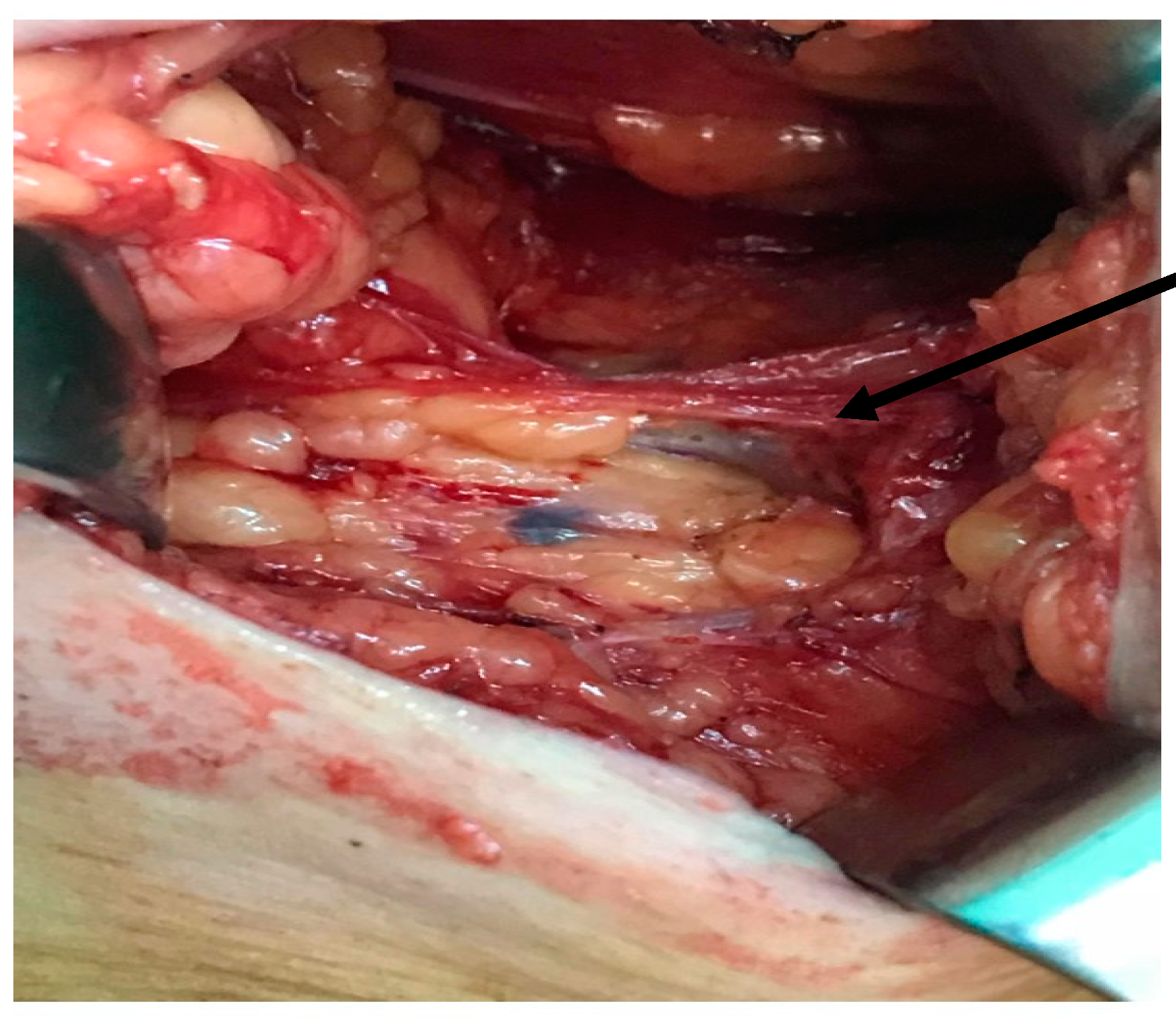
2.2. Surgical Technique
2.3. Studied Parameters
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Identification of ARM Nodes
3.3. Metastatic Involvement of ARM Nodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Inokuchi, M.; Noguchi, M.; Morioka, E.; Ohno, Y.; Kurita, T. Axillary surgery for breast cancer: Past, present, and future. Breast Cancer 2021, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.A.; Peng, J.; He, Y.; Chen, J.; Cen, Y. Clinical application of axillary reverse mapping in patients with breast cancer: A systematic review and meta-analysis. Breast 2020, 53, 189–200. [Google Scholar] [CrossRef]
- Abdelhamid, M.I.; Bari, A.A.; Farid, M.I.; Nour, H. Evaluation of axillary reverse mapping (ARM) in clinically axillary node negative breast cancer patients—Randomised controlled trial. Int. J. Surg. 2020, 75, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ogawa, Y.; Komatsu, H.; Mori, Y.; Ishikawa, A.; Nakajima, T.; Oohira, G.; Tokunaga, S.; Fukushima, H.; Inoue, T. Evaluation of the metastatic status of lymph nodes identified using axillary reverse mapping in breast cancer patients. World J. Surg. Oncol. 2012, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisti, A.; Huayllani, M.T.; Boczar, D.; Restrepo, D.J.; Spaulding, A.C.; Emmanuel, G.; Bagaria, S.P.; McLaughlin, S.A.; Parker, A.S.; Forte, A.J. Breast cancer in women: A descriptive analysis of the national cancer database. Acta Biomed. 2020, 91, 332–341. [Google Scholar] [PubMed]
- Kuusk, U.; Seyednejad, N.; McKevitt, E.C.; Dingee, C.K.; Wiseman, S.M. Axillary reverse mapping in breast cancer: A Canadian experience. J. Surg. Oncol. 2014, 110, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Zhuang, D.; Zhou, P.; Zheng, L.; Fan, Z.; Zhu, J.; Hou, L.; Yu, F.; Dong, X.; Xiao, L.; et al. A Prospective Study to Assess the Feasibility of Axillary Reverse Mapping and Evaluate Its Effect on Preventing Lymphedema in Breast Cancer Patients. Clin. Breast Cancer 2015, 15, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tausch, C.; Baege, A.; Dietrich, D.; Vergin, I.; Heuer, H.; Heusler, R.H.; Rageth, C. Can axillary reverse mapping avoid lymphedema in node positive breast cancer patients? Eur. J. Surg. Oncol. 2013, 39, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Nos, C.; Lesieur, B.; Clough, K.B.; Lecuru, F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann. Surg. Oncol. 2007, 14, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Ngui, N.K.; French, J.; Kilbu, C.J.; Pathmanathan, N.; Elder, E.E. Axillary Reverse Mapping in Patients With Breast Cancer: Is it Oncologically Safe? J. Surg. Oncol. 2016, 113, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Beek, A.M.; Gobardhan, P.D.; Schoenmaeckers, E.J.P.; Klompenhouwer, E.G.; Rutten, H.J.T.; Voogd, A.C. Axillary reverse mapping in axillary surgery for breast cancer: An update of the current status. Breast Cancer Res. Treat. 2016, 158, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Yang, B.; Zuo, W.S.; Zheng, G.; Yang, L.; Zheng, M.Z. The Feasibility and Oncological Safety of Axillary Reverse Mapping in Patients with Breast Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0150285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Korourian, S.; Henry-Tillman, R.; Adkins, L.; Mumford, S.; Westbrook, K.C.; Klimberg, V.S. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann. Surg. Oncol. 2007, 14, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Ponzone, R.; Cont, N.T.; Maggiorotto, F.; Cassina, E.; Mininanni, P.; Biglia, N.; Sismondi, P. Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. J. Clin. Oncol. 2009, 27, 5547–5551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.; Sun, B.; Shen, Y. Axillary reverse mapping (ARM): Where to go. Breast Cancer 2019, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, N.; Kuusk, U.; Wiseman, S.M. Axillary reverse lymphatic mapping in breast cancer surgery: A comprehensive review. Expert Rev. Anticancer Ther. 2014, 14, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Cebrecos, I.; Peg, V.; Esgueva, A.; Mendoza, C.; Cortadellas, T.; Cordoba, O.; Espinosa-Bravo, M.; Xercavins, J. Extensive Nodal Involvement Increases the Positivity of Blue Nodes in the Axillary Reverse Mapping Procedure in Patients with Breast Cancer. J. Surg. Oncol. 2012, 106, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Seo, Y.J.; Choi, J.E.; Kang, S.H.; Bae, Y.K.; Lee, S.J. The Efficacy of Arm Node Preserving Surgery Using Axillary Reverse Mapping for Preventing Lymphedema in Patients with Breast Cancer. J. Breast Cancer 2012, 15, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Population Study | Identified ARM Nodes (n = 18) | Unidentified ARM Nodes (n = 12) | p-Value |
|---|---|---|---|---|
| Age * | 56.73 ± 12.56 | 56.67 ± 12.61 | 56.83 ± 13.05 | 0.068 |
| BMI * | 27.08 ± 5.63 | 25.06 ± 4.78 | 30.12 ± 5.61 | 0.013 |
| Tumor size ** | 0.35 | |||
| T1 | 12 | 8 | 4 | |
| T2 | 13 | 7 | 6 | |
| T3 | 2 | 1 | 1 | |
| T4 | 3 | 2 | 1 | |
| Pathological lymph node status (pN) ** | 0.0001 | |||
| pN0 | 14 | 10 | 4 | |
| pN1 | 9 | 4 | 5 | |
| pN2 | 5 | 2 | 3 | |
| pN3 | 2 | 2 | 0 | |
| Hystological type ** | 0.54 | |||
| NST | 25 | 15 | 10 | |
| Lobular | 4 | 3 | 1 | |
| Other | 1 | 0 | 1 | |
| Intrinsic subtype ** | 0.39 | |||
| Luminal A | 16 | 5 | 11 | |
| Luminl B Her 2− | 13 | 7 | 6 | |
| Luminal B Her 2+ | 6 | 4 | 2 | |
| HER 2 overexpressed | 1 | 1 | 0 | |
| Triple negative | 3 | 1 | 2 | |
| Grade ** | 0.338 | |||
| G1 | 4 | 2 | 2 | |
| G2 | 18 | 12 | 6 | |
| G3 | 8 | 4 | 4 | |
| Neoadjuvant treatment ** | 0.51 | |||
| ChT | 15 | 7 | 8 | |
| HT | 4 | 2 | 2 | |
| Localization ** | 0.52 | |||
| UOQ | 15 | 9 | 6 | |
| UIQ | 2 | 1 | 1 | |
| LOQ | 3 | 2 | 1 | |
| LIQ | 2 | 1 | 1 | |
| Central | 2 | 1 | 1 | |
| Multicentric/multifocal | 6 | 4 | 2 |
| Factor | Positive ARM Nodes | Negative ARM Nodes | p |
|---|---|---|---|
| Average age * | 53.79 ± 10.05 | 66.75 ± 16.99 | 0.068 |
| Average BMI * | 24.67 ± 4.13 | 26.42 ± 7.23 | 0.53 |
| Axillary lymph node status ** | 0.0001 | ||
| pN0 | 10 | 0 | |
| pN1 | 4 | 0 | |
| pN2 | 0 | 2 | |
| pN3 | 0 | 2 | |
| Tumor size ** | 0.35 | ||
| T1 | 7 | 1 | |
| T2 | 5 | 2 | |
| T3 | 1 | 0 | |
| T4 | 1 | 1 | |
| Histological grade ** | 0.338 | ||
| G1 | 2 | 0 | |
| G2 | 9 | 3 | |
| G3 | 3 | 1 | |
| Neoadjuvant chemotherapy ** | 6 | 1 | 0.51 |
| Histological type ** | 0.54 | ||
| NST | 12 | 3 | |
| Lobular | 2 | 1 | |
| Molecular subtype ** | 0.39 | ||
| Triple negative | 1 | 0 | |
| Luminal A | 4 | 1 | |
| Luminal B Her2 positive | 3 | 1 | |
| Luminal B Her2 negative | 6 | 1 | |
| Her2 overexpression | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caziuc, A.; Schlanger, D.; Amarinei, G.; Fagarasan, V.; Andras, D.; Dindelegan, G.C. Preventing Breast Cancer-Related Lymphedema: Feasibility of Axillary Reverse Mapping Technique. J. Clin. Med. 2021, 10, 5707. https://doi.org/10.3390/jcm10235707
Caziuc A, Schlanger D, Amarinei G, Fagarasan V, Andras D, Dindelegan GC. Preventing Breast Cancer-Related Lymphedema: Feasibility of Axillary Reverse Mapping Technique. Journal of Clinical Medicine. 2021; 10(23):5707. https://doi.org/10.3390/jcm10235707
Chicago/Turabian StyleCaziuc, Alexandra, Diana Schlanger, Giorgiana Amarinei, Vlad Fagarasan, David Andras, and George Calin Dindelegan. 2021. "Preventing Breast Cancer-Related Lymphedema: Feasibility of Axillary Reverse Mapping Technique" Journal of Clinical Medicine 10, no. 23: 5707. https://doi.org/10.3390/jcm10235707
APA StyleCaziuc, A., Schlanger, D., Amarinei, G., Fagarasan, V., Andras, D., & Dindelegan, G. C. (2021). Preventing Breast Cancer-Related Lymphedema: Feasibility of Axillary Reverse Mapping Technique. Journal of Clinical Medicine, 10(23), 5707. https://doi.org/10.3390/jcm10235707






