E-Cadherin, Integrin Alpha2 (Cd49b), and Transferrin Receptor-1 (Tfr1) Are Promising Immunohistochemical Markers of Selected Adverse Pathological Features in Patients Treated with Radical Prostatectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candidate Protein Selection
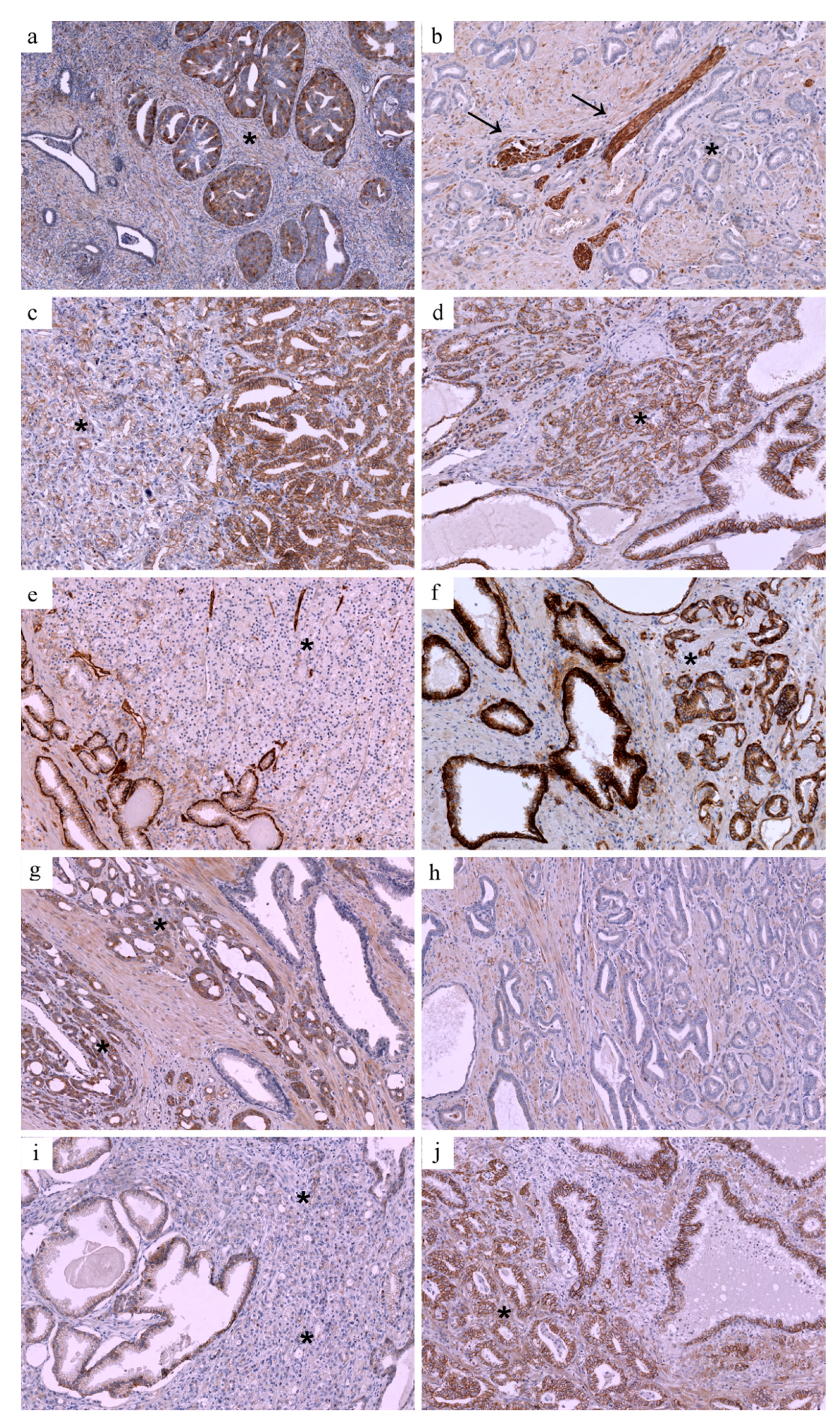
2.2. Immunohistochemical Validation
2.3. Statistical Analysis
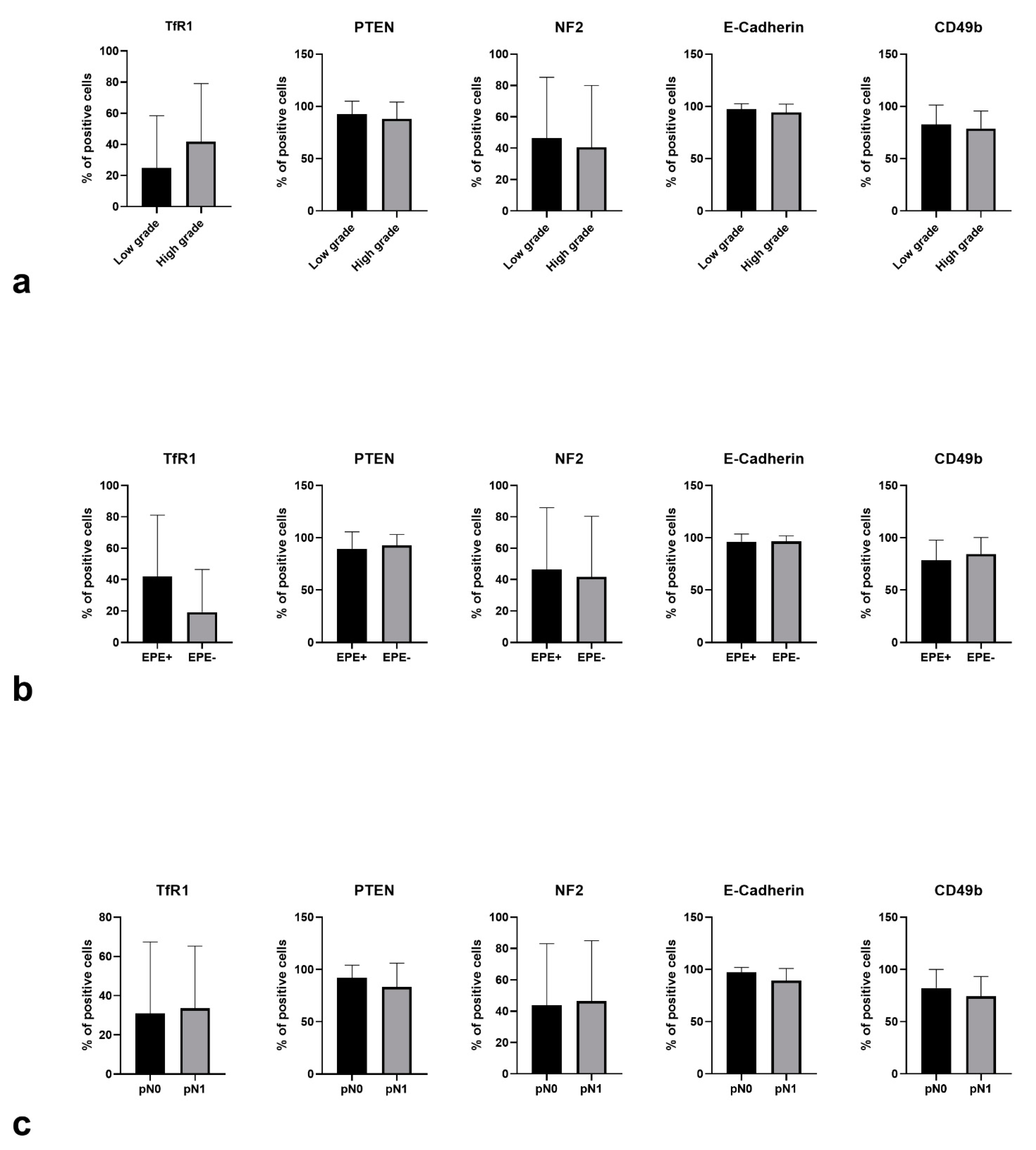
3. Results
3.1. Expression Patterns vs. Adverse Pathological Features
3.2. Multivariable Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moris, L.; Cumberbatch, M.G.; Van den Broeck, T.; Gandaglia, G.; Fossati, N.; Kelly, B.; Pal, R.; Briers, E.; Cornford, P.; De Santis, M.; et al. Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review. Eur. Urol. 2020, 77, 614–627. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Seisen, T.; Abdollah, F.; Briganti, A.; Fonteyne, V.; James, N.; Roach, M.; Thalmann, G.N.; Touijer, K.; Chen, R.C.; et al. A Systematic Review of the Role of Definitive Local Treatment in Patients with Clinically Lymph Node-positive Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 294–301. [Google Scholar] [CrossRef]
- Hinev, A.I.; Anakievski, D.; Kolev, N.H.; Hadjiev, V.I. Validation of nomograms predicting lymph node involvement in patients with prostate cancer undergoing extended pelvic lymph node dissection. Urol. Int. 2014, 92, 300–305. [Google Scholar] [CrossRef]
- Neill, M.G.; Louie-Johnsun, M.; Chabert, C.; Eden, C. Does intrafascial dissection during nerve-sparing laparoscopic radical prostatectomy compromise cancer control? BJU Int. 2009, 104, 1730–1733. [Google Scholar] [CrossRef]
- Sayyid, R.; Perlis, N.; Ahmad, A.; Evans, A.; Toi, A.; Horrigan, M.; Finelli, A.; Zlotta, A.; Kulkarni, G.; Hamilton, R.; et al. Development and external validation of a biopsy-derived nomogram to predict risk of ipsilateral extraprostatic extension. BJU Int. 2017, 120, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Ohori, M.; Kattan, M.W.; Koh, H.; Maru, N.; Slawin, K.M.; Shariat, S.; Muramoto, M.; Reuter, V.E.; Wheeler, T.M.; Scardino, P.T. Predicting the presence and side of extracapsular extension: A nomogram for staging prostate cancer. J. Urol. 2004, 171, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Chun, F.K.-H.; Salonia, A.; Gallina, A.; Farina, E.; Da Pozzo, L.F.; Rigatti, P.; Montorsi, F.; Karakiewicz, P.I. Validation of a nomogram predicting the probability of lymph node invasion based on the extent of pelvic lymphadenectomy in patients with clinically localized prostate cancer. BJU Int. 2006, 98, 788–793. [Google Scholar] [CrossRef]
- Bandini, M.; Marchioni, M.; Pompe, R.S.; Tian, Z.; Gandaglia, G.; Fossati, N.; Abdollah, F.; Graefen, M.; Montorsi, F.; Saad, F.; et al. First North American validation and head-to-head comparison of four preoperative nomograms for prediction of lymph node invasion before radical prostatectomy. BJU Int. 2018, 121, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Trapani, E.; Luzzago, S.; Peveri, G.; Catellani, M.; Ferro, M.; Cordima, G.; Mistretta, F.A.; Bianchi, R.; Cozzi, G.; Alessi, S.; et al. A novel nomogram predicting lymph node invasion among patients with prostate cancer: The importance of extracapsular extension at multiparametric magnetic resonance imaging. Urol Oncol. 2021, 39, 431.e15–431.e22. [Google Scholar] [CrossRef] [PubMed]
- Diamand, R.; Ploussard, G.; Roumiguié, M.; Oderda, M.; Benamran, D.; Fiard, G.; Quackels, T.; Assenmacher, G.; Simone, G.; Van Damme, J.; et al. External Validation of a Multiparametric Magnetic Resonance Imaging-based Nomogram for the Prediction of Extracapsular Extension and Seminal Vesicle Invasion in Prostate Cancer Patients Undergoing Radical Prostatectomy. Eur. Urol. 2021, 79, 180–185. [Google Scholar] [CrossRef]
- Gandaglia, G.; Martini, A.; Ploussard, G.; Fossati, N.; Stabile, A.; De Visschere, P.; Borgmann, H.; Heidegger, I.; Steinkohl, F.; Kretschmer, A.; et al. External Validation of the 2019 Briganti Nomogram for the Identification of Prostate Cancer Patients Who Should Be Considered for an Extended Pelvic Lymph Node Dissection. Eur. Urol. 2020, 78, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zapała, P.; Dybowski, B.; Bres-Niewada, E.; Lorenc, T.; Powała, A.; Lewandowski, Z.; Gołębiowski, M.; Radziszewski, P. Predicting side-specific prostate cancer extracapsular extension: A simple decision rule of PSA, biopsy, and MRI parameters. Int. Urol. Nephrol. 2019, 51, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapała, P.; Kozikowski, M. External validation of a magnetic resonance imaging-based algorithm for prediction of side-specific extracapsular exten-sion in prostate cancer. Cent. Eur. J. Urol. 2021, 74, 327–333. [Google Scholar]
- Watson, K.; Wang, C.; Yilmaz, A.; Bismar, T.A.; Trpkov, K. Use of immunohistochemistry in routine workup of prostate needle biopsies: A tertiary academic institution experience. Arch. Pathol. Lab. Med. 2013, 137, 541–545. [Google Scholar] [CrossRef]
- Picanço-Albuquerque, C.G.; Morais, C.L.; Carvalho, F.L.F.; Peskoe, S.B.; Hicks, J.L.; Ludkovski, O.; Vidotto, T.; Fedor, H.; Humphreys, E.; Han, M.; et al. In prostate cancer needle biopsies, detections of PTEN loss by fluorescence in situ hybridization (FISH) and by immunohistochemistry (IHC) are concordant and show consistent association with upgrading. Virchows Arch. Int. J. Pathol. 2016, 468, 607–617. [Google Scholar] [CrossRef]
- Verrill, C.; Cerundolo, L.; Mckee, C.; White, M.; Kartsonaki, C.; Fryer, E.; Morris, E.; Brewster, S.; Ratnayaka, I.; Marsden, L.; et al. Altered expression of epithelial-to-mesenchymal transition proteins in extraprostatic prostate cancer. Oncotarget 2016, 7, 1107–1119. [Google Scholar] [CrossRef] [Green Version]
- Maruta, S.; Sakai, H.; Kanda, S.; Hayashi, T.; Kanetake, H.; Miyata, Y. E1AF expression is associated with extra-prostatic growth and matrix metalloproteinase-7 expression in prostate cancer. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2009, 117, 791–796. [Google Scholar] [CrossRef]
- Lokman, U.; Erickson, A.M.; Vasarainen, H.; Rannikko, A.S.; Mirtti, T. PTEN Loss but Not ERG Expression in Diagnostic Biopsies Is Associated with Increased Risk of Progression and Adverse Surgical Findings in Men with Prostate Cancer on Active Surveillance. Eur. Urol. Focus. 2018, 4, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Hamid, A.A.; Gray, K.P.; Huang, Y.; Bowden, M.; Pomerantz, M.; Loda, M.; Sweeney, C.J. Loss of PTEN Expression Detected by Fluorescence Immunohistochemistry Predicts Lethal Prostate Cancer in Men Treated with Prostatectomy. Eur. Urol. Oncol. 2019, 2, 475–482. [Google Scholar] [CrossRef]
- Lotan, T.L.; Heumann, A.; Rico, S.D.; Hicks, J.; Lecksell, K.; Koop, C.; Sauter, G.; Schlomm, T.; Simon, R. PTEN loss detection in prostate cancer: Comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget 2017, 8, 65566–65576. [Google Scholar] [CrossRef]
- Harmon, S.A.; Gesztes, W.; Young, D.; Mehralivand, S.; McKinney, Y.; Sanford, T.; Sackett, J.; Cullen, J.; Rosner, I.L.; Srivastava, S.; et al. Prognostic Features of Biochemical Recurrence of Prostate Cancer Following Radical Prostatectomy Based on Multiparametric MRI and Immunohistochemistry Analysis of MRI-guided Biopsy Specimens. Radiology 2021, 299, 613–623. [Google Scholar] [CrossRef]
- Krohn, A.; Freudenthaler, F.; Harasimowicz, S.; Kluth, M.; Fuchs, S.; Burkhardt, L.; Stahl, P.; Tsourlakis, M.C.; Bauer, M.; Tennstedt, P.; et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod. Pathol. 2014, 27, 1612–1620. [Google Scholar] [CrossRef] [Green Version]
- Bedolla, R.; Prihoda, T.J.; Kreisberg, J.I.; Malik, S.N.; Krishnegowda, N.K.; Troyer, D.A.; Ghosh, P.M. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3860–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonarakis, E.S.; Keizman, D.; Zhang, Z.; Gurel, B.; Lotan, T.L.; Hicks, J.L.; Fedor, H.L.; Carducci, M.A.; De Marzo, A.M.; Eisenberger, M.A. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 2012, 118, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, T.U.; Pettersson, A.; Ebot, E.M.; Gerke, T.; Graff, R.E.; Morais, C.L.; Hicks, J.L.; Wilson, K.M.; Rider, J.R.; Sesso, H.D.; et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J. Natl. Cancer Inst. 2015, 108, djv346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraldeschi, R.; Nava Rodrigues, D.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Rescigno, P.; Ravi, P.; Pezaro, C.; Omlin, A.; Lorente, D.; et al. PTEN Protein Loss and Clinical Outcome from Castration-resistant Prostate Cancer Treated with Abiraterone Acetate. Eur. Urol. 2015, 67, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, A.; Zheng, R.; Shen, R.; Nanus, D.M. Inactivation of the NF2 tumor suppressor protein merlin in DU145 prostate cancer cells. Prostate 2008, 68, 975–984. [Google Scholar] [CrossRef]
- Van Slambrouck, S.; Jenkins, A.R.; Romero, A.E.; Steelant, W.F.A. Reorganization of the integrin alpha2 subunit controls cell adhesion and cancer cell invasion in prostate cancer. Int. J. Oncol. 2009, 34, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Ziaee, S.; Chung, L.W.K. Induction of integrin α2 in a highly bone metastatic human prostate cancer cell line: Roles of RANKL and AR under three-dimensional suspension culture. Mol. Cancer 2014, 13, 208. [Google Scholar] [CrossRef] [Green Version]
- Colombel, M.; Eaton, C.L.; Hamdy, F.; Ricci, E.; van der Pluijm, G.; Cecchini, M.; Mege-Lechevallier, F.; Clezardin, P.; Thalmann, G. Increased expression of putative cancer stem cell markers in primary prostate cancer is associated with progression of bone metastases. Prostate 2012, 72, 713–720. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Ukai, R.; Johnston, D.; Troncoso, P.; Fidler, I.J.; Pettaway, C.A. The relative mRNA expression levels of matrix metalloproteinase to E-cadherin in prostate biopsy specimens distinguishes organ-confined from advanced prostate cancer at radical prostatectomy. Clin. Cancer Res. 2003, 9, 2185–2194. [Google Scholar]
- Richmond, P.J.; Karayiannakis, A.J.; Nagafuchi, A.; Kaisary, A.V.; Pignatelli, M. Aberrant E-cadherin and alpha-catenin expression in prostate cancer: Correlation with patient survival. Cancer Res. 1997, 57, 3189–3193. [Google Scholar]
- Aaltomaa, S.; Lipponen, P.; Viitanen, J.; Kankkunen, J.P.; Ala-Opas, M.; Kosma, V.M. Prognostic value of CD44 standard, variant isoforms 3 and 6 and -catenin expression in local prostate cancer treated by radical prostatectomy. Eur. Urol. 2000, 38, 555–562. [Google Scholar] [CrossRef]
- Aaltomaa, S.; Lipponen, P.; Ala-Opas, M.; Eskelinen, M.; Kosma, V.M. Alpha-catenin expression has prognostic value in local and locally advanced prostate cancer. Br. J. Cancer 1999, 80, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.; Lobo, J.; Antunes, L.; Lopes, P.; Jerónimo, C.; Henrique, R. Differential expression of E-cadherin and P-cadherin in pT3 prostate cancer: Correlation with clinical and pathological features. Virchows Arch. Int. J. Pathol. 2018, 473, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Manz, D.H.; Torti, S.V.; Torti, F.M. Iron-responsive element-binding protein 2 plays an essential role in regulating prostate cancer cell growth. Oncotarget 2017, 8, 82231–82243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keer, H.N.; Kozlowski, J.M.; Tsai, Y.C.; Lee, C.; McEwan, R.N.; Grayhack, J.T. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J. Urol. 1990, 143, 381–385. [Google Scholar] [CrossRef]
- Herman, J.G.; Meadows, G.G. Transferrin reverses the anti-invasive activity of human prostate cancer cells that overexpress sema3E. Int. J. Oncol. 2007, 31, 1267–1272. [Google Scholar]
| Univariate | Multivariate | ||
|---|---|---|---|
| Proteins preselected from TCGA development subset * | p | OR (95% CI) | p |
| CD49b | 0.0001 | 0.06 (0.01–0.41) | 0.0008 |
| E-Cadherin | 0.0036 | ||
| Heregulin | 0.0274 | ||
| NF2 | <0.0001 | 0.17 (0.04–0.67) | <0.0001 |
| PTEN | 0.0015 | 0.50 (0.27–0.94) | 0.0287 |
| TfR1 | 0.0001 | 1.83 (1.11–3.03) | 0.0137 |
| Variable | Number (%) | |
|---|---|---|
| <10 ng/mL | 26 (49.06%) | |
| PSA | 10–20 ng/mL | 19 (35.85%) |
| >20 ng/mL | 8 (15.09%) | |
| I | 14 (27.45%) | |
| II | 15 (29.41%) | |
| Biopsy prognostic group | III | 12 (23.53%) |
| IV | 8 (15.69%) | |
| V | 2 (3.92%) | |
| T1c | 21 (41.18%) | |
| T2a | 19 (37.25%) | |
| cT | T2b | 4 (7.84%) |
| T2c | 6 (11.76%) | |
| T3 | 1 (1.96%) |
| Variable | Number (%) | p | ||
|---|---|---|---|---|
| TCGA cohort | Validation cohort | |||
| Postprostatectomy prognostic group | I | 44 (8.87%) | 3 (5.66%) | 0.22 |
| II | 148 (29.84%) | 16 (30.19%) | ||
| III | 101 (20.36%) | 14 (26.42%) | ||
| IV | 64 (12.9%) | 11 (20.75%) | ||
| V | 139 (28.02%) | 9 (16.98%) | ||
| Nodal involvement | 79 (18.63%) | 7 (13.21%) | 0.44 | |
| Extracapsular extension | 158 (31.79%) | 14 (26.42%) | 0.53 | |
| Seminal vesicle invasion | 134 (26.96%) | 14 (26.42%) | 0.99 | |
| Positive surgical margins | 151 (36.26%) | 26 (49.06%) | 0.038 | |
| (a) Extraprostatic Disease (AUROC = 0.879) | |||
| Variable | OR (95% CI) | p | |
| TfR1 status | Heterogenic | 7.54 (0.95–59.65) | 0.015 |
| Positive | 13.74 (1.48–127.54) | ||
| CD49b status | Reduced | 10.15 (1.37–75.43) | 0.013 |
| PSA (ng/mL) | 1.29 (1.11–1.51) | 0.013 | |
| (b) nodal involvement (AUROC = 0.888) | |||
| e-cadherin status | Reduced | 10.22 (0.74–142.11) | 0.005 |
| CD49b status | Reduced | 24.44 (0.79–756.37) | 0.017 |
| PSA (ng/mL) | 1.18 (0.96–1.45) | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapała, P.; Fus, Ł.; Lewandowski, Z.; Garbas, K.; Zapała, Ł.; Górnicka, B.; Radziszewski, P. E-Cadherin, Integrin Alpha2 (Cd49b), and Transferrin Receptor-1 (Tfr1) Are Promising Immunohistochemical Markers of Selected Adverse Pathological Features in Patients Treated with Radical Prostatectomy. J. Clin. Med. 2021, 10, 5587. https://doi.org/10.3390/jcm10235587
Zapała P, Fus Ł, Lewandowski Z, Garbas K, Zapała Ł, Górnicka B, Radziszewski P. E-Cadherin, Integrin Alpha2 (Cd49b), and Transferrin Receptor-1 (Tfr1) Are Promising Immunohistochemical Markers of Selected Adverse Pathological Features in Patients Treated with Radical Prostatectomy. Journal of Clinical Medicine. 2021; 10(23):5587. https://doi.org/10.3390/jcm10235587
Chicago/Turabian StyleZapała, Piotr, Łukasz Fus, Zbigniew Lewandowski, Karolina Garbas, Łukasz Zapała, Barbara Górnicka, and Piotr Radziszewski. 2021. "E-Cadherin, Integrin Alpha2 (Cd49b), and Transferrin Receptor-1 (Tfr1) Are Promising Immunohistochemical Markers of Selected Adverse Pathological Features in Patients Treated with Radical Prostatectomy" Journal of Clinical Medicine 10, no. 23: 5587. https://doi.org/10.3390/jcm10235587
APA StyleZapała, P., Fus, Ł., Lewandowski, Z., Garbas, K., Zapała, Ł., Górnicka, B., & Radziszewski, P. (2021). E-Cadherin, Integrin Alpha2 (Cd49b), and Transferrin Receptor-1 (Tfr1) Are Promising Immunohistochemical Markers of Selected Adverse Pathological Features in Patients Treated with Radical Prostatectomy. Journal of Clinical Medicine, 10(23), 5587. https://doi.org/10.3390/jcm10235587






