Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Randomization
2.3. Procedure
2.4. Data Collection
2.5. Outcomes
2.6. Statistical Analysis
2.6.1. Sample Size Calculation
2.6.2. Statistical Methods
3. Results
3.1. Patients
3.2. Patients’ Characteristics at Entry
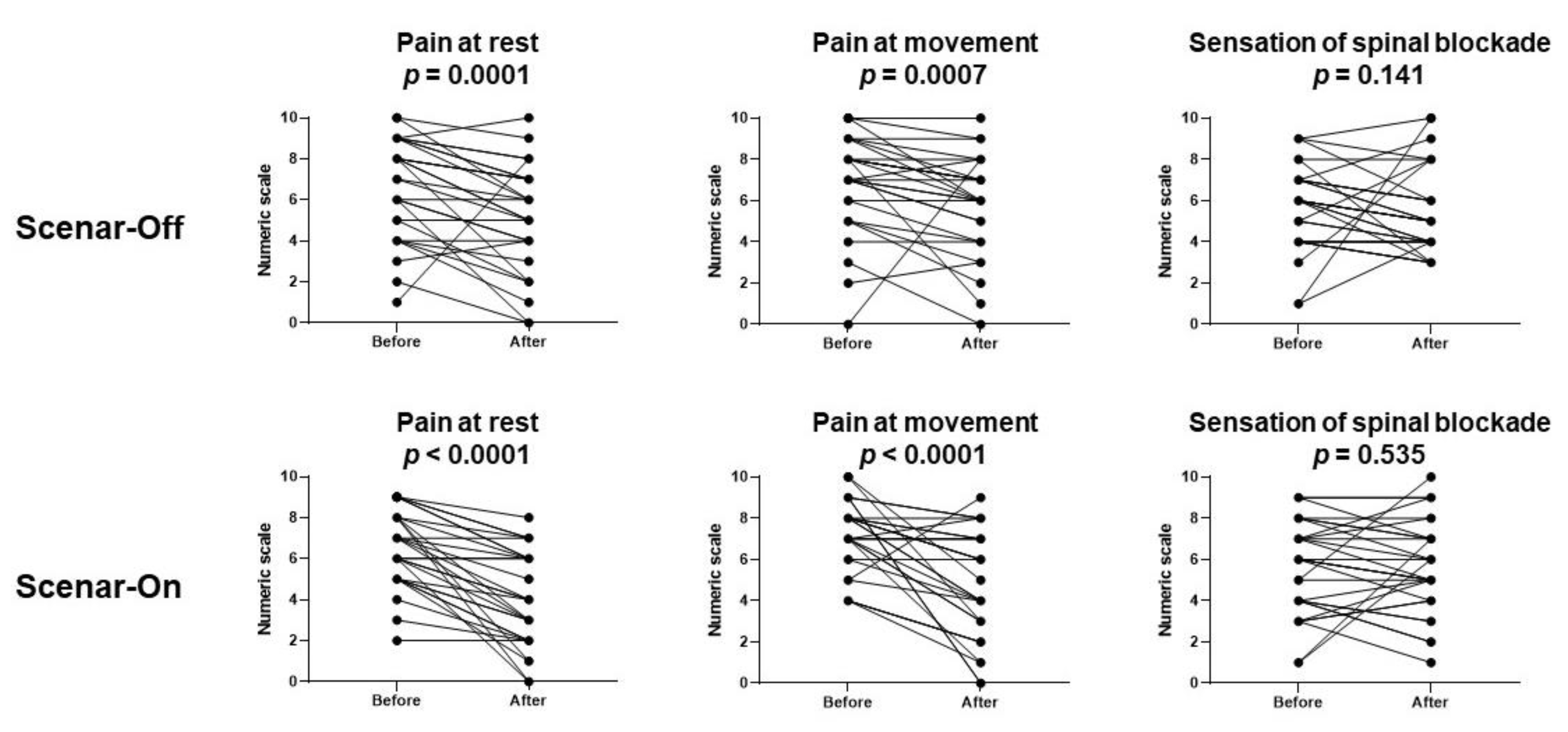
3.3. Effects of the ScenarTM Sessions
3.4. Adverse Effects or Events
3.5. Follow-Up
4. Discussion
Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.L.; Bonello, R.; Pollard, H. The effectiveness of ENAR for the treatment of chronic neck pain in Australian adults: A preliminary single-blind, randomised controlled trial. Chiropr. Osteopat. 2007, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selfe, T.K.; Bourguignon, C.; Taylor, A.G. Effects of noninvasive interactive neurostimulation on symptoms of osteoarthritis of the knee: A randomized, sham-controlled pilot study. J. Altern. Complement. Med. 2008, 14, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Cannan, A.; Mullens, R.; Dunphy, M.; Pearson, T.; Lau, C.; Chipchase, L.S. The effect of interactive neurostimulation therapy on myofascial trigger points associated with mechanical neck pain: A preliminary randomized, sham-controlled trial. J. Altern. Complement. Med. 2012, 18, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Han, I. A comparative study of the efficacy between self-controlled energo-neuro-adaptive regulator and transcutaneous electrical nerve stimulation for whiplash injury. Nerve 2016, 2, 33–37. [Google Scholar] [CrossRef]
- Udina-Cortés, C.; Fernández-Carnero, J.; Romano, A.A.; Cuenca-Zaldívar, J.N.; Villafañe, J.H.; Castro-Marrero, J.; Alguacil-Diego, I.M. Effects of neuro-adaptive electrostimulation therapy on pain and disability in fibromyalgia: A prospective, randomized, double-blind study. Medicine 2020, 99, e23785. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bonello, R.P.; Cohen, M.; Reece, J.; Aggarwal, A.; Rigney, C. A postmarket surveillance study on electro-neuro-adaptive-regulator therapy. Evid. Based Complement. Alternat. Med. 2014, 2014, 341256. [Google Scholar] [CrossRef] [PubMed]
- Vigotsky, A.D.; Bruhns, R.P. The role of descending modulation in manual therapy and its analgesic implications: A narrative review. Pain Res. Treat. 2015, 2015, 292805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscemi, A.; Martino, S.; Scire Campisi, S.; Rapisarda, A.; Coco, M. Endocannabinoids release after Osteopathic Manipulative Treatment. A brief review. J. Complement. Integr. Med. 2020, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Finniss, D.G. Placebo effects: Historical and modern evaluation. Int. Rev. Neurobiol. 2018, 139, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.V.; Hatsiopoulou, O.; Koch, T.; Berbaum, K.; Lutgendorf, S.; Kettenmann, E.; Logan, H.; Kaptchuk, T.J. Can words hurt? Patient-provider interactions during invasive procedures. Pain 2005, 114, 303–309. [Google Scholar] [CrossRef] [PubMed]
| Scenar-Off Group (n = 30) | Scenar-On Group (n = 30) | p | |
|---|---|---|---|
| Age (years) | 48 ± 14 | 51 ± 13 | 0.41 |
| Sex female | 23 (76.7%) | 23 (76.7%) | 1 |
| Body Mass Index | 26.5 (6.2) | 25.0 (5.0) | 0.13 |
| Pain | |||
| More than 3 months of pain * | 16 (53.3%) | 12 (40.0%) | 0.48 |
| Location | 0.60 | ||
| Lumbar pain | 15 (50.0%) | 18 (60.0%) | |
| Cervical pain | 15 (50.0%) | 12 (40.0%) | |
| Lumbar pain irradiation | 0.73 | ||
| No irradiation | 7 (23.3%) | 7 (23.3%) | |
| Irradiation | 8 (26.7%) | 11 (36.7%) | |
| Cervical pain irradiation | 1 | ||
| No irradiation | 3 (10.0%) | 2 (6.7%) | |
| Irradiation | 12 (40.0%) | 10 (33.3%) | |
| Concomitant analgesics | |||
| WHO analgesic level 1 * | 18 (60.0%) | 13 (43.3%) | 0.30 |
| WHO analgesic level 2 * | 14 (46.7%) | 10 (33.3%) | 0.43 |
| WHO analgesic level 3 * | 1 (3.3%) | 1 (3.33%) | 1 |
| Benzodiazepine | 11 (36.7%) | 6 (20.0%) | 0.25 |
| Antidepressant | 11 (36.7%) | 7 (23.3%) | 0.40 |
| Antiepileptic | 7 (23.3%) | 3 (10.0%) | 0.30 |
| Lidocaine 5% medicated plaster | 3 (10.0%) | 0 (0.0%) | 0.24 |
| NSAIDS | 10 (33.3%) | 5 (16.7%) | 0.23 |
| Other | 1 (3.3%) | 0 (0%) | 1 |
| Complementary therapies | |||
| Transcutaneous electrical nerve stimulation | 8 (26.7%) | 5 (16.7%) | 0.53 |
| Auriculotherapy | 2 (6.7%) | 2 (6.7%) | 1 |
| Acupuncture | 0 (0%) | 2 (6.7%) | 0.49 |
| Physiotherapy | 14 (46.7%) | 17 (56.7%) | 0.61 |
| Psychocorporal techniques (relaxation, hypnosis and mindfulness) | 7 (23.3%) | 6 (20.0%) | 1 |
| Pain ** | |||
| At rest | 8 (4) | 7 (3) | 0.51 |
| During movement | 8 (2) | 7 (2) | 0.96 |
| Anxiety *** | 5 (6) | 4 (5) | 0.15 |
| Scenar-Off Group (n = 30) | Scenar-On Group(n = 30) | Mixed Model | |||
|---|---|---|---|---|---|
| Group Effect | Time Effect | Group-Time Effect | |||
| Pain at rest * | F = 2.527 p = 0.12 | F = 36.445 p < 0.0001 | F = 1.910 p = 0.15 | ||
| Basal | 8 (4) | 7 (3) | |||
| Postprocedure | 5 (3) | 4 (4) | |||
| Day 1 | 6 (4) | 4 (4) | |||
| Pain at movement * | F = 1.742 p = 0.19 | F = 17.981 p < 0.0001 | F = 2.613 p = 0.08 | ||
| Basal | 8 (2) | 7 (2) | |||
| Postprocedure | 6 (4) | 5 (4) | |||
| Day 1 | 7 (4) | 6 (4) | |||
| Stiffness ** | F = 0.437 p = 0.51 | F = 2.860 p = 0.44 | F = 0.711 p = 0.49 | ||
| Basal | 6 (3) | 6 (3) | |||
| Postprocedure | 5 (3) | 6 (3) | |||
| Day 1 | 5 (3) | 5 (2) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel-Cherqui, M.; Guirimand, A.; Szekely, B.; Kennel, T.; Fischler, M.; Le Guen, M. Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial. J. Clin. Med. 2021, 10, 5570. https://doi.org/10.3390/jcm10235570
Michel-Cherqui M, Guirimand A, Szekely B, Kennel T, Fischler M, Le Guen M. Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial. Journal of Clinical Medicine. 2021; 10(23):5570. https://doi.org/10.3390/jcm10235570
Chicago/Turabian StyleMichel-Cherqui, Mireille, Avit Guirimand, Barbara Szekely, Titouan Kennel, Marc Fischler, and Morgan Le Guen. 2021. "Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial" Journal of Clinical Medicine 10, no. 23: 5570. https://doi.org/10.3390/jcm10235570
APA StyleMichel-Cherqui, M., Guirimand, A., Szekely, B., Kennel, T., Fischler, M., & Le Guen, M. (2021). Effects of a Single Application of ScenarTM, a Low-Frequency Modulated Electric Current Therapy, for Pain Relief in Patients with Low Back and Neck Pain: A Randomized Single Blinded Trial. Journal of Clinical Medicine, 10(23), 5570. https://doi.org/10.3390/jcm10235570






