Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan
Abstract
:1. Introduction
2. Patients and Methods
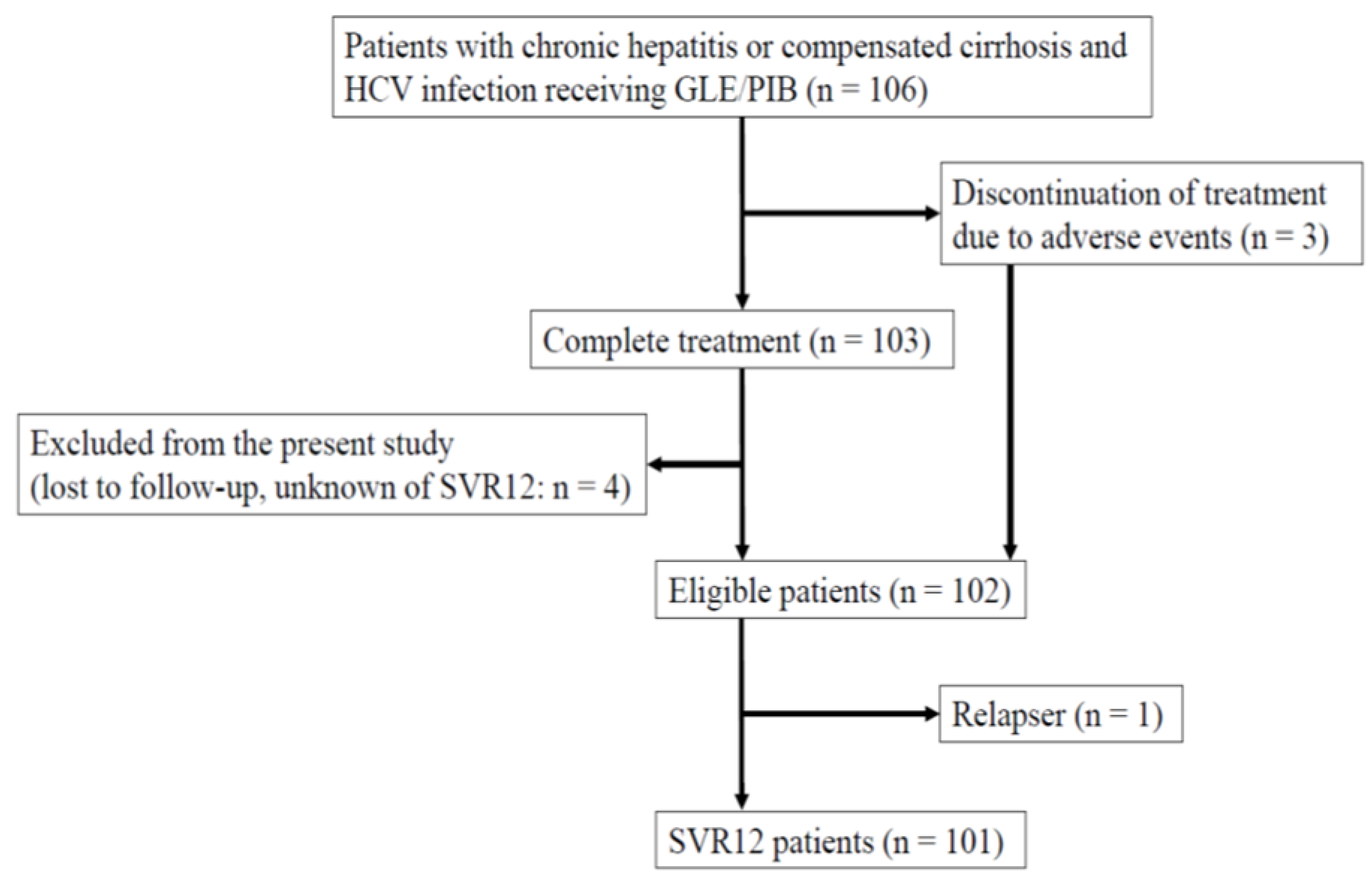
2.1. Study Design and Patients
2.2. Serum Biochemical Tests and Hematological Tests
2.3. Measurement of HCV RNA Levels and Determination of HCV Genotypes
2.4. Assessment of Advanced Liver Fibrosis and Diagnosis of Cirrhosis and HCC
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
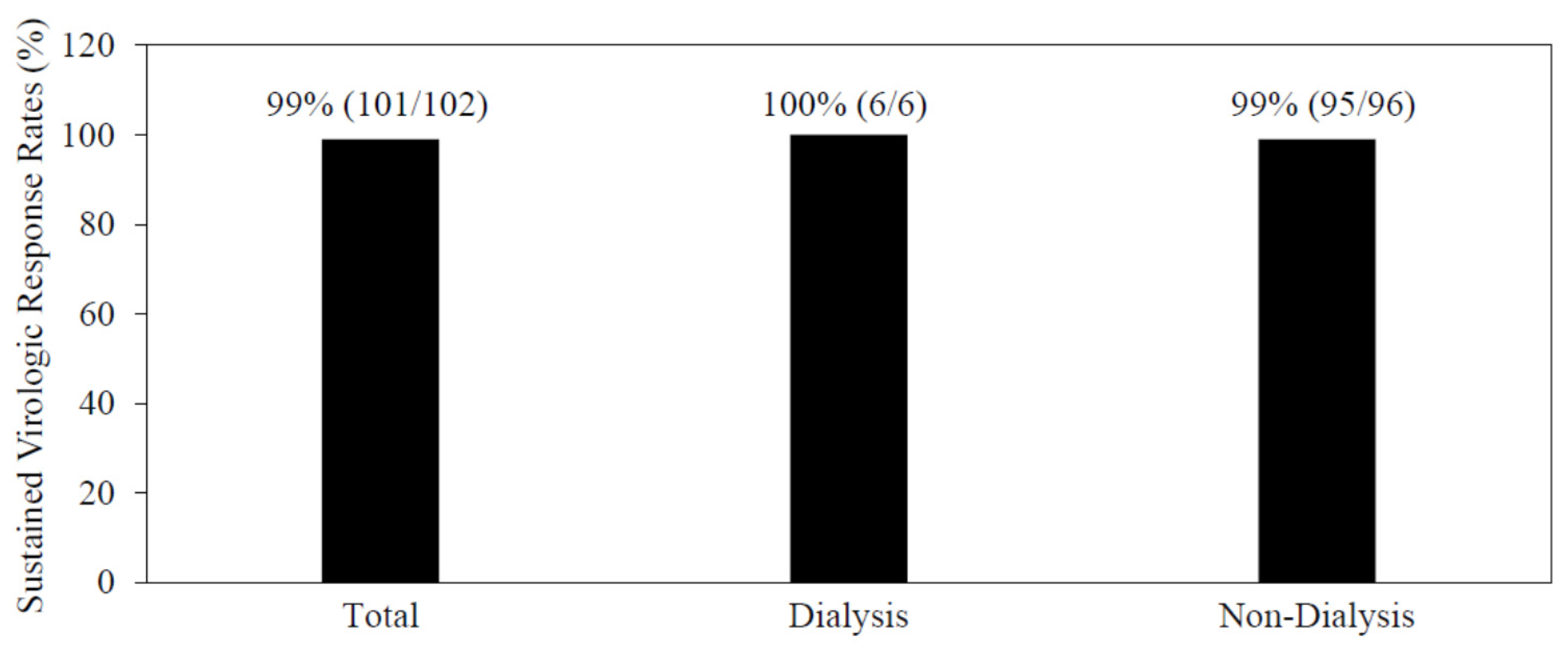
3.2. The Efficacy and Safety of the 8- or 12-Week Combination Treatment of Glecaprevir/Pibrentasvir
3.3. Twelve-Week Combination of Glecaprevir/Pibrentasvir for DAA-Failure Patients
3.4. Combination Treatment of Glecaprevir/Pibrentasvir for Patients Undergoing Artificial Dialysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef]
- Tada, T.; Toyoda, H.; Yasuda, S.; Miyake, N.; Kumada, T.; Kurisu, A.; Ohisa, M.; Akita, T.; Tanaka, J. Natural history of liver-related disease in patients with chronic hepatitis C virus infection: An analysis using a Markov chain model. J. Med. Virol. 2019, 91, 1837–1844. [Google Scholar] [CrossRef]
- Mizokami, M.; Yokosuka, O.; Takehara, T.; Sakamoto, N.; Korenaga, M.; Mochizuki, H.; Nakane, K.; Enomoto, H.; Ikeda, F.; Yanase, M.; et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: An open-label, randomised, phase 3 trial. Lancet. Infect. Dis. 2015, 15, 645–653. [Google Scholar] [CrossRef]
- Kumada, H.; Suzuki, Y.; Karino, Y.; Chayama, K.; Kawada, N.; Okanoue, T.; Itoh, Y.; Mochida, S.; Toyoda, H.; Yoshiji, H.; et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: A randomized phase II/III study. J. Gastroenterol. 2017, 52, 520–533. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Ooka, Y.; Ogasawara, S.; Chiba, T.; Saito, T.; Haga, Y.; et al. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int. J. Mol. Sci. 2017, 18, 906. [Google Scholar] [CrossRef]
- Kaneko, T.; Kanda, T.; Nirei, K.; Matsumoto, N.; Yamazaki, M.; Shibata, T.; Tamura, A.; Ogawa, M.; Nakajima, N.; Matsuoka, S.; et al. Follow-up Results of HCV GT2 Patients After Sofosbuvir/Ribavirin Therapy: Careful Attention to Occurrence of HCC. Anticancer Res. 2019, 39, 3855–3862. [Google Scholar] [CrossRef] [Green Version]
- Zeuzem, S.; Foster, G.R.; Wang, S.; Asatryan, A.; Gane, E.; Feld, J.J.; Asselah, T.; Bourlière, M.; Ruane, P.J.; Wedemeyer, H.; et al. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 2018, 378, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.I.; Krishnan, P.; Pilot-Matias, T.; Kati, W.; Schnell, G.; Beyer, J.; Reisch, T.; Lu, L.; Dekhtyar, T.; Irvin, M.; et al. In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS5A Inhibitor Pibrentasvir. Antimicrob. Agents Chemother. 2017, 61, e02558-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.I.; Tripathi, R.; Reisch, T.; Lu, L.; Middleton, T.; Hopkins, T.A.; Pithawalla, R.; Irvin, M.; Dekhtyar, T.; Krishnan, P.; et al. In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS3/4A Protease Inhibitor Glecaprevir. Antimicrob. Agents Chemother. 2017, 62, e01620-17. [Google Scholar] [CrossRef] [Green Version]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Bräu, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef]
- Izumi, N.; Takehara, T.; Chayama, K.; Yatsuhashi, H.; Takaguchi, K.; Ide, T.; Kurosaki, M.; Ueno, Y.; Toyoda, H.; Kakizaki, S.; et al. Sofosbuvir-velpatasvir plus ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol. Int. 2018, 12, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Nirei, K.; Kanda, T.; Masuzaki, R.; Mizutani, T.; Moriyama, M. Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients. Medicina 2021, 57, 761. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tsukiyama-Kohara, K.; Yamaguchi, K.; Yagi, S.; Tanaka, S.; Hasegawa, A.; Ohta, Y.; Hattori, N.; Kohara, M. Significance of specific antibody assay for genotyping of hepatitis C virus. Hepatology 1994, 19, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Ohno, O.; Mizokami, M.; Wu, R.R.; Saleh, M.G.; Ohba, K.; Orito, E.; Mukaide, M.; Williams, R.; Lau, J.Y. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J. Clin. Microbiol. 1997, 35, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Haga, Y.; Sasaki, R.; Wu, S.; Nakamoto, S.; Imazeki, F.; et al. Daclatasvir plus Asunaprevir Treatment for Real-World HCV Genotype 1-Infected Patients in Japan. Int. J. Med. Sci. 2016, 13, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Nirei, K.; Nakamura, H.; Matsuoka, S.; Yamana, Y.; Yoda, S.; Hirayama, A.; Moriyama, M. Ventricular Tachycardia as a Complication of Ledipasvir and Sofosbuvir Treatment for HCV Infection. Intern. Med. 2017, 56, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL clinical practice recommendation: How to treat HCV-infected patients with renal impairment? Hepatol. Int. 2019, 13, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Ghany, M.G.; Morgan, T.R.; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Lau, G.; Benhamou, Y.; Chen, G.; Li, J.; Shao, Q.; Ji, D.; Li, F.; Li, B.; Liu, J.; Hou, J.; et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: A phase 2, open-label, proof-of-concept study. Lancet Gastroenterol. Hepatol. 2016, 1, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Kumada, H.; Watanabe, T.; Suzuki, F.; Ikeda, K.; Sato, K.; Toyoda, H.; Atsukawa, M.; Ido, A.; Takaki, A.; Enomoto, N.; et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J. Gastroenterol. 2018, 53, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, J.M.; Pham, L.V.; Mikkelsen, L.S.; Ghanem, L.; Ramirez, S.; Scheel, T.K.H.; Carlsen, T.H.R.; Bukh, J. Efficacy of NS5A Inhibitors Against Hepatitis C Virus Genotypes 1-7 and Escape Variants. Gastroenterology 2018, 154, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.H.; O’Boyle, D.R., 2nd; Nower, P.; Valera, L.; Roberts, S.; Fridell, R.A.; Gao, M. Persistence of resistant variants in hepatitis C virus-infected patients treated with the NS5A replication complex inhibitor daclatasvir. Antimicrob. Agents Chemother. 2013, 57, 2054–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, A.; Hikita, H.; Sakamori, R.; Tahata, Y.; Kai, Y.; Yamada, R.; Yakushijin, T.; Mita, E.; Ohkawa, K.; Imai, Y.; et al. Nonstructural protein 5A/P32 deletion after failure of ledipasvir/sofosbuvir in hepatitis C virus genotype 1b infection. Hepatology 2018, 68, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Uemura, H.; Uchida, Y.; Kouyama, J.I.; Naiki, K.; Tsuji, S.; Sugawara, K.; Nakao, M.; Motoya, D.; Nakayama, N.; Imai, Y.; et al. NS5A-P32 deletion as a factor involved in virologic failure in patients receiving glecaprevir and pibrentasvir. J. Gastroenterol. 2019, 54, 459–470. [Google Scholar] [CrossRef]
- Wei, L.; Wang, G.; Alami, N.N.; Xie, W.; Heo, J.; Xie, Q.; Zhang, M.; Kim, Y.J.; Lim, S.G.; Fredrick, L.M.; et al. Glecaprevir-pibrentasvir to treat chronic hepatitis C virus infection in Asia: Two multicentre, phase 3 studies- a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol. Hepatol. 2020, 5, 839–849. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Toyoda, H.; Takaguchi, K.; Nakamuta, M.; Watanabe, T.; Michitaka, K.; Ikegami, T.; Nozaki, A.; Uojima, H.; et al. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment: A prospective, multicenter study. Aliment. Pharmacol. Ther. 2019, 49, 1230–1241. [Google Scholar] [CrossRef]
- Okuda, K.; Hayashi, H.; Yokozeki, K.; Kobayashi, S.; Kashima, T.; Irie, Y. Acute hepatitis C among renal failure patients on chronic haemodialysis. J. Gastroenterol. Hepatol. 1998, 13, 62–67. [Google Scholar] [CrossRef]
- Sugiura, A.; Joshita, S.; Umemura, T.; Yamazaki, T.; Fujimori, N.; Kimura, T.; Matsumoto, A.; Igarashi, K.; Usami, Y.; Wada, S.; et al. Past history of hepatocellular carcinoma is an independent risk factor of treatment failure in patients ith chronic hepatitis C virus infection receiving direct-acting antivirals. J. Viral Hepat. 2018, 25, 1462–1471. [Google Scholar] [CrossRef]
- Takehara, T.; Sakamoto, N.; Nishiguchi, S.; Ikeda, F.; Tatsumi, T.; Ueno, Y.; Yatsuhashi, H.; Takikawa, Y.; Kanda, T.; Sakamoto, M.; et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: An open-label phase 3 trial. J. Gastroenterol. 2019, 54, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Mauss, S.; Persico, M.; Barclay, S.T.; Marx, S.; Lohmann, K.; Bondin, M.; Zhang, Z.; Marra, F.; Belperio, P.S.; et al. Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis. Adv. Ther. 2020, 37, 4033–4042. [Google Scholar] [CrossRef]

| Characteristics | All (n = 102) |
|---|---|
| Age (years) | 62.7 ± 12.1 |
| Gender (male/female) | 41/61 |
| Interferon (naïve/experienced) | 88/14 |
| DAAs (naïve/experienced) | 93/9 |
| HCV genotypes (1/2/3) | 54/45/3 |
| Pretreatment HCV RNA (LIU/mL) | 6.0 ± 1.2 |
| Body weight (kg) | 58.0 ± 12.9 |
| Body length (m) | 1.60 ± 0.10 |
| History of HCC (+/−) | 5/97 |
| Chronic hepatitis/cirrhosis | 74/28 |
| Liver stiffness (kPa) | 9.9 ± 7.9 |
| AST (IU/L) | 50.7 ± 30.4 |
| ALT (IU/L) | 51.4 ± 39.1 |
| Hemoglobin (g/dL) | 13.5 ± 1.6 |
| Platelets (×104/μL) | 17.6 ± 6.3 |
| eGFR (mL/min/1.73 m2) | 67.6 ± 26.8 |
| Characteristics | A Relapser at Week 12 after Treatment |
|---|---|
| Age (years) | 65 |
| Gender | Male |
| Interferon | Naive |
| Interferon-free DAAs | Naive |
| HCV genotypes | 1b |
| Pretreatment HCV RNA (LIU/mL) | 5.4 |
| Body weight (kg) | 51 |
| Body length (m) | 1.58 |
| History of HCC | No |
| Chronic hepatitis or cirrhosis | Chronic hepatitis |
| Liver stiffness (kPa) | 7.9 |
| AST (IU/L) | 91 |
| ALT (IU/L) | 80 |
| Hemoglobin (g/dL) | 14.1 |
| Platelets (×104/μL) | 23.8 |
| eGFR (mL/min/1.73 m2) | 64.4 |
| Adherence > 80% | Yes |
| * NS5A-L31 | Wild |
| * NS5A-Y93 | Wild |
| Characteristics | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 |
|---|---|---|---|---|---|---|
| Age (years) | 82 | 84 | 55 | 57 | 56 | 64 |
| Gender | Male | Female | Male | Male | Male | Male |
| Interferon | Experienced | Naive | Naive | Naive | Naive | Naive |
| Interferon-free DAAs | Naive | Naive | Naive | Naive | Naive | Naive |
| HCV GTs | 1b | 1b | 2b | 2a | 2 | 2b |
| Pretreatment HCV RNA (LIU/mL) | 6.8 | 6.3 | 4.8 | 3.9 | 3.3 | 5.3 |
| Body weight (kg) | 58.4 | 36.5 | 88.4 | 67.5 | 71.9 | 64.5 |
| Body length (m) | 1.60 | 1.48 | 1.73 | 169 | 1.79 | 1.64 |
| History of HCC | No | No | No | No | No | No |
| CH or LC | LC | CH | CH | CH | CH | CH |
| Liver stiffness (kPa) | 13.6 | 8.3 | 11.5 | 11.8 | 6.1 | 4.4 |
| AST (IU/L) | 50 | 22 | 72 | 27 | 15 | 16 |
| ALT (IU/L) | 63 | 10 | 80 | 24 | 17 | 13 |
| Hemoglobin (g/dL) | 14.2 | 8.8 | 9.2 | 10.4 | 13.7 | 10.4 |
| Platelets (x 104/μL) | 18.2 | 14.3 | 16.1 | 17.3 | 18.6 | 15.5 |
| eGFR (mL/min/1.73 m2) | 7.5 | 7.9 | 3.8 | 5 | 5 | 6.4 |
| Type of dialysis | HD | HD | PD | HD | HD | HD |
| Duration of dialysis (years) | 0.5 | 3.5 | 2 | 4.5 | 5 | 7 |
| DM | No | Yes | Yes | Yes | Yes | Yes |
| Number of drugs under treatment | 8 | 13 | 17 | 12 | 10 | 15 |
| Nalfurafine hydrochloride | Yes | Yes | Yes | Yes | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamana, Y.; Kanda, T.; Matsumoto, N.; Honda, M.; Kumagawa, M.; Sasaki, R.; Kanezawa, S.; Mizutani, T.; Yamagami, H.; Masuzaki, R.; et al. Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan. J. Clin. Med. 2021, 10, 5529. https://doi.org/10.3390/jcm10235529
Yamana Y, Kanda T, Matsumoto N, Honda M, Kumagawa M, Sasaki R, Kanezawa S, Mizutani T, Yamagami H, Masuzaki R, et al. Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan. Journal of Clinical Medicine. 2021; 10(23):5529. https://doi.org/10.3390/jcm10235529
Chicago/Turabian StyleYamana, Yoichiro, Tatsuo Kanda, Naoki Matsumoto, Masayuki Honda, Mariko Kumagawa, Reina Sasaki, Shini Kanezawa, Taku Mizutani, Hiroaki Yamagami, Ryota Masuzaki, and et al. 2021. "Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan" Journal of Clinical Medicine 10, no. 23: 5529. https://doi.org/10.3390/jcm10235529
APA StyleYamana, Y., Kanda, T., Matsumoto, N., Honda, M., Kumagawa, M., Sasaki, R., Kanezawa, S., Mizutani, T., Yamagami, H., Masuzaki, R., Ishii, T., Nirei, K., & Moriyama, M. (2021). Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan. Journal of Clinical Medicine, 10(23), 5529. https://doi.org/10.3390/jcm10235529








