Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function
Abstract
1. Introduction
2. Materials and Methods
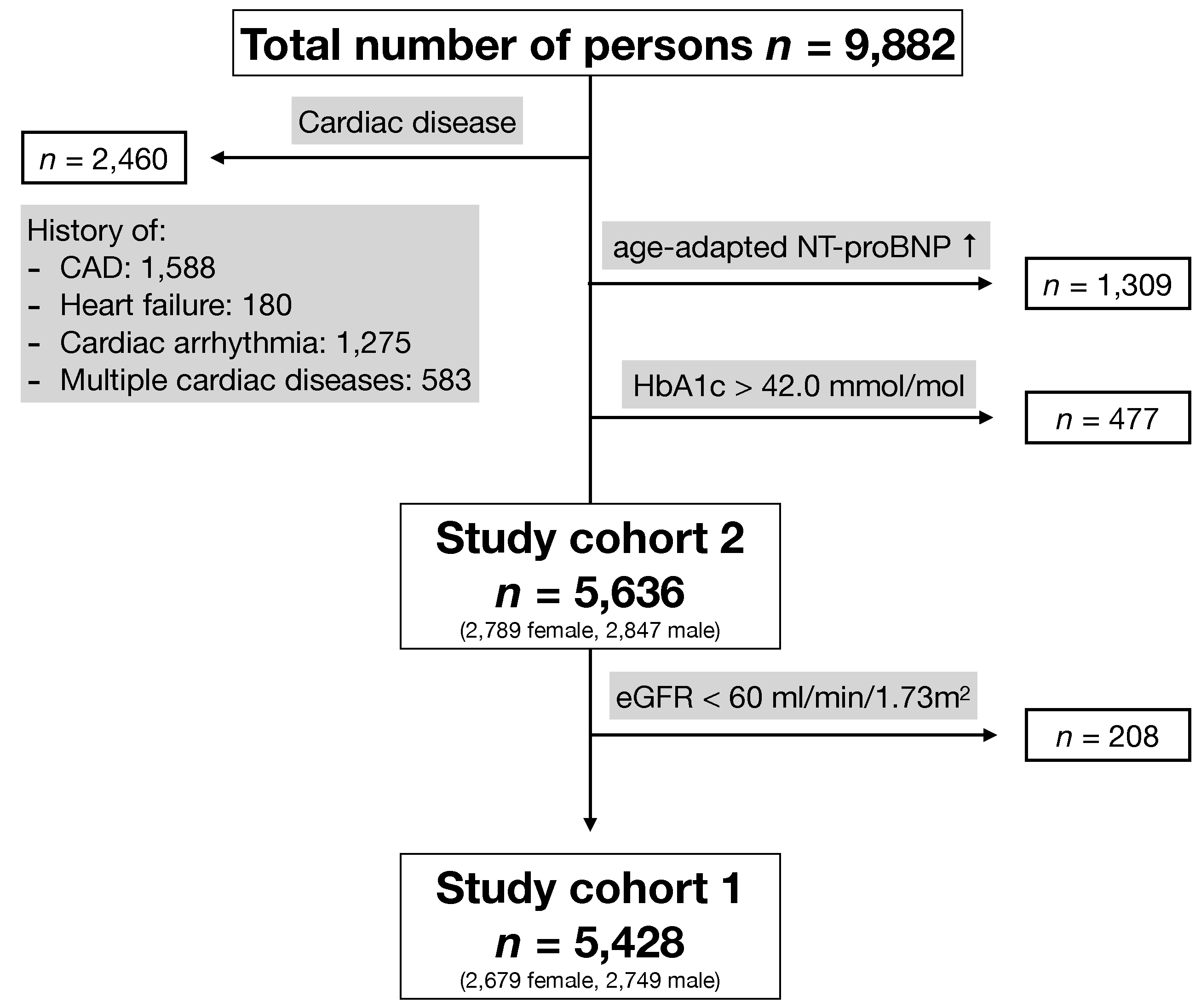
2.1. Study Population
2.2. Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
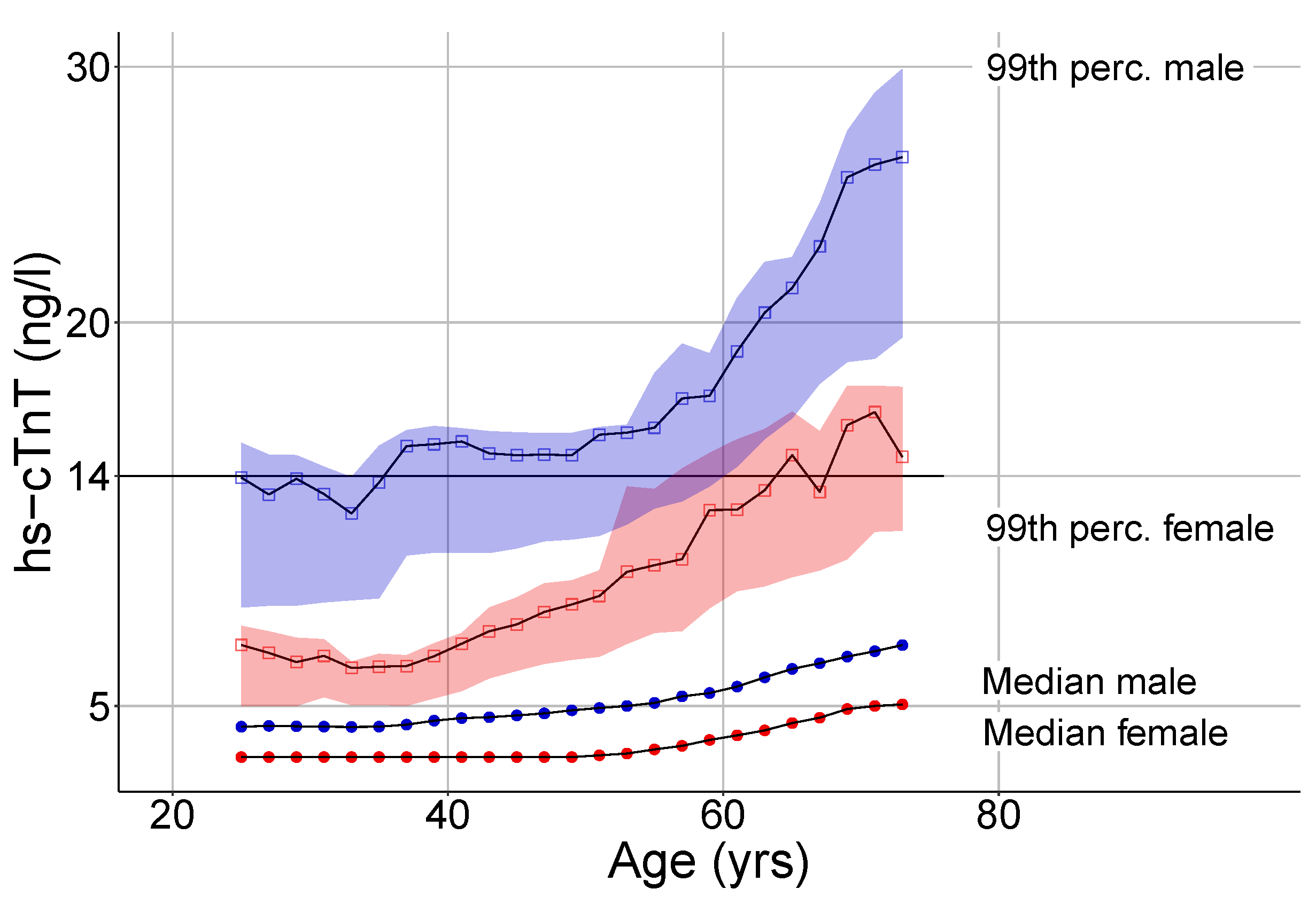
3.2. Hs-cTnT Upper Reference Limits in Relation to Age and Sex
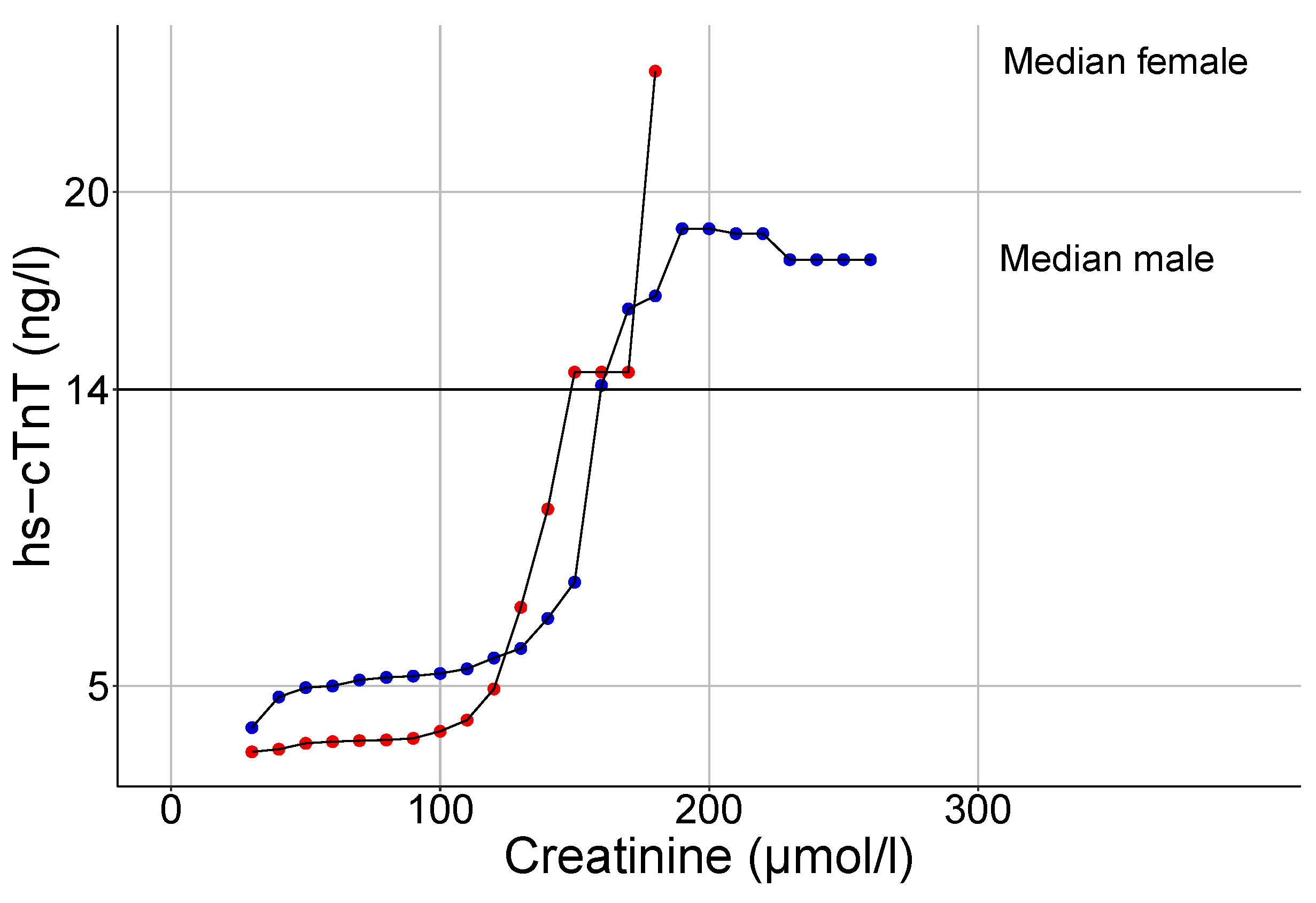
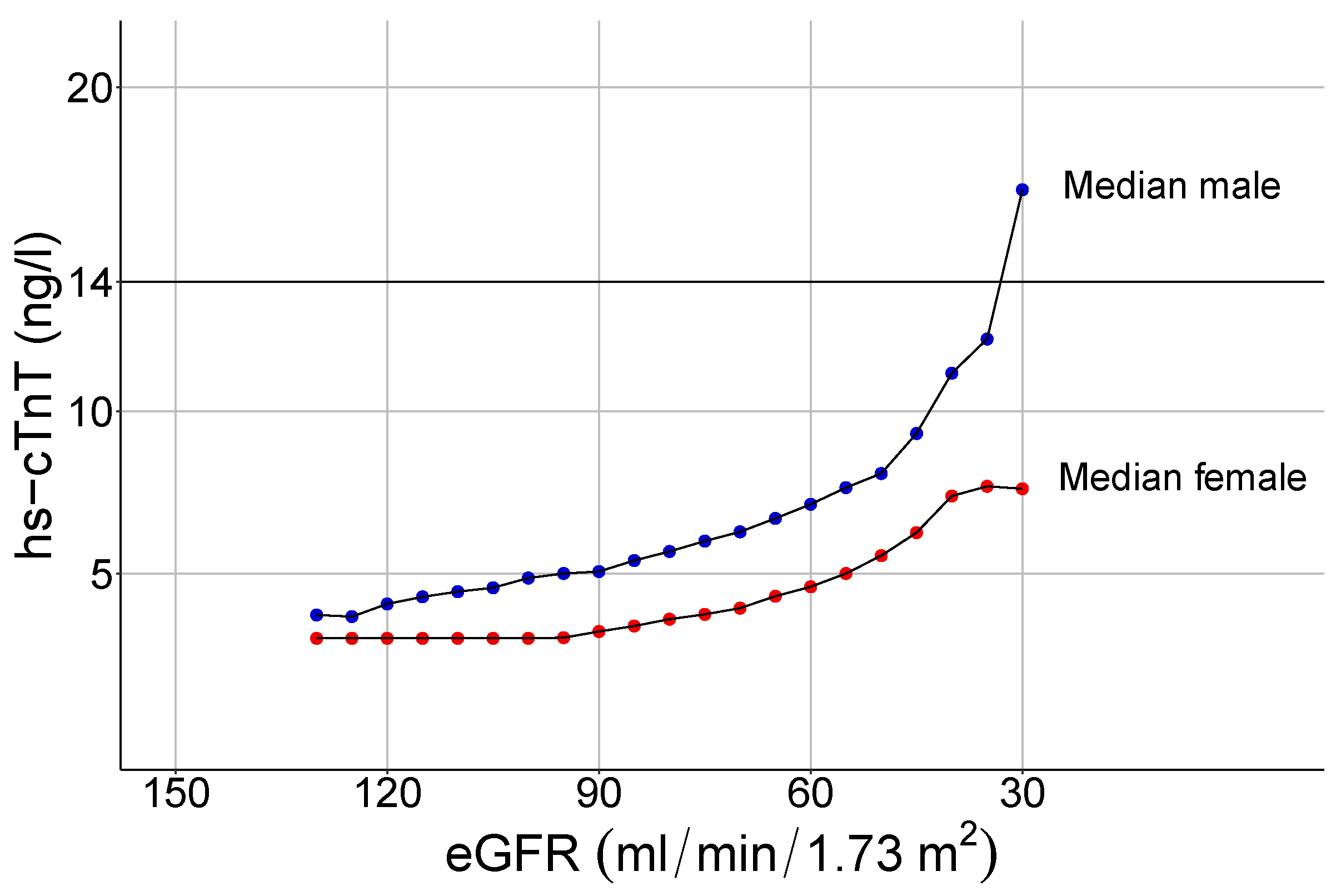
3.3. Influence of Renal Function on hs-cTnT Values
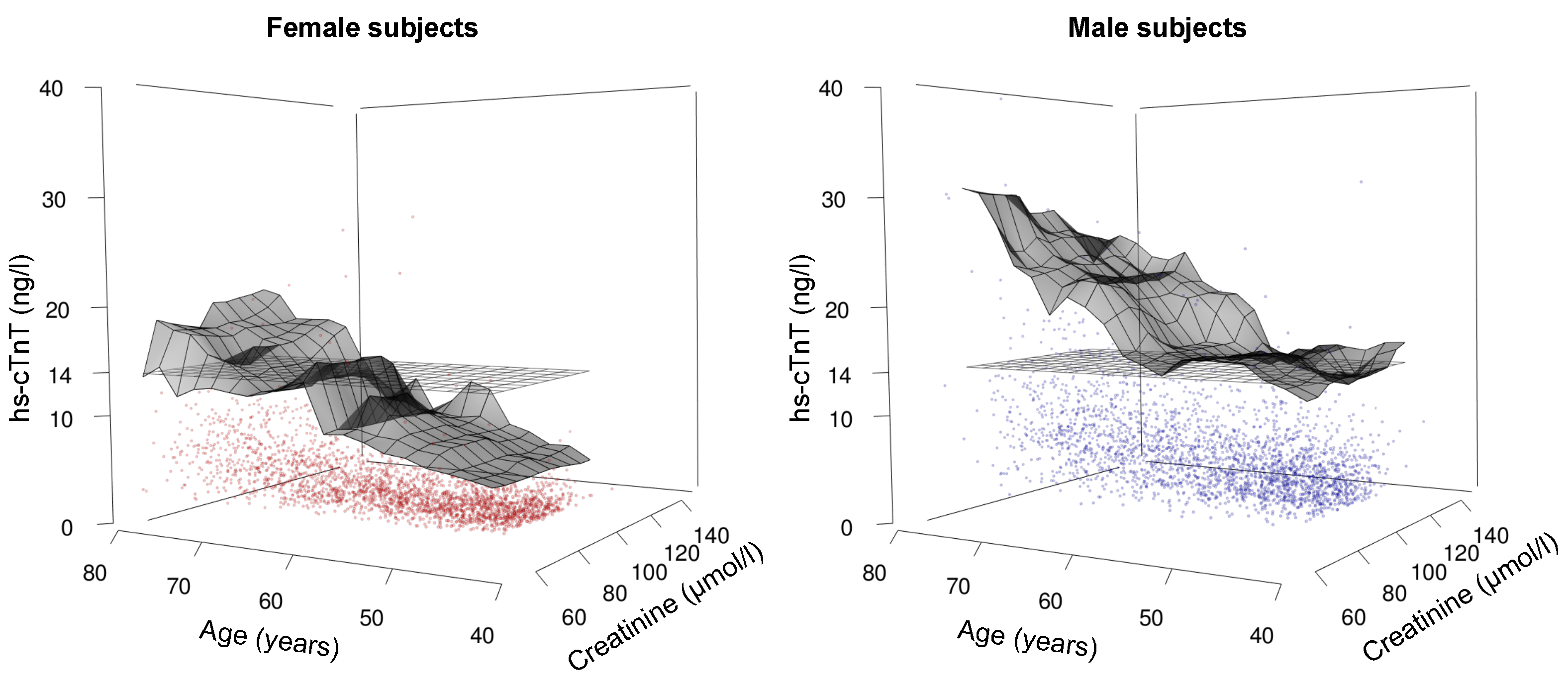
3.4. Hs-cTnT Levels in Relation to Age, Sex, and Renal Function—A Three-Dimensional Analysis
4. Discussion
4.1. The Impact of Sex on Hs-cTnT Plasma Levels
4.2. The Impact of Age on Hs-cTnT Plasma Levels
4.3. The Impact of Renal Function on Hs-cTnT Plasma Levels
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AACC | Academy of the American Association for Clinical Chemistry |
| AMI | Acute myocardial infarction |
| CAD | Coronary artery disease |
| CKD-EPI | Chronic kidney disease epidemiology collaboration |
| ECLIA | Electrochemiluminescence immunoassay |
| eGFR | Estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| HbA1c | Hemoglobin A1c |
| hs-cTnT | Highly sensitive cardiac troponin T |
| IFCC | International Federation of Clinical Chemistry and Laboratory Medicine |
| NT-proBNP | Amino-terminus pro B-type natriuretic peptide |
| TINIA | Turbidimetric inhibition immuno assay |
| TRAPID-AMI | High Sensitivity Cardiac Troponin T assay for rapid Rule-out of Acute Myocardial Infarction Study |
References
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Saenger, A.K.; Beyrau, R.; Braun, S.; Cooray, R.; Dolci, A.; Freidank, H.; Giannitsis, E.; Gustafson, S.; Handy, B.; Katus, H.; et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin. Chim. Acta 2011, 412, 748–754. [Google Scholar] [CrossRef]
- Gore, M.O.; Seliger, S.L.; Defilippi, C.R.; Nambi, V.; Christenson, R.H.; Hashim, I.A.; Hoogeveen, R.C.; Ayers, C.R.; Sun, W.; McGuire, D.K.; et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J. Am. Coll. Cardiol. 2014, 63, 1441–1448. [Google Scholar] [CrossRef]
- Franzini, M.; Lorenzoni, V.; Masotti, S.; Prontera, C.; Chiappino, D.; Latta, D.D.; Daves, M.; Deluggi, I.; Zuin, M.; Ferrigno, L.; et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: A multicenter study in Italy. Clin. Chim. Acta 2015, 438, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kuster, N.; Monnier, K.; Baptista, G.; Dupuy, A.M.; Badiou, S.; Bargnoux, A.S.; Jeandel, C.; Cristol, J.P. Estimation of age- and comorbidities-adjusted percentiles of high-sensitivity cardiac troponin T levels in the elderly. Clin. Chem. Lab. Med. 2015, 53, 691–698. [Google Scholar] [CrossRef]
- Orlev, A.; Klempfner, R.; Rott, D. Serum Cardiac Troponin T Levels in Asymptomatic Elderly Nursing Home Residents. Am. J. Med. 2018, 131, 842–845. [Google Scholar] [CrossRef]
- Gaggin, H.K.; Dang, P.V.; Do, L.D.; deFilippi, C.R.; Christenson, R.H.; Lewandrowski, E.L.; Lewandrowski, K.B.; Truong, B.Q.; Pham, V.Q.; Vu, V.H.; et al. Reference interval evaluation of high-sensitivity troponin T and N-terminal B-type natriuretic peptide in Vietnam and the US: The North South East West Trial. Clin. Chem. 2014, 60, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kimenai, D.M.; Henry, R.M.; van der Kallen, C.J.; Dagnelie, P.C.; Schram, M.T.; Stehouwer, C.D.; van Suijlen, J.D.; Niens, M.; Bekers, O.; Sep, S.J.; et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 2016, 102, 610–616. [Google Scholar] [CrossRef]
- Ungerer, J.P.J.; Tate, J.R.; Pretorius, C.J. Discordance with 3 Cardiac Troponin I and T Assays: Implications for the 99th Percentile Cutoff. Clin. Chem. 2016, 62, 1106–1114. [Google Scholar] [CrossRef]
- Aw, T.-C.; Phua, S.-K.; Lam, C.-W.; Wong, M.-S. What are normal high-sensitivity troponin-T values in a large multi-ethnic Asian population? Blood Heart Circ. 2017, 1, 1–4. [Google Scholar] [CrossRef][Green Version]
- Giannitsis, E.; Mueller-Hennessen, M.; Zeller, T.; Schuebler, A.; Aurich, M.; Biener, M.; Vafaie, M.; Stoyanov, K.M.; Ochs, M.; Riffel, J.; et al. Gender-specific reference values for high-sensitivity cardiac troponin T and I in well-phenotyped healthy individuals and validity of high-sensitivity assay designation. Clin. Biochem. 2020, 78, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sandstede, J.; Lipke, C.; Beer, M.; Hofmann, S.; Pabst, T.; Kenn, W.; Neubauer, S.; Hahn, D. Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur. Radiol. 2000, 10, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Wingren, C.J.; Ottosson, A. Postmortem heart weight modelled using piecewise linear regression in 27,645 medicolegal autopsy cases. Forensic. Sci. Int. 2015, 252, 157–162. [Google Scholar] [CrossRef]
- Aksoy, N.; Ozer, O.; Sari, I.; Sucu, M.; Aksoy, M.; Geyikli, I. Contribution of renal function impairment to unexplained troponin T elevations in congestive heart failure. Ren. Fail. 2009, 31, 272–277. [Google Scholar] [CrossRef]
- Chotivanawan, T.; Krittayaphong, R. Normal range of serum highly-sensitive troponin-T in patients with chronic kidney disease stage 3–5. J Med. Assoc. Thai. 2012, 95 (Suppl. 2), S127–S132. [Google Scholar] [PubMed]
- Pfortmueller, C.A.; Funk, G.C.; Marti, G.; Leichtle, A.B.; Fiedler, G.M.; Schwarz, C.; Exadaktylos, A.K.; Lindner, G. Diagnostic performance of high-sensitive troponin T in patients with renal insufficiency. Am. J. Cardiol. 2013, 112, 1968–1972. [Google Scholar] [CrossRef]
- Dubin, R.F.; Li, Y.; He, J.; Jaar, B.G.; Kallem, R.; Lash, J.P.; Makos, G.; Rosas, S.E.; Soliman, E.Z.; Townsend, R.R.; et al. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: A cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013, 14, 229. [Google Scholar] [CrossRef]
- Chung, J.Z.Y.; Dallas Jones, G.R. Effect of renal function on serum cardiac troponin T–Population and individual effects. Clin. Biochem. 2015, 48, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Gimenez, M.R.; Reiter, M.; Reichlin, T.; Walukiewicz, A.; Gugala, M.; Krivoshei, L.; Marti, N.; et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients with Renal Dysfunction. Circulation 2015, 131, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Chesnaye, N.C.; Szummer, K.; Bárány, P.; Heimbürger, O.; Magin, H.; Almquist, T.; Uhlin, F.; Dekker, F.W.; Wanner, C.; Jager, K.J.; et al. Association between Renal Function and Troponin T Over Time in Stable Chronic Kidney Disease Patients. J. Am. Heart Assoc. 2019, 8, e013091. [Google Scholar] [CrossRef]
- Twerenbold, R.; Badertscher, P.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Puelacher, C.; Sabti, Z.; Rubini Gimenez, M.; Tschirky, S.; du Fay de Lavallaz, J.; et al. 0/1-Hour Triage Algorithm for Myocardial Infarction in Patients with Renal Dysfunction. Circulation 2018, 137, 436–451. [Google Scholar] [CrossRef]
- Omar, A.S.; Mahmoud, K.; Hanoura, S.; Osman, H.; Sivadasan, P.; Sudarsanan, S.; Shouman, Y.; Singh, R.; AlKhulaifi, A. Acute kidney injury induces high-sensitivity troponin measurement changes after cardiac surgery. BMC Anesthesiol. 2017, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Engel, C.; Ahnert, P.; Alfermann, D.; Arelin, K.; Baber, R.; Beutner, F.; Binder, H.; Brähler, E.; Burkhardt, R.; et al. The LIFE-Adult-Study: Objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015, 15, 691. [Google Scholar] [CrossRef]
- Wu, A.H.B.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2018, 64, 645–655. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- GitHub: QuantileCI. Available online: https://github.com/hoehleatsu/quantileCI (accessed on 7 September 2021).
- R Cor Team. A Language and Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Adler, D.; Murdoch, D. Rgl: 3D Visualization Using OpenGL. 2017. Available online: https://www.researchgate.net/publication/318392813_Rgl_3D_Visualization_Using_OpenGL (accessed on 14 September 2021).
- van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Shah, A.S.V.; Griffiths, M.; Lee, K.K.; McAllister, D.A.; Hunter, A.L.; Ferry, A.V.; Cruikshank, A.; Reid, A.; Stoddart, M.; Strachan, F.; et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: Prospective cohort study. BMJ 2015, 350, g7873. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Lindahl, B. Impact of Sex on Cardiac Troponin Concentrations—A Critical Appraisal. Clin. Chem. 2017, 63, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Hennessen, M.; Lindahl, B.; Giannitsis, E.; Biener, M.; Vafaie, M.; Defilippi, C.R.; Christ, M.; Santalo-Bel, M.; Panteghini, M.; Plebani, M.; et al. Diagnostic and prognostic implications using age- and gender-specific cut-offs for high-sensitivity cardiac troponin T—Sub-analysis from the TRAPID-AMI study. Int. J. Cardiol. 2016, 209, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rubini Giménez, M.; Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Puelacher, C.; Hillinger, P.; Wildi, K.; Jaeger, C.; Grimm, K.; Heitzelmann, K.F.; et al. Clinical Effect of Sex-Specific Cutoff Values of High-Sensitivity Cardiac Troponin T in Suspected Myocardial Infarction. JAMA Cardiol. 2016, 1, 912–920. [Google Scholar] [CrossRef]
- Enzenbach, C.; Wicklein, B.; Wirkner, K.; Loeffler, M. Evaluating selection bias in a population-based cohort study with low baseline participation: The LIFE-Adult-Study. BMC Med. Res. Methodol. 2019, 19, 135. [Google Scholar] [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 136–150. Available online: http://ijpog.org/downloads/9/81_90.pdf (accessed on 7 September 2021).
| Study Cohort 1 | Study Cohort 2 | |
|---|---|---|
| female (n, %) | 2,679 (49.4%) | 2,789 (49.5%) |
| Age (years) | 52.2 (45.0–62.3) | 52.9 (45.2–63.3) |
| hs-cTnT (ng/L) | 4.3 (3.0–6.0) | 4.4 (3–6.2) |
| Creatinine (mol/L) | 77.0 (69.0–87.0) | 78.0 (69–88) |
| HbA1c (mmol/mol) | 34.4 ( 31.3–36.6) | 34.4 (32.2–36.6) |
| NT-proBNP (ng/L) | 48.0 (28.0–75.3) | 49.1 (28.5–76.6) |
| eGFR (mL/min/1.73 m) | 87.1 (77.2–97.0) | 86.4 (76.0–96.5) |
| Age (Years) | Count (%) | Median hs-cTnT (ng/L) | 99th Percentile hs-cTnT (ng/L) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| 20–40 | 196 (47.6) | 217 (52.4) | 3.0 | 4.2 | 6.6 [5.0, 18.0] | 13.7 [10.9, 16.5] | <0.001 |
| 41–50 | 992 (51.2) | 945 (48.8) | 3.0 | 4.5 | 7.4 [6.7, 9.7] | 14.4 [12.1, 20.7] | <0.001 |
| 51–60 | 724 (50.3) | 714 (49.7) | 3.4 | 5.2 | 12.2 [8.8, 46.4] | 14.7 [13.2, 20.0] | <0.001 |
| 61–70 | 522 (49.9) | 525 (50.1) | 4.5 | 6.4 | 12.5 [10.5, 18.2] | 22.4 [18.4, 34.9] | <0.001 |
| 71–80 | 245 (41.3) | 348 (58.7) | 6.0 | 8.3 | 17.8 [14.2, 54.3] | 30.1 [23.4, 40.0] | <0.001 |
| Creatinine (mol/L) | Count | Median Age (Years) | Median Hs-cTnT (ng/L) | 99th Perc. hs-cTnT (ng/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| 0–50 | 45 | 1 | 52 (48–62) | 47 (47) | 3.0 | 6.2 | – | – |
| 51–100 | 2723 | 2467 | 53 (45–62) | 52 (45–63) | 3.4 | 5.2 | 13.2 [12.0, 16.5] | 20.2 [18.0, 23.1] |
| 101–150 | 20 | 374 | 72 (61–75) | 58 (47–70) | 7.4 | 6.2 | – | 23.4 [19.8, 126.0] |
| 151–250 | 1 | 5 | 79 (79) | 76 (69–76) | 23.7 | 18.9 | – | – |
| eGFR (mL/min/1.73 m) | Count | Median Hs-cTnT (ng/L) | 99th Perc. hs-cTnT (ng/L) | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| 121–150 | 21 | 15 | 3.0 | 4.0 | – | – |
| 91–120 | 1018 | 1181 | 3.0 | 4.6 | 9.3 [8.0, 15.7] | 16.6 [14.0, 20.7] |
| 61–90 | 1640 | 1553 | 3.7 | 6.1 | 13.2 [12.0, 17.6] | 22.1 [19.4, 26.2] |
| 31–60 | 109 | 97 | 6.3 | 9.9 | – | – |
| 0–30 | 1 | 1 | 23.7 | 17.9 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gärtner, C.; Langhammer, R.; Schmidt, M.; Federbusch, M.; Wirkner, K.; Löffler, M.; Isermann, B.; Laufs, U.; Wachter, R.; Kaiser, T. Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function. J. Clin. Med. 2021, 10, 5508. https://doi.org/10.3390/jcm10235508
Gärtner C, Langhammer R, Schmidt M, Federbusch M, Wirkner K, Löffler M, Isermann B, Laufs U, Wachter R, Kaiser T. Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function. Journal of Clinical Medicine. 2021; 10(23):5508. https://doi.org/10.3390/jcm10235508
Chicago/Turabian StyleGärtner, Christiane, Romy Langhammer, Maria Schmidt, Martin Federbusch, Kerstin Wirkner, Markus Löffler, Berend Isermann, Ulrich Laufs, Rolf Wachter, and Thorsten Kaiser. 2021. "Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function" Journal of Clinical Medicine 10, no. 23: 5508. https://doi.org/10.3390/jcm10235508
APA StyleGärtner, C., Langhammer, R., Schmidt, M., Federbusch, M., Wirkner, K., Löffler, M., Isermann, B., Laufs, U., Wachter, R., & Kaiser, T. (2021). Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function. Journal of Clinical Medicine, 10(23), 5508. https://doi.org/10.3390/jcm10235508







