Effect of Hyperbaric Oxygenation on Blood Cytokines and Arginine Derivatives; No Evidence for Induction of Inflammation or Endothelial Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Enzyme-Linked Immunoabsorbent Assay
2.3. Mass Spectrometry
2.4. Statistical Analysis
3. Results
3.1. Quantification of Arginine Derivatives in the Serum of Patients Treated with HBOT
- homoarginine concentrations before HBOT between ABN and ISSNHL (p = 0.027, Cohen’s d = 0.067) and between NSTI and ISSNHL (p = 0.025, Cohen’s d = 0.064) and after 15 HBOT sessions between NSTI and ISSNHL (p = 0.012, Cohen’s d = 0.072);
- L-NMMA concentrations between ISSNHL and NSTI after 15 HBOT sessions (p = 0.002, Cohen’s d = 2.323);
- ADMA concentrations between ISSNHL and ABN after 15 HBOT sessions (p = 0.036, Cohen’s d = 0.385).
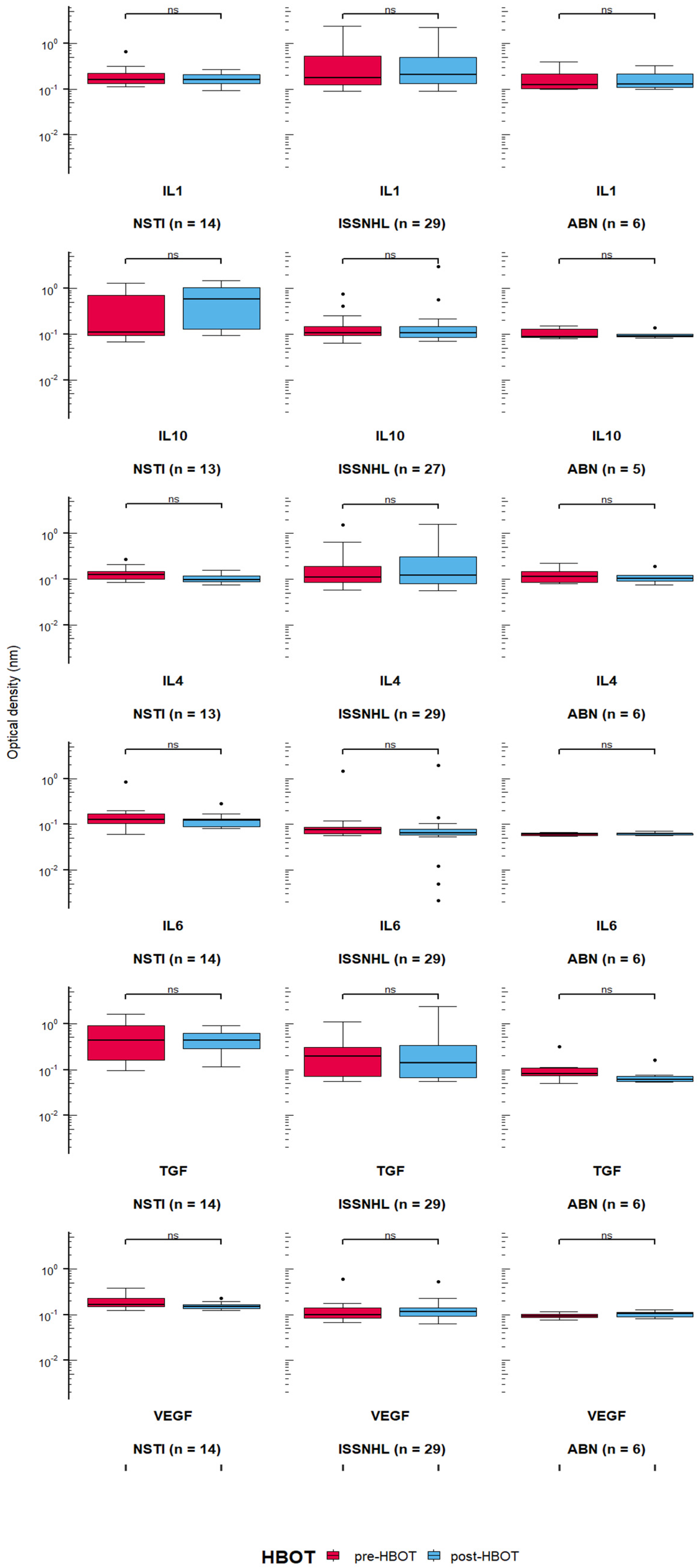
3.2. Quantification of Cytokines in the Serum of Patients Treated with HBOT
- IL-10 level after 15 HBOT sessions between NSTI and ISSNHL (p = 0.005, Cohen’s d = 0.0859), and between NSTI and ABN (p = 0.01, Cohen’s d = 0.102);
- IL-6 level before HBOT between ISSNHL and ABN (p = 0.012, Cohen’s d = 0.209), NSTI and ABN (p = 0.0002, Cohen’s d = 0.293), and between ISSNHL and NSTI before (p = 0.0002, Cohen’s d = 0.207) and after 15 HBO sessions (p = 0.0007, Cohen’s d = 0.1705);
- TGF-β level between ISSNHL and NSTI before HBOT (p = 0.005, Cohen’s d = 0.131) and after 15 HBO sessions (p = 0.004, Cohen’s d = 0.128), between NSTI and ABN before HBOT (p = 0.002, Cohen’s d = 0.108) and after 15 HBO sessions (p = 0.0003, Cohen’s d = 0.193), and between ISSNHL and ABN after 15 HBO sessions (p = 0.03, Cohen’s d = 0.123);
- VEGF level between ISSNHL and NSTI before HBOT (p = 0.002, Cohen’s d = 0.516) and after 15 HBO sessions (p = 0.001, Cohen’s d = 0.683), and between NSTI and ABN before HBOT (p = 0.0001, Cohen’s d = 0.632) and after 15 HBO sessions (p = 0.0009, Cohen’s d = 1.375).
3.3. Correlations between Levels of Arginine Derivatives and Cytokines in Patients Treated with HBOT
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European consensus conference on hyperbaric medicine: Recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- Camporesi, E.M.; Bosco, G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2014, 41, 247–252. [Google Scholar]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef]
- Choudhury, R. Hypoxia and hyperbaric oxygen therapy: A review. Int. J. Gen. Med. 2018, 11, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Sen, S. Therapeutic effects of hyperbaric oxygen: Integrated review. Med. Gas Res. 2021, 11, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Semadi, I.N. The role of VEGF and TNF-Alpha on epithelialization of diabetic foot ulcers after hyperbaric oxygen therapy. Open Access Maced. J. Med Sci. 2019, 7, 3177–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, E.; Sabatier, J.-M.; Behdani, M.; Irani, S.; Kazemi-Lomedasht, F. A nanobody-derived mimotope against VEGF inhibits cancer angiogenesis. J. Enzym. Inhib. Med. Chem. 2020, 35, 1233–1239. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 2018, 173, 117–129.e14. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.I.R.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and its receptor (vegfr) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Ziche, M.; Morbidelli, L. Nitric oxide and angiogenesis. J. Neuro-Oncol. 2000, 50, 139–148. [Google Scholar] [CrossRef]
- Wieczór, A.M.; Wieczór, R.; Kulwas, A.; Rość, D. Asymmetric dimethylarginine and angiogenesis: Biological significance. Int. Angiol. 2018, 37, 431–436. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Benson, R.M.; Minter, L.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin. Exp. Immunol. 2003, 134, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedetoft, M.; Garred, P.; Madsen, M.B.; Hyldegaard, O. Hyperbaric oxygen treatment is associated with a decrease in cytokine levels in patients with necrotizing soft-tissue infection. Physiol. Rep. 2021, 9, e14757. [Google Scholar] [CrossRef] [PubMed]
- Heyboer, M.; Sharma, D.; Santiago, W.; McCulloch, N. Hyperbaric oxygen therapy: Side effects defined and quantified. Adv. Wound Care 2017, 6, 210–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolenska, Z.; Zabielska-Kaczorowska, M.; Wojteczek, A.; Kutryb-Zajac, B.; Zdrojewski, Z. Metabolic pattern of systemic sclerosis: Association of changes in plasma concentrations of amino acid-related compounds with disease presentation. Front. Mol. Biosci. 2020, 7, 585161. [Google Scholar] [CrossRef] [PubMed]
- Charytan, D.M.; Cinelli, A.; Zeisberg, E.M. Association of circulating angiogenesis inhibitors and asymmetric dimethyl arginine with coronary plaque burden. Fibrogenesis Tissue Repair 2015, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Loscalzo, J.; Jin, R.C. Vascular nitric oxide: Formation and function. J. Blood Med. 2010, 1, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Kielstein, J.T.; Salpeter, S.R.; Bode-Boeger, S.M.; Cooke, J.; Fliser, D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—A meta-analysis. Nephrol. Dial. Transplant. 2006, 21, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Buijs, N.; Oosterink, J.E.; Jessup, M.; Schierbeek, H.; Stolz, D.B.; Houdijk, A.P.; Geller, D.A.; Van Leeuwen, P.A. A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: Dimethylarginine dimethylaminohydrolase 1. Angiogenesis 2017, 20, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Achan, V.; Ho, H.K.; Heeschen, C.; Stuehlinger, M.; Jang, J.J.; Kimoto, M.; Vallance, P.; Cooke, J.P. ADMA regulates angiogenesis: Genetic and metabolic evidence. Vasc. Med. 2005, 10, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, H.; Rodionov, R.N.; Lynch, C.; Cooke, J.P.; Arning, E.; Bottiglieri, T.; Lentz, S.R.; Faraci, F.M. Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine–induced endothelial dysfunction in the cerebral circulation. Stroke 2008, 39, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Shashar, M.; Chernichovski, T.; Pasvolsky, O.; Levi, S.; Grupper, A.; Hershkovitz, R.; Weinstein, T.; Schwartz, I.F. Vascular endothelial growth factor augments arginine transport and nitric oxide generation via a kdr receptor signaling pathway. Kidney Blood Press. Res. 2017, 42, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Oyaizu, T.; Enomoto, M.; Horie, M.; Yuasa, M.; Okawa, A.; Yagishita, K. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep. 2020, 10, 2744. [Google Scholar] [CrossRef]



| n | Median Age (IQR) | Median HBO Sessions (Min–Max) | ||
|---|---|---|---|---|
| Aseptic bone necrosis (ABN) | Males | 3 | 39 (28–54) | 60 (60) |
| Females | 3 | 57 (52–58) | 60 (60) | |
| All | 6 | 52 (39–57) | 60 (60) | |
| Necrotizing soft tissue infection (NSTI) | Males | 9 | 54 (19–70) | 30 (15–30) |
| Females | 9 | 58 (49–84) | 30 (30–60) | |
| All | 18 | 56 (49–70) | 30 (15–60) | |
| Idiopathic sudden sensori-neural hearing loss (ISSNHL) | Males | 30 | 54 (37–69) | 15 (8–30) |
| Females | 15 | 41 (40–47) | 15 (15) | |
| All | 45 | 47 (37–62) | 15 (15–30) |
| H | df | p | Cohen’s D | ||
|---|---|---|---|---|---|
| Arginine | pre-HBOT | 8.999 | 2 | 0.011 | 0.0015 |
| Homoarginine | pre-HBOT | 8.154 | 2 | 0.017 | 0.066 |
| post-HBOT | 6.196 | 2 | 0.045 | 0.0732 | |
| L-NMMA | pre-HBOT | 7.085 | 2 | 0. 029 | 1.95 |
| post-HBOT | 14.005 | 2 | 0.0009 | 2.4 |
| H | df | p | Cohen’s D | ||
|---|---|---|---|---|---|
| IL-6 | pre-HBOT | 31.731 | 2 | <0.00001 | 0.220 |
| post-HBOT | 22.959 | 2 | 0.00001 | 0.182 | |
| TGF-β | pre-HBOT | 10.94 | 2 | 0.004 | 0.137 |
| post-HBOT | 15.65 | 2 | 0.0004 | 0.134 | |
| VEGF | pre-HBOT | 10.507 | 2 | 0.005 | 0.540 |
| post-HBOT | 10.033 | 2 | 0.007 | 0.716 | |
| IL-10 | post-HBOT | 13.945 | 2 | 0.0009 | 0.0904 |
| Group | Estimate | SE | CI Upper | CI Lower | p | |
|---|---|---|---|---|---|---|
| Arginine | NSTI | −13.087 | 4.888 | −4.142 | −22.252 | 0.002 |
| VEGF | NSTI | 0.042 | 0.019 | 0.0815 | 0.010 | 0.008 |
| ADMA | NSTI | −0.102 | 0.058 | −0.018 | −0.233 | 0.018 |
| Homoarginine | ABN | −0.332 | 0.154 | −0.090 | −0.641 | 0.02 |
| IL4 | NSTI | 0.031 | 0.015 | 0.064 | 0.006 | 0.022 |
| SDMA | NSTI | −0.122 | 0.076 | −0.018 | −0.292 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siewiera, J.; Smoleński, M.; Jermakow, N.; Kot, J.; Brodaczewska, K.; Turyn, J.; Zabielska-Kaczorowska, M.A.; Ludwig, N.; Szczepański, M.J. Effect of Hyperbaric Oxygenation on Blood Cytokines and Arginine Derivatives; No Evidence for Induction of Inflammation or Endothelial Injury. J. Clin. Med. 2021, 10, 5488. https://doi.org/10.3390/jcm10235488
Siewiera J, Smoleński M, Jermakow N, Kot J, Brodaczewska K, Turyn J, Zabielska-Kaczorowska MA, Ludwig N, Szczepański MJ. Effect of Hyperbaric Oxygenation on Blood Cytokines and Arginine Derivatives; No Evidence for Induction of Inflammation or Endothelial Injury. Journal of Clinical Medicine. 2021; 10(23):5488. https://doi.org/10.3390/jcm10235488
Chicago/Turabian StyleSiewiera, Jacek, Michał Smoleński, Natalia Jermakow, Jacek Kot, Klaudia Brodaczewska, Jacek Turyn, Magdalena A. Zabielska-Kaczorowska, Nils Ludwig, and Mirosław J. Szczepański. 2021. "Effect of Hyperbaric Oxygenation on Blood Cytokines and Arginine Derivatives; No Evidence for Induction of Inflammation or Endothelial Injury" Journal of Clinical Medicine 10, no. 23: 5488. https://doi.org/10.3390/jcm10235488
APA StyleSiewiera, J., Smoleński, M., Jermakow, N., Kot, J., Brodaczewska, K., Turyn, J., Zabielska-Kaczorowska, M. A., Ludwig, N., & Szczepański, M. J. (2021). Effect of Hyperbaric Oxygenation on Blood Cytokines and Arginine Derivatives; No Evidence for Induction of Inflammation or Endothelial Injury. Journal of Clinical Medicine, 10(23), 5488. https://doi.org/10.3390/jcm10235488








