Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Chemistry
2.2. Measurement of Circulating Growth Factors and Vasoactive Peptides
2.3. Human Coronary Artery Smooth Muscle Cell Culture
2.4. Basal Cell Growth of Vascular Smooth Muscle Cells
2.5. Vascular Smooth Muscle Cell Proliferation
2.6. Apoptosis
2.7. Cell Migration
2.8. Steady-State Gene Expression Analysis
2.9. Statistics
3. Results
3.1. Clinical Parameters of Cirrhotic Patients and Healthy Controls
3.2. Clinical Chemistry
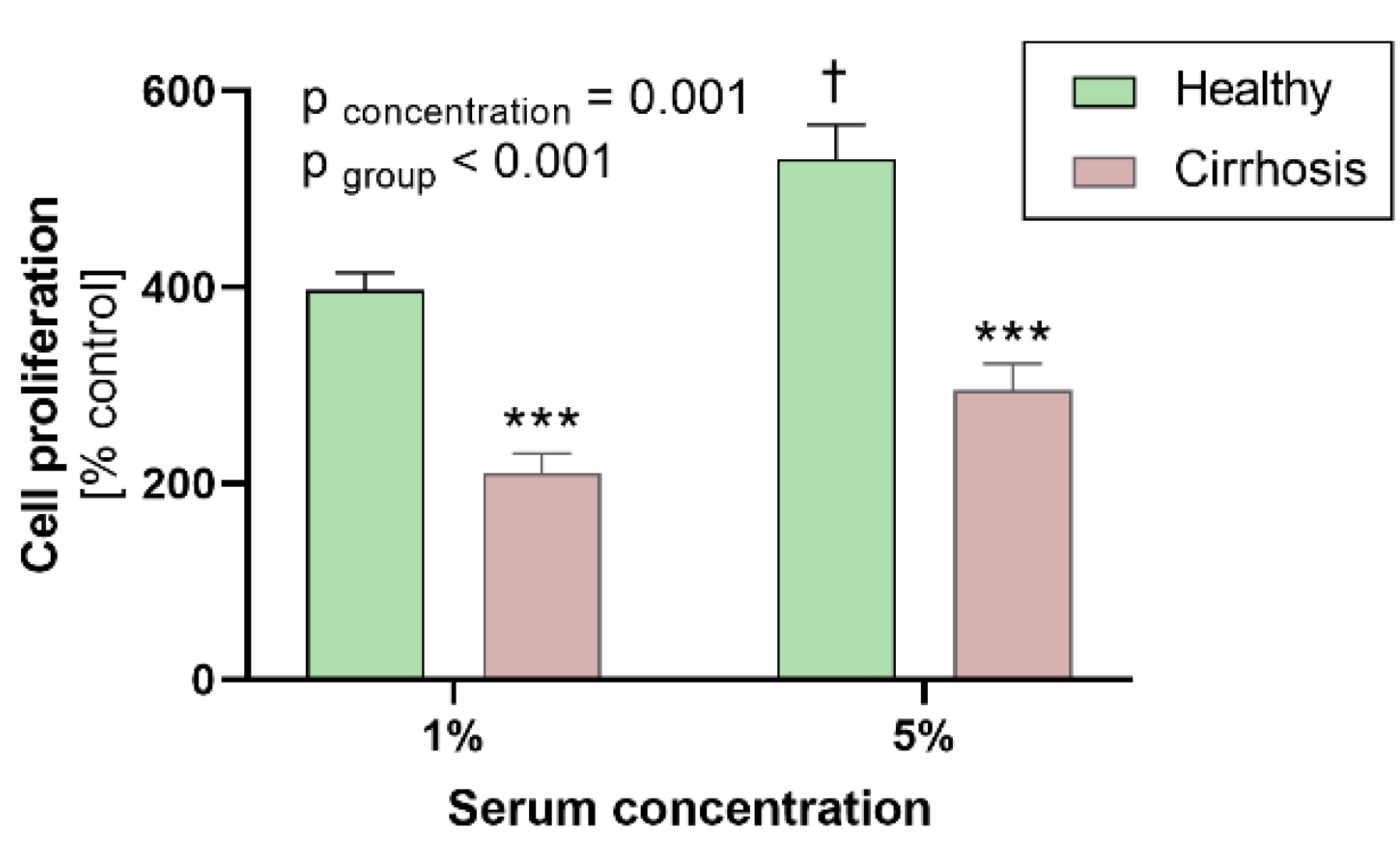
3.3. Effects of Sera on Human Coronary Artery Smooth Muscle Cell Proliferation
3.4. Effects of Sera on HCASMC Apoptosis
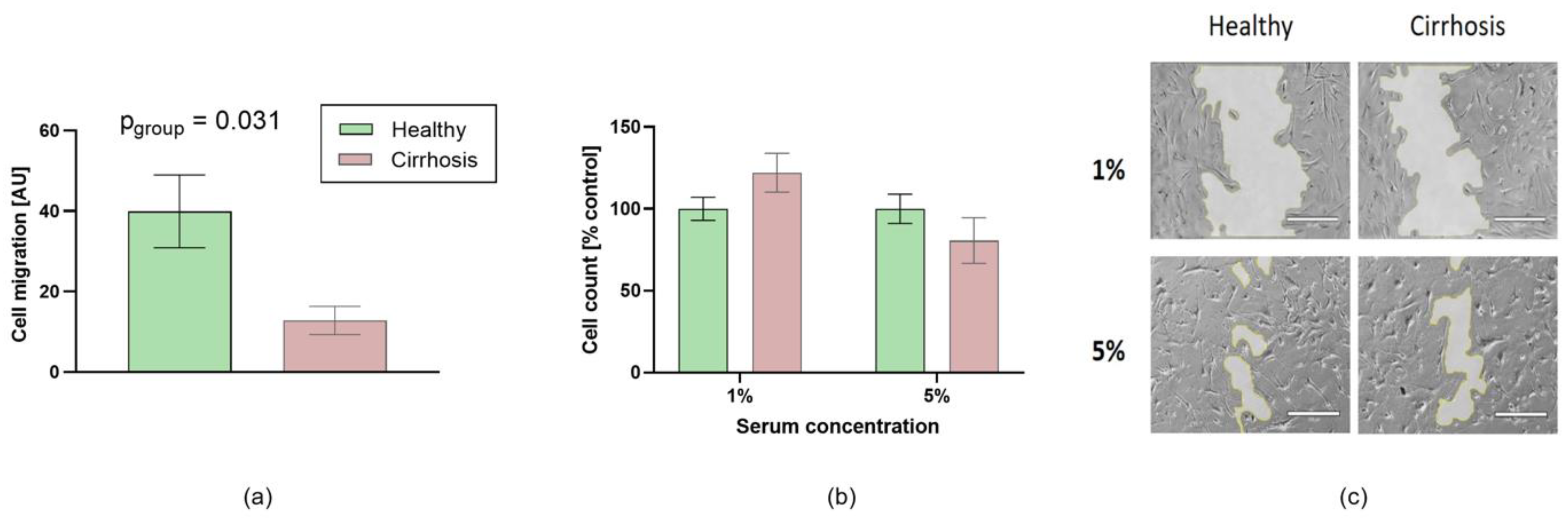
3.5. Effects of Patient Sera on Cell Migration
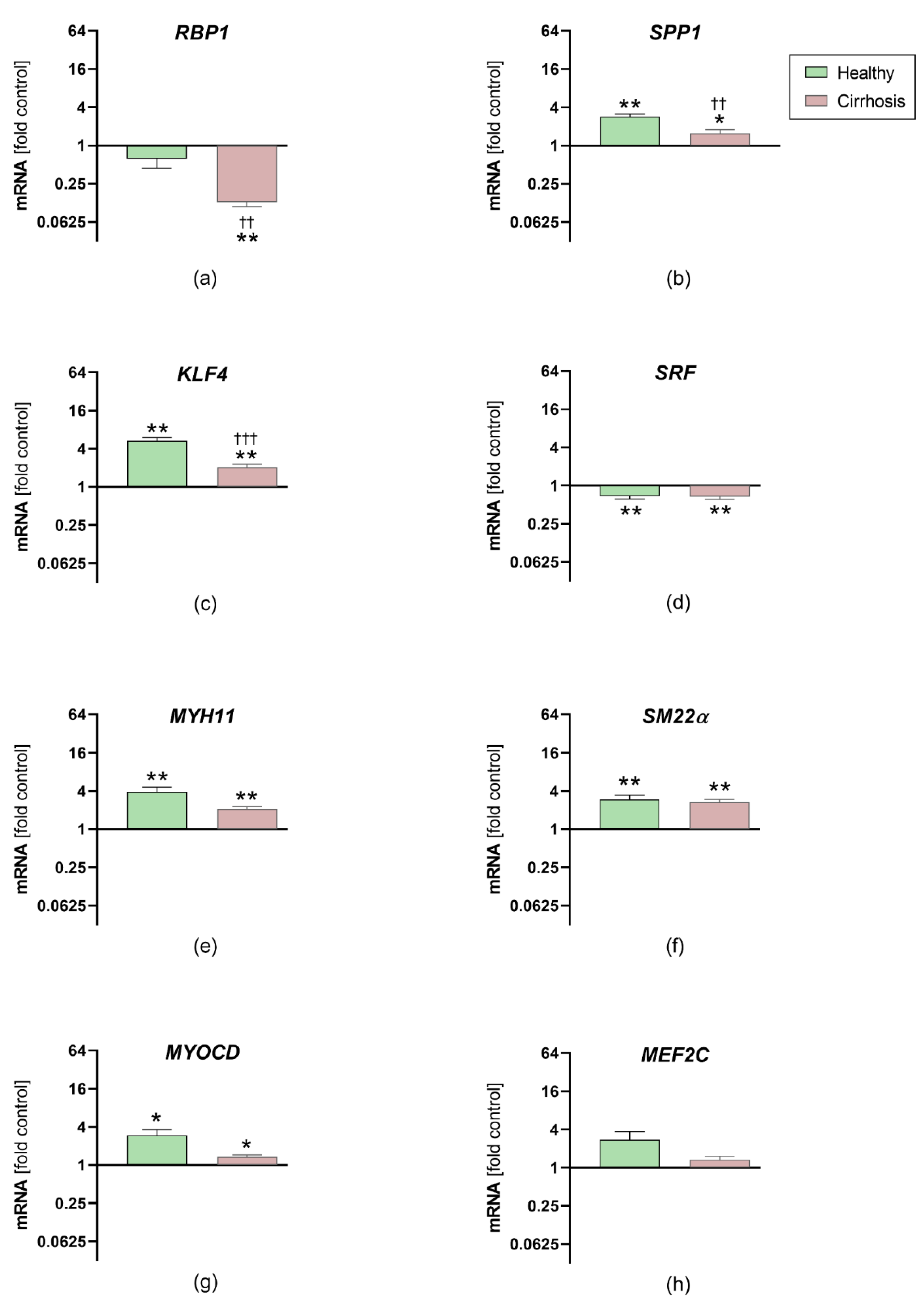
3.6. Effects of Sera on HCASMC Gene Expression Analysis
4. Discussion
4.1. Effects on Cell Proliferation, Migration and Gene Expression in HCASMC
4.2. Potential Factors Contributing to Differences in Cell Proliferation and Migration
4.2.1. Circulating Levels of PDGF
4.2.2. Inflammatory Factors
4.2.3. Bilirubin
4.3. Clinical Impact of Alcolholic Cirrhosis on CAD
4.4. Possible Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennings, J.; Faselis, C.; Yao, M.D. NAFLD-NASH: An under-recognized epidemic. Curr. Vasc. Pharmacol. 2018, 16, 209–213. [Google Scholar] [CrossRef]
- Carey, W.D.; Dumot, J.A.; Pimentel, R.R.; Barnes, D.S.; Hobbs, R.E.; Henderson, J.M.; Vogt, D.P.; Mayes, J.T.; Westveer, M.K.; Easley, K.A. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation 1995, 59, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, B.G.; Valantine, H.; Keeffe, E.B. Detection and treatment of coronary artery disease in liver transplant candidates. Liver Transpl. 2001, 7, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Ehtisham, J.; Altieri, M.; Salame, E.; Saloux, E.; Ollivier, I.; Hamon, M. Coronary artery disease in orthotopic liver transplantation: Pretransplant assessment and management. Liver Transpl. 2010, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Arnett, D.K.; Goodman, R.A.; Halperin, J.L.; Anderson, J.L.; Parekh, A.K.; Zoghbi, W.A. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: From the American Heart Association, American College of Cardiology, and U.S. Department of Health and Human Services. J. Am. Coll. Cardiol. 2014, 64, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.A.M.; Kaplan, G.G.; Hubbard, J.N.; Myers, R.P. Morbidity and mortality following coronary artery bypass graft surgery in patients with cirrhosis: A population-based study. Liver Int 2009, 29, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Marui, A.; Kimura, T.; Tanaka, S.; Miwa, S.; Yamazaki, K.; Minakata, K.; Nakata, T.; Ikeda, T.; Furukawa, Y.; Kita, T.; et al. Coronary revascularization in patients with liver cirrhosis. Ann. Thorac. Surg. 2011, 91, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Steitieh, D.; Feldman, D.N.; Cheung, J.W.; Wong, S.C.; Halazun, H.; Halazun, K.J.; Amin, N.; Wang, J.; Chae, J.; et al. Impact of cirrhosis on 90-day outcomes after percutaneous coronary intervention (from A Nationwide Database). Am. J. Cardiol. 2020, 125, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Chen, M.; Dai, R.; Zhang, Y.; Zhao, H.; Song, Z.; Zhang, L.; Li, Z.; Feng, Y.; Gao, H.; et al. SRSF1 promotes vascular smooth muscle cell proliferation through a Delta133p53/EGR1/KLF5 pathway. Nat. Commun. 2017, 8, 16016. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, C.; Otsuka, F.; Virmani, R.; Bochaton-Piallat, M.L. Biological responses in stented arteries. Cardiovasc. Res. 2013, 99, 353–363. [Google Scholar] [CrossRef]
- Kadayifci, A.; Tan, V.; Ursell, P.C.; Merriman, R.B.; Bass, N.M. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: A comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J. Hepatol. 2008, 49, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E.; Bjornsson, E. Coronary artery disease in liver cirrhosis: Does the aetiology of liver disease matter? J. Hepatol. 2009, 51, 962–963, Author Reply 963–964. [Google Scholar] [CrossRef][Green Version]
- Gologorsky, E.; Pretto, E.A., Jr.; Fukazawa, K. Coronary artery disease and its risk factors in patients presenting for liver transplantation. J. Clin. Anesth. 2013, 25, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kiefer, T.L.; Ahmed, A.; Ali, Z.A.; Tremmel, J.A.; Lee, D.P.; Yeung, A.C.; Fearon, W.F. Comparison of the frequency of coronary artery disease in alcohol-related versus non-alcohol-related endstage liver disease. Am. J. Cardiol. 2011, 108, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Shim, J.H.; Kim, S.O.; Lee, D.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Chung, Y.H.; Lee, Y.S. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: A registry-based matched case-control study. Circulation 2014, 130, 1353–1362. [Google Scholar] [CrossRef]
- Hafizi, S.; Mordi, V.N.; Andersson, K.M.; Chester, A.H.; Yacoub, M.H. Differential effects of rapamycin, cyclosporine A, and FK506 on human coronary artery smooth muscle cell proliferation and signalling. Vasc. Pharmacol. 2004, 41, 167–176. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Barlic, J.; Zhu, W.; Murphy, P.M. Atherogenic lipids induce high-density lipoprotein uptake and cholesterol efflux in human macrophages by up-regulating transmembrane chemokine CXCL16 without engaging CXCL16-dependent cell adhesion. J. Immunol. 2009, 182, 7928–7936. [Google Scholar] [CrossRef]
- van der Meer, J.J.; de Boer, O.J.; Teeling, P.; van der Loos, C.M.; Dessing, M.C.; van der Wal, A.C. Smooth muscle homeostasis in human atherosclerotic plaques through interleukin 15 signalling. Int. J. Clin. Exp. Pathol. 2011, 4, 287–294. [Google Scholar] [PubMed]
- Chen, Z.; Ichetovkin, M.; Kurtz, M.; Zycband, E.; Kawka, D.; Woods, J.; He, X.; Plump, A.S.; Hailman, E. Cholesterol in human atherosclerotic plaque is a marker for underlying disease state and plaque vulnerability. Lipids Health Dis. 2010, 9, 61. [Google Scholar] [CrossRef]
- Mia, M.M.; Bank, R.A. The IkappaB kinase inhibitor ACHP strongly attenuates TGFbeta1-induced myofibroblast formation and collagen synthesis. J. Cell Mol. Med. 2015, 19, 2780–2792. [Google Scholar] [CrossRef]
- Cindhuchao, N.; Quinn, D.A.; Garg, H.G.; Hales, C.A. Heparin inhibits SMC growth in the presence of human and fetal bovine serum. Biochem. Biophys. Res. Commun. 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Bu, D.; Liu, A.D.; Holmberg, L.; Jia, Y.; Tang, C.; Du, J.; Jin, H. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling. Cell Death Dis. 2014, 5, e1251. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Ahmetaj-Shala, B.; Mitchell, J.; Abdul-Salam, V.B.; Mahomed, A.S.; Aldabbous, L.; Oliver, E.; Iannone, L.; Dubois, O.D.; Storck, E.M.; et al. Tipifarnib prevents development of hypoxia-induced pulmonary hypertension. Cardiovasc. Res. 2017, 113, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Lu, Y.B.; Shi, C.; Yang, B.; Lu, Z.F.; Wu, Y.L.; Zhang, R.Y.; He, X.; Li, L.M.; Hu, B.; Hu, Y.W.; et al. Long noncoding RNA ZNF800 suppresses proliferation and migration of vascular smooth muscle cells by upregulating PTEN and inhibiting AKT/mTOR/HIF-1alpha signaling. Atherosclerosis 2020, 312, 43–53. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, L.; Jiang, R.; Zheng, L.; Liu, D. Oxidized high-density lipoprotein induces the proliferation and migration of vascular smooth muscle cells by promoting the production of ROS. J. Atheroscler Thromb. 2014, 21, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Fenu, M.; Bettermann, T.; Vogl, C.; Darwish-Miranda, N.; Schramel, J.; Jenner, F.; Ribitsch, I. A novel magnet-based scratch method for standardisation of wound-healing assays. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Rangrez, A.Y.; M’Baya-Moutoula, E.; Metzinger-Le Meuth, V.; Hénaut, L.; Djelouat, M.S.e.I.; Benchitrit, J.; Massy, Z.A.; Metzinger, L. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: Evidence for the involvement of miR-223. PLoS ONE 2012, 7, e47807. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel "gene expression’s CT difference" formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Gabbiani, G.; Bochaton-Piallat, M.L. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler Thromb. Vasc. Biol. 2003, 23, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Liu, Y.; Sinha, S.; McDonald, O.G.; Shang, Y.; Hoofnagle, M.H.; Owens, G.K. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 2005, 280, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Pagiatakis, C.; Gordon, J.W.; Ehyai, S.; McDermott, J.C. A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway regulates vascular smooth muscle cell gene expression. J. Biol. Chem. 2012, 287, 8361–8370. [Google Scholar] [CrossRef]
- Orlandi, A.; Francesconi, A.; Cocchia, D.; Corsini, A.; Spagnoli, L.G. Phenotypic heterogeneity influences apoptotic susceptibility to retinoic acid and cis-platinum of rat arterial smooth muscle cells in vitro: Implications for the evolution of experimental intimal thickening. Arterioscler Thromb. Vasc. Biol. 2001, 21, 1118–1123. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Shah, V.; Rockey, D.C. Vascular pathobiology in chronic liver disease and cirrhosis—current status and future directions. J. Hepatol. 2014, 61, 912–924. [Google Scholar] [CrossRef] [PubMed]
- De Donatis, A.; Comito, G.; Buricchi, F.; Vinci, M.C.; Parenti, A.; Caselli, A.; Camici, G.; Manao, G.; Ramponi, G.; Cirri, P. Proliferation versus migration in platelet-derived growth factor signaling: The key role of endocytosis. J. Biol. Chem. 2008, 283, 19948–19956. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, D.; Cartland, S.P.; Bursill, C.A.; Kavurma, M.M. Broad-spectrum chemokine inhibition blocks inflammation-induced angiogenesis, but preserves ischemia-driven angiogenesis. FASEB J. 2019, fj201900232RR. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, C.; Andersen, O.; Krag, A.; Bendtsen, F.; Moller, S. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur. J. Gastroenterol. Hepatol. 2012, 24, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Chapman, G.B.; Wang, H.; Durante, W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation 2002, 105, 79–84. [Google Scholar] [CrossRef]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef]
- Wray, C.; Scovotti, J.C.; Tobis, J.; Niemann, C.U.; Planinsic, R.; Walia, A.; Findlay, J.; Wagener, G.; Cywinski, J.B.; Markovic, D.; et al. Liver transplantation outcome in patients with angiographically proven coronary artery disease: A multi-institutional study. Am. J. Transpl. 2013, 13, 184–191. [Google Scholar] [CrossRef]


| Characteristics | Patients with Alcoholic Liver Cirrhosis n = 10 | Healthy Controls n = 10 | p-Value |
|---|---|---|---|
| Age (years) | 50.9 ± 1.5 | 50.4 ± 2.0 | 0.847 |
| Women (n) | 5 | 8 | 0.178 |
| MELD score (points) | 17.7 ± 0.6 | n/a | - |
| Child-Pugh A, B, C (n) | 0, 3, 7 | n/a | - |
| Initial diagnosis (years ago) | 8.6 ± 6.6 | n/a | - |
| Spironolactone (n) | 9 | 0 | - |
| Propranolol/carvedilol (n) | 6 | 0 | - |
| Diabetes (n) | 2 | 0 | - |
| Hypertension (n) | 1 | 0 | - |
| Smoking (n) | 4 | 0 | - |
| CAD (n) | 1 | 0 | - |
| PAD (n) | 1 | 0 | - |
| CKD III° or IV° (n) | 3 | 0 | - |
| BMI > 25 kg/m2 (n) | 3 | 0 | - |
| Serum Concentration | Patient with Liver Cirrhosis | Healthy Controls | p-Value |
|---|---|---|---|
| Bilirubin (mg/dL) | 3.87 ± 0.59 | 0.52 ± 0.09 | 0.002 |
| AST (U/L) | 70 ± 15 | 25 ± 2 | 0.015 |
| ALT (U/L) | 35 ± 5 | 25 ± 3 | 0.123 |
| Creatinine (mg/dL) | 1.33 ± 0.15 | 0.97 ± 0.10 | 0.062 |
| Albumin (g/dL) | 3.0 ± 0.2 | 5.5 ± 0.2 | <0.001 |
| CRP (mg/L) | 27.1 ± 4.7 | 1.5 ± 0.5 | <0.001 |
| Cholesterol (mg/dL) | 178 ± 23 | 203 ± 9 | 0.342 |
| Triglycerides (mg/dL) | 126 ± 22 | 111 ± 13 | 0.554 |
| LDL cholesterol (mg/dL) | 107 ± 11 | 116 ± 5 | 0.457 |
| IL-6 (mg/dL) | 41.5 ± 11.7 | 2.1 ± 0.4 | 0.008 |
| TNFα (pg/mL) | 13.3 ± 1.3 | 4.5 ± 0.3 | <0.001 |
| PDGF-BB (pg/mL) | 1160 ± 187 | 2411 ± 265 | 0.001 |
| VEGF (pg/mL) | 52.9 ± 10.2 | 87.1 ± 15.7 | 0.085 |
| Angiotensin II (pg/mL) | 286.1 ± 60.9 | 406.0 ± 111.1 | 0.356 |
| bFGF (pg/mL) | 20.09 ± 7.64 | 5.02 ± 1.42 | 0.134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechelinck, M.; Peschel, M.; Habigt, M.A.; Kroy, D.; Lehrke, M.; Helmedag, M.J.; Rossaint, R.; Barton, M.; Hein, M. Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells. J. Clin. Med. 2021, 10, 5471. https://doi.org/10.3390/jcm10235471
Mechelinck M, Peschel M, Habigt MA, Kroy D, Lehrke M, Helmedag MJ, Rossaint R, Barton M, Hein M. Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells. Journal of Clinical Medicine. 2021; 10(23):5471. https://doi.org/10.3390/jcm10235471
Chicago/Turabian StyleMechelinck, Mare, Miriam Peschel, Moriz A. Habigt, Daniela Kroy, Michael Lehrke, Marius J. Helmedag, Rolf Rossaint, Matthias Barton, and Marc Hein. 2021. "Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells" Journal of Clinical Medicine 10, no. 23: 5471. https://doi.org/10.3390/jcm10235471
APA StyleMechelinck, M., Peschel, M., Habigt, M. A., Kroy, D., Lehrke, M., Helmedag, M. J., Rossaint, R., Barton, M., & Hein, M. (2021). Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells. Journal of Clinical Medicine, 10(23), 5471. https://doi.org/10.3390/jcm10235471






