Quantitative Detection of Disseminated Melanoma Cells by Trp-1 Transcript Analysis Reveals Stochastic Distribution of Pulmonary Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Ex Vivo Admixture Experiments
2.3. Quantitative Real Time PCR (qRT-PCR)
2.4. Animals
2.5. Spontaneous Metastasis Assay
2.6. Autochthonous Metastasis Model
2.7. Hematoxylin-Eosin Staining
2.8. Statistical Analysis
3. Results
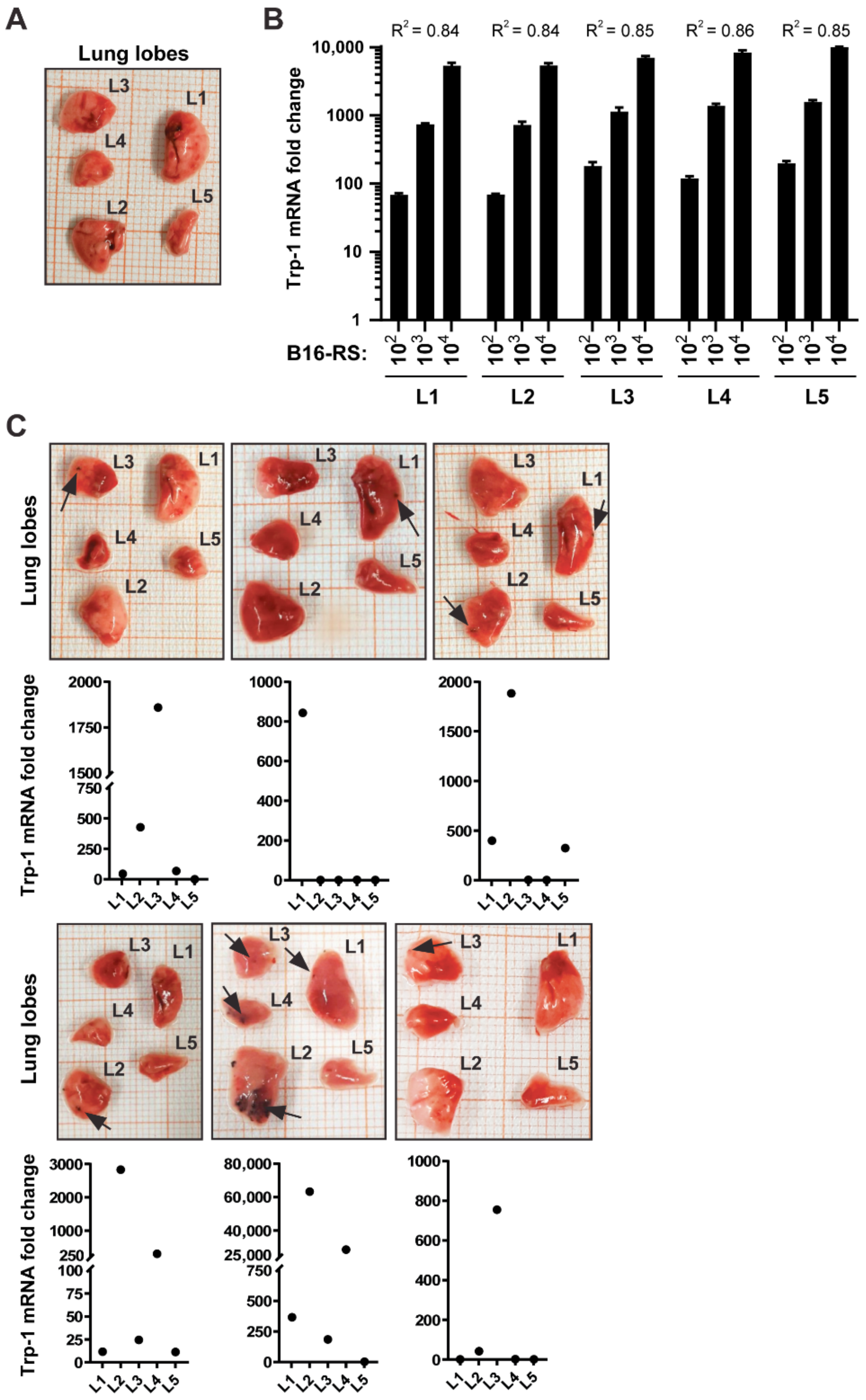
3.1. Trp-1 and Trp-2 Transcript Levels Correlate with Melanoma Cell Numbers Admixed with Lung Tissue Ex Vivo
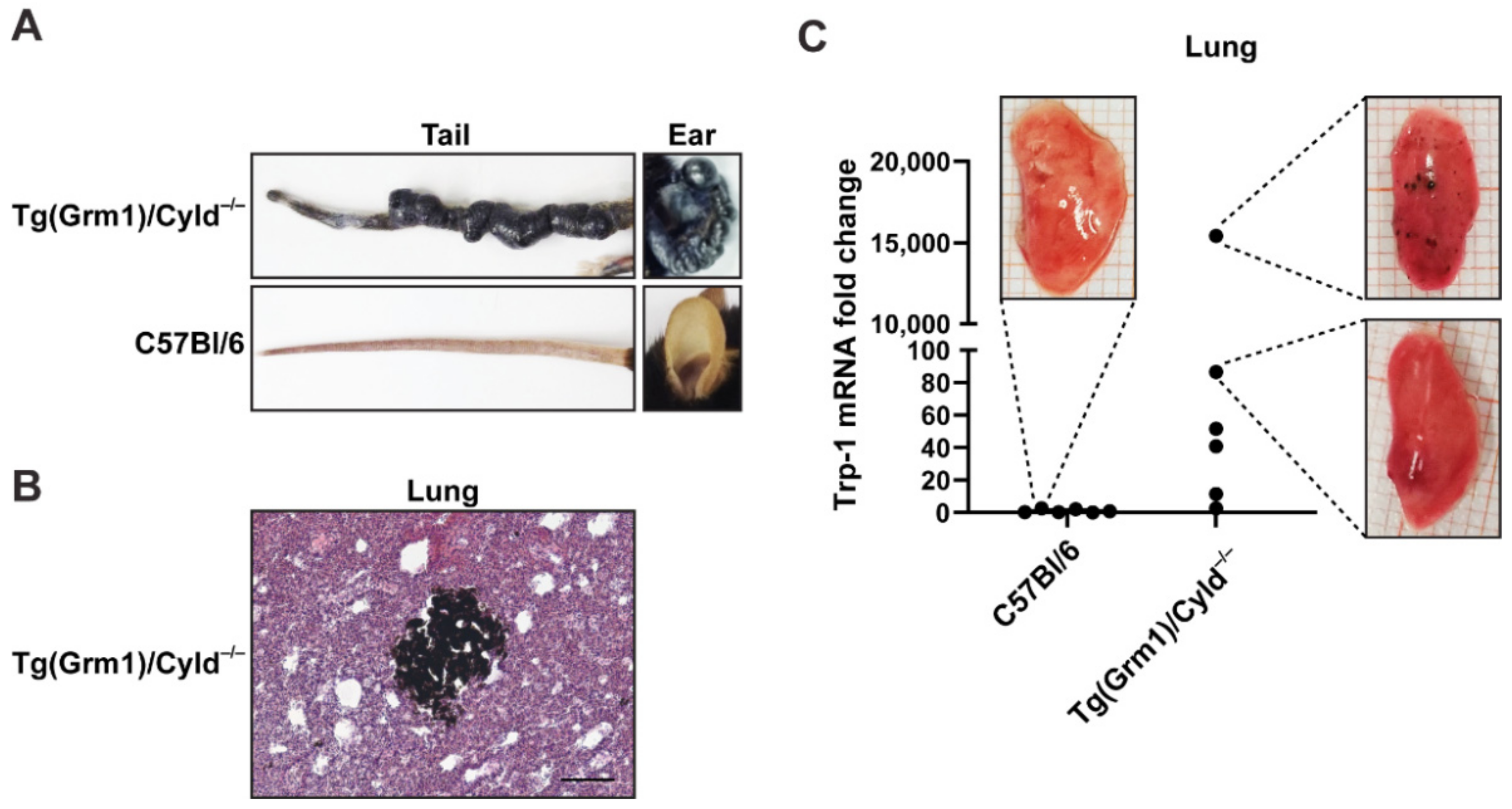
3.2. B16-RS Lung Metastases Are Distributed Stochastically between the Lobes of the Mouse Lung
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braeuer, R.R.; Watson, I.R.; Wu, C.J.; Mobley, A.K.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014, 27, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Abrao, F.C.; Gross, J. Pulmonary metastasectomy for malignant melanoma: Prognostic factors for long-term survival. Melanoma Res. 2013, 23, 307–311. [Google Scholar] [CrossRef]
- Soliman, M.; Petrella, T.; Tyrrell, P.; Wright, F.; Look Hong, N.J.; Lu, H.; Zezos, P.; Jimenez-Juan, L.; Oikonomou, A. The clinical significance of indeterminate pulmonary nodules in melanoma patients at baseline and during follow-up chest CT. Eur. J. Radiol. Open 2019, 6, 85–90. [Google Scholar] [CrossRef]
- Borghesi, A.; Tironi, A.; Michelini, S.; Scrimieri, A.; Benetti, D.; Maroldi, R. Two synchronous lung metastases from malignant melanoma: The same patient but different morphological patterns. Eur. J. Radiol. Open 2019, 6, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Erler, J.T. Quantification of Lung Metastases from In Vivo Mouse Models. Adv. Exp. Med. Biol. 2016, 899, 245–251. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Steinbauer, M.; Guba, M.; Cernaianu, G.; Kohl, G.; Cetto, M.; Kunz-Schughart, L.A.; Geissler, E.K.; Falk, W.; Jauch, K.W. GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term tumor development studies in immunocompetent mice. Clin. Exp. Metastasis 2003, 20, 135–141. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Kilpelainen, A.; Petkov, S.; Abakumov, M.A.; Grinenko, N.F.; Yusubalieva, G.M.; Latanova, A.A.; Gubskiy, I.L.; Zabozlaev, F.G.; Starodubova, E.S.; et al. Luciferase Expression Allows Bioluminescence Imaging But Imposes Limitations on the Orthotopic Mouse (4T1) Model of Breast Cancer. Sci. Rep. 2017, 7, 7715. [Google Scholar] [CrossRef] [PubMed]
- Banan, B.; Beckstead, J.A.; Dunavant, L.E.; Sohn, Y.; Adcock, J.M.; Nomura, S.; Abumrad, N.; Goldenring, J.R.; Fingleton, B. Development of a novel murine model of lymphatic metastasis. Clin. Exp. Metastasis 2020, 37, 247–255. [Google Scholar] [CrossRef]
- Moriyama, E.H.; Niedre, M.J.; Jarvi, M.T.; Mocanu, J.D.; Moriyama, Y.; Subarsky, P.; Li, B.; Lilge, L.D.; Wilson, B.C. The influence of hypoxia on bioluminescence in luciferase-transfected gliosarcoma tumor cells in vitro. Photochem. Photobiol. Sci. 2008, 7, 675–680. [Google Scholar] [CrossRef]
- Becker, M.; Nitsche, A.; Neumann, C.; Aumann, J.; Junghahn, I.; Fichtner, I. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. Br. J. Cancer 2002, 87, 1328–1335. [Google Scholar] [CrossRef]
- Schneider, T.; Osl, F.; Friess, T.; Stockinger, H.; Scheuer, W.V. Quantification of human Alu sequences by real-time PCR--an improved method to measure therapeutic efficacy of anti-metastatic drugs in human xenotransplants. Clin. Exp. Metastasis 2002, 19, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, B.L.; Parker, B.S.; van Laar, R.K.; Restall, C.M.; Natoli, A.L.; Tavaria, M.D.; Stanley, K.L.; Sloan, E.K.; Moseley, J.M.; Anderson, R.L. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol. Cancer Res. 2005, 3, 1–13. [Google Scholar] [PubMed]
- Malek, A.; Catapano, C.V.; Czubayko, F.; Aigner, A. A sensitive polymerase chain reaction-based method for detection and quantification of metastasis in human xenograft mouse models. Clin. Exp. Metastasis 2010, 27, 261–271. [Google Scholar] [CrossRef]
- Sorensen, M.R.; Pedersen, S.R.; Lindkvist, A.; Christensen, J.P.; Thomsen, A.R. Quantification of B16 melanoma cells in lungs using triplex Q-PCR--a new approach to evaluate melanoma cell metastasis and tumor control. PLoS ONE 2014, 9, e87831. [Google Scholar] [CrossRef]
- Abt, M.A.; Grek, C.L.; Ghatnekar, G.S.; Yeh, E.S. Evaluation of Lung Metastasis in Mouse Mammary Tumor Models by Quantitative Real-time PCR. J. Vis. Exp. 2016, e53329. [Google Scholar] [CrossRef]
- Schwartz, H.; Blacher, E.; Amer, M.; Livneh, N.; Abramovitz, L.; Klein, A.; Ben-Shushan, D.; Soffer, S.; Blazquez, R.; Barrantes-Freer, A.; et al. Incipient Melanoma Brain Metastases Instigate Astrogliosis and Neuroinflammation. Cancer Res. 2016, 76, 4359–4371. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; McLaughlin, S.L.; Klinke, D.J. Quantifying spontaneous metastasis in a syngeneic mouse melanoma model using real time PCR. Analyst 2017, 142, 2945–2953. [Google Scholar] [CrossRef]
- del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Ghanem, G.; Fabrice, J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol. Oncol. 2011, 5, 150–155. [Google Scholar] [CrossRef]
- Wang, R.F.; Robbins, P.F.; Kawakami, Y.; Kang, X.Q.; Rosenberg, S.A. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J. Exp. Med. 1995, 181, 799–804. [Google Scholar] [CrossRef]
- Orlow, S.J.; Hearing, V.J.; Sakai, C.; Urabe, K.; Zhou, B.K.; Silvers, W.K.; Mintz, B. Changes in expression of putative antigens encoded by pigment genes in mouse melanomas at different stages of malignant progression. Proc. Natl. Acad. Sci. USA 1995, 92, 10152–10156. [Google Scholar] [CrossRef]
- Chu, W.; Pak, B.J.; Bani, M.R.; Kapoor, M.; Lu, S.J.; Tamir, A.; Kerbel, R.S.; Ben-David, Y. Tyrosinase-related protein 2 as a mediator of melanoma specific resistance to cis-diamminedichloroplatinum(II): Therapeutic implications. Oncogene 2000, 19, 395–402. [Google Scholar] [CrossRef]
- Bloom, M.B.; Perry-Lalley, D.; Robbins, P.F.; Li, Y.; el-Gamil, M.; Rosenberg, S.A.; Yang, J.C. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J. Exp. Med. 1997, 185, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wallbaum, S.; Grau, N.; Schmid, A.; Frick, K.; Neeb, A.; Sleeman, J.P. Cell cycle quiescence can suppress transcription from an ecdysone receptor-based inducible promoter in mammalian cells. Biotechniques 2009, 46, 433–440. [Google Scholar] [CrossRef] [PubMed]
- de Jel, M.M.; Schott, M.; Lamm, S.; Neuhuber, W.; Kuphal, S.; Bosserhoff, A.K. Loss of CYLD accelerates melanoma development and progression in the Tg(Grm1) melanoma mouse model. Oncogenesis 2019, 8, 56. [Google Scholar] [CrossRef]
- Pollock, P.M.; Cohen-Solal, K.; Sood, R.; Namkoong, J.; Martino, J.J.; Koganti, A.; Zhu, H.; Robbins, C.; Makalowska, I.; Shin, S.S.; et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 2003, 34, 108–112. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Suarez, C.J.; Dintzis, S.M.; Frevert, C.W. 9-Respiratory System. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 147–162. [Google Scholar]
- Schiffner, S.; Chen, S.; Becker, J.C.; Bosserhoff, A.K. Highly pigmented Tg(Grm1) mouse melanoma develops non-pigmented melanoma cells in distant metastases. Exp. Dermatol. 2012, 21, 786–788. [Google Scholar] [CrossRef]
- Taus, L.J.; Flores, R.E.; Seyfried, T.N. Quantification of metastatic load in a syngeneic murine model of metastasis. Cancer Lett. 2017, 405, 56–62. [Google Scholar] [CrossRef]
- Orlow, S.J.; Silvers, W.K.; Zhou, B.K.; Mintz, B. Comparative decreases in tyrosinase, TRP-1, TRP-2, and Pmel 17/silver antigenic proteins from melanotic to amelanotic stages of syngeneic mouse cutaneous melanomas and metastases. Cancer Res. 1998, 58, 1521–1523. [Google Scholar] [PubMed]
- Meeth, K.; Wang, J.X.; Micevic, G.; Damsky, W.; Bosenberg, M.W. The YUMM lines: A series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment. Cell Melanoma Res. 2016, 29, 590–597. [Google Scholar] [CrossRef]
- Fang, D.; Hallman, J.; Sangha, N.; Kute, T.E.; Hammarback, J.A.; White, W.L.; Setaluri, V. Expression of microtubule-associated protein 2 in benign and malignant melanocytes: Implications for differentiation and progression of cutaneous melanoma. Am. J. Pathol. 2001, 158, 2107–2115. [Google Scholar] [CrossRef]
- Lenggenhager, D.; Curioni-Fontecedro, A.; Storz, M.; Shakhova, O.; Sommer, L.; Widmer, D.S.; Seifert, B.; Moch, H.; Dummer, R.; Mihic-Probst, D. An Aggressive Hypoxia Related Subpopulation of Melanoma Cells is TRP-2 Negative. Transl. Oncol. 2014, 7, 206–212. [Google Scholar] [CrossRef][Green Version]
- Bolander, A.; Agnarsdottir, M.; Stromberg, S.; Ponten, F.; Hesselius, P.; Uhlen, M.; Bergqvist, M. The protein expression of TRP-1 and galectin-1 in cutaneous malignant melanomas. Cancer Genom. Proteom. 2008, 5, 293–300. [Google Scholar]
- Journe, F.; Id Boufker, H.; Van Kempen, L.; Galibert, M.D.; Wiedig, M.; Sales, F.; Theunis, A.; Nonclercq, D.; Frau, A.; Laurent, G.; et al. TYRP1 mRNA expression in melanoma metastases correlates with clinical outcome. Br. J. Cancer. 2011, 105, 1726–1732. [Google Scholar] [CrossRef]
- El Hajj, P.; Gilot, D.; Migault, M.; Theunis, A.; van Kempen, L.C.; Sales, F.; Fayyad-Kazan, H.; Badran, B.; Larsimont, D.; Awada, A.; et al. SNPs at miR-155 binding sites of TYRP1 explain discrepancy between mRNA and protein and refine TYRP1 prognostic value in melanoma. Br. J. Cancer. 2015, 113, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tief, K.; Hahne, M.; Schmidt, A.; Beermann, F. Tyrosinase, the key enzyme in melanin synthesis, is expressed in murine brain. Eur. J. Biochem. 1996, 241, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.D.; Merchant, R.E.; Rand, R.; Conrad, A.J.; Garrison, D.; Turner, R.; Morton, D.L.; Hoon, D.S. Molecular detection of tumor-associated antigens shared by human cutaneous melanomas and gliomas. Am. J. Pathol. 1997, 150, 2143–2152. [Google Scholar]
- Gautron, A.; Migault, M.; Bachelot, L.; Corre, S.; Galibert, M.D.; Gilot, D. Human TYRP1: Two functions for a single gene? Pigment Cell Melanoma Res. 2021, 34, 836–852. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyjacova, L.; Saup, R.; Rothley, M.; Schmaus, A.; Wagner, T.; Boßerhoff, A.; Garvalov, B.K.; Thiele, W.; Sleeman, J.P. Quantitative Detection of Disseminated Melanoma Cells by Trp-1 Transcript Analysis Reveals Stochastic Distribution of Pulmonary Metastases. J. Clin. Med. 2021, 10, 5459. https://doi.org/10.3390/jcm10225459
Kyjacova L, Saup R, Rothley M, Schmaus A, Wagner T, Boßerhoff A, Garvalov BK, Thiele W, Sleeman JP. Quantitative Detection of Disseminated Melanoma Cells by Trp-1 Transcript Analysis Reveals Stochastic Distribution of Pulmonary Metastases. Journal of Clinical Medicine. 2021; 10(22):5459. https://doi.org/10.3390/jcm10225459
Chicago/Turabian StyleKyjacova, Lenka, Rafael Saup, Melanie Rothley, Anja Schmaus, Tabea Wagner, Anja Boßerhoff, Boyan K. Garvalov, Wilko Thiele, and Jonathan P. Sleeman. 2021. "Quantitative Detection of Disseminated Melanoma Cells by Trp-1 Transcript Analysis Reveals Stochastic Distribution of Pulmonary Metastases" Journal of Clinical Medicine 10, no. 22: 5459. https://doi.org/10.3390/jcm10225459
APA StyleKyjacova, L., Saup, R., Rothley, M., Schmaus, A., Wagner, T., Boßerhoff, A., Garvalov, B. K., Thiele, W., & Sleeman, J. P. (2021). Quantitative Detection of Disseminated Melanoma Cells by Trp-1 Transcript Analysis Reveals Stochastic Distribution of Pulmonary Metastases. Journal of Clinical Medicine, 10(22), 5459. https://doi.org/10.3390/jcm10225459





