Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis
Abstract
:1. Introduction
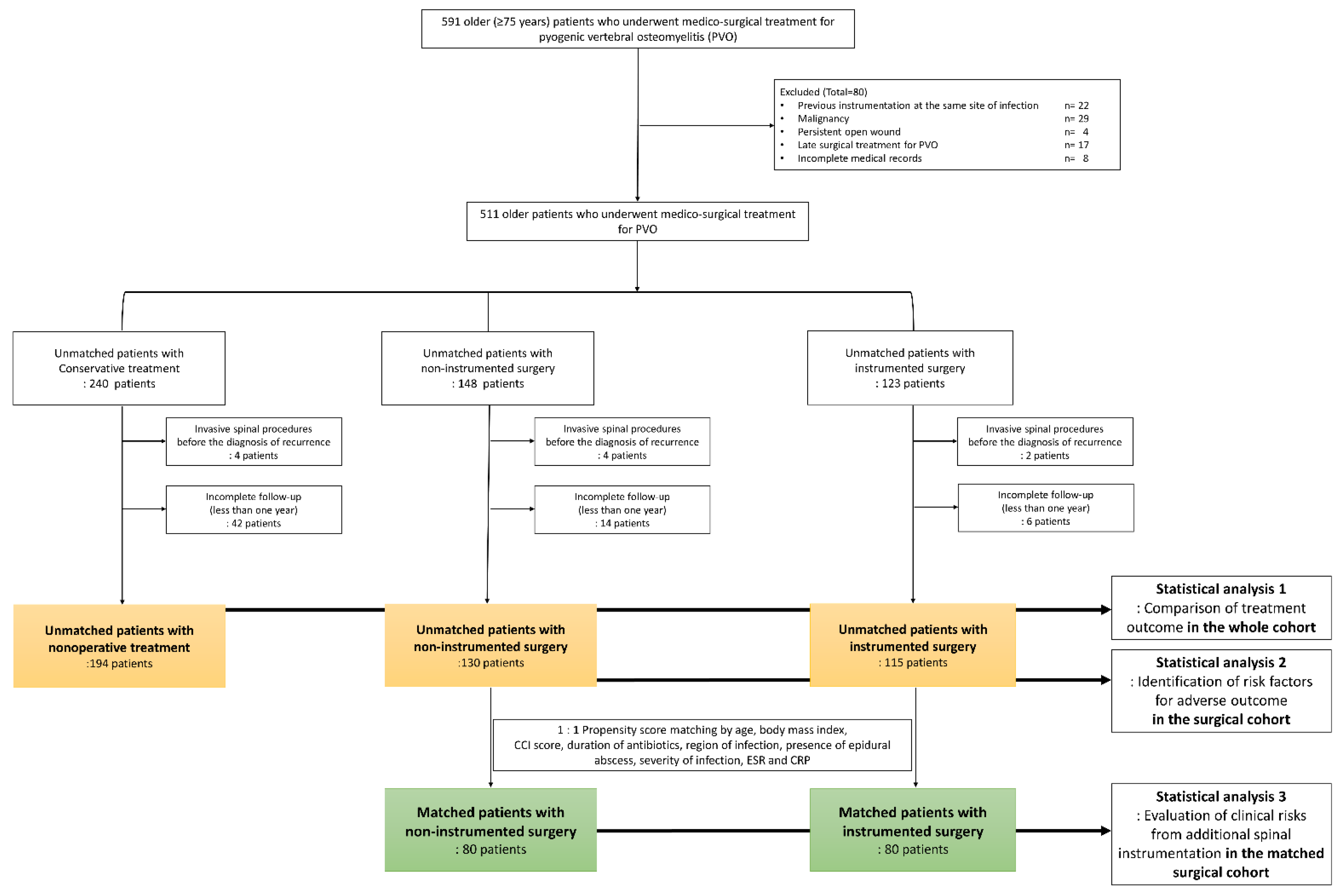
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Covariables and Treatment Outcomes
2.3. Protocol for Surgical Treatment of Older Patients with PVO
2.4. Statistical Analysis 1: Comparison of Treatment Outcomes According to the Three Treatment Groups in the Whole Cohort
2.5. Statistical Analysis 2: Identification of Risk Factors for Adverse Outcomes in the Surgical Cohort
2.6. Statistical Analysis 3: Evaluation of Clinical Risks from Additional Spinal Instrumentation in the Matched Surgical Cohort
3. Results
3.1. Demographics and Completeness of Follow-Up
3.2. Clinical Characteristics of the Three Treatment Groups
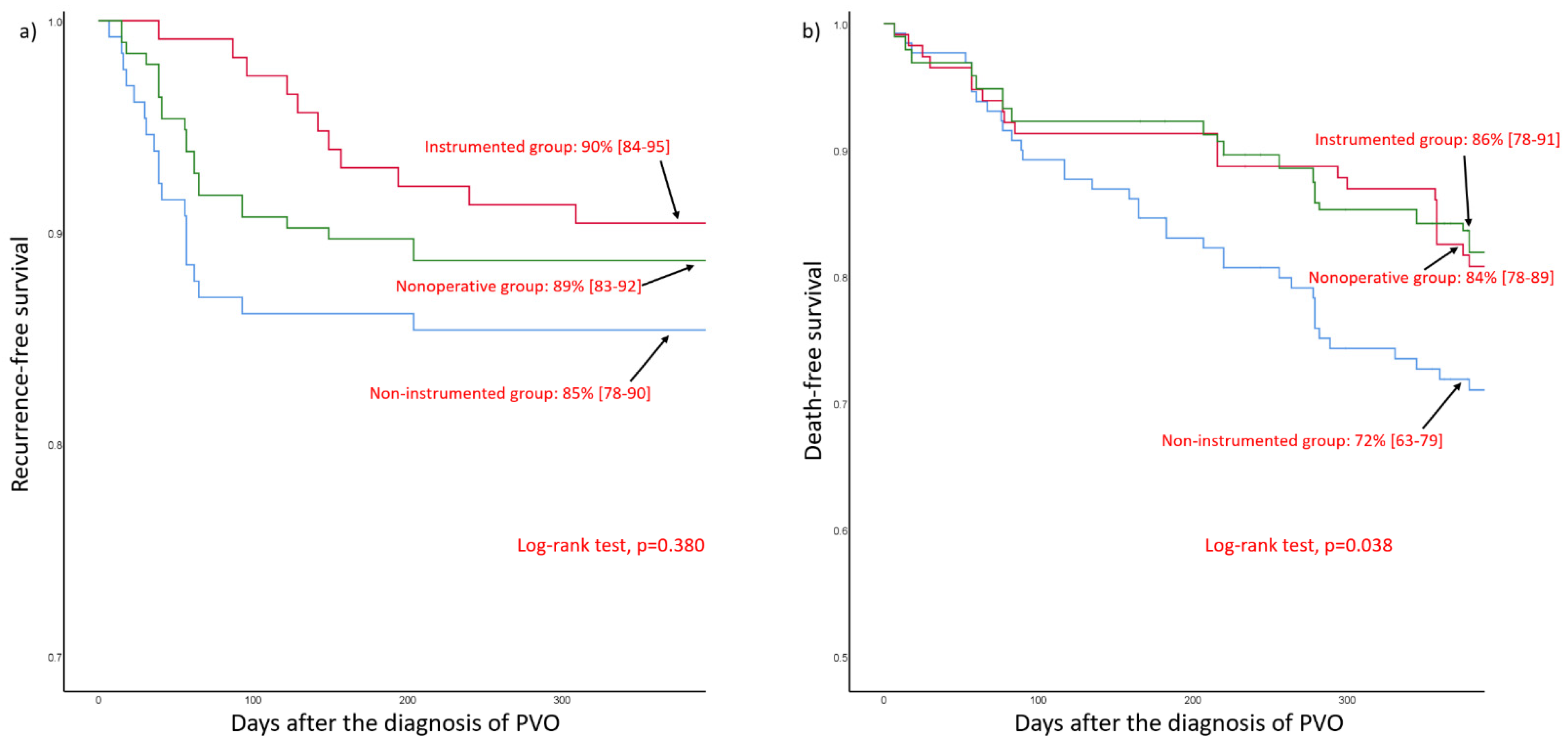
3.3. Comparison of Treatment Outcome According to the Three Treatment Groups in the Whole Cohort
3.4. Identification of Risk Factors for Recurrence or Mortality in the Surgical Cohort
3.5. Evaluation of Clinical Risks of Additional Spinal Instrumentation in the Matched Surgical Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kremers, H.M.; Nwojo, M.E.; Ransom, J.E.; Wood-Wentz, C.M.; Melton, L.J., 3rd; Huddleston, P.M., 3rd. Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J. Bone Joint. Surg. Am. 2015, 97, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehrer, M.; Pedersen, C.; Jensen, T.G.; Lassen, A.T. Increasing incidence of pyogenic spondylodiscitis: A 14-year population-based study. J. Infect. 2014, 68, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, P.; Rivero, A.; del Castillo, N.; Jarque, A.; Getino, M.A.; Garcia-Perez, J.; Navarro-Gonzalez, J.F. Infectious spondylodiscitis in hemodialysis. Semin. Dial. 2010, 23, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.; Sun, W.C.; Lu, Y.A.; Chen, C.Y.; Kao, H.K.; Lin, Y., Jr.; Chen, Y.C.; Hung, C.C.; Tian, Y.C.; Hsu, H.H. Chronic dialysis patients with infectious spondylodiscitis have poorer outcomes than non-dialysis populations. Ther. Clin. Risk. Manag. 2018, 14, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, E.K.; Sinha, R. Vertebral osteomyelitis in adults: An update. Br. Med. Bull. 2016, 117, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Pourtaheri, S.; Issa, K.; Stewart, T.; Shafa, E.; Ajiboye, R.; Buerba, R.A.; Lord, E.; Hwang, K.; Mangels, D.; Emami, A. Comparison of Instrumented and Noninstrumented Surgical Treatment of Severe Vertebral Osteomyelitis. Orthopedics 2016, 39, e504–e508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.; Kim, T.H. Treatment Guideline for Patients with Native Culture-negative Pyogenic Vertebral Osteomyelitis. Clin. Orthop. Relat. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Cho, O.H.; Lee, Y.M.; Moon, C.; Park, S.Y.; Moon, S.M.; Lee, J.H.; Park, J.S.; Ryu, K.N.; Kim, S.H.; et al. Therapeutic outcomes of hematogenous vertebral osteomyelitis with instrumented surgery. Clin. Infect. Dis. 2015, 60, 1330–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jang, S.B.; Kim, S.W.; Oh, J.-K.; Kim, T.-H. Clinical effect of early bisphosphonate treatment for pyogenic vertebral osteomyelitis with osteoporosis: An analysis by the Cox proportional hazard model. Spine J. 2019, 19, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Jung, J.; Kim, S.; Oh, J.; Park, M.; Chang, H.; Kim, T.J.B.J.J. The outcome following spinal instrumentation in haemodialyzed patients with pyogenic spondylodiscitis. Bone Joint J. 2019, 101, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Cho, O.-H.; Lee, J.H.; Park, J.S.; Ryu, K.N.; Park, S.Y.; Lee, Y.-M.; Chong, Y.P.; Kim, S.-H.; Lee, S.-O.; et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin. Infect. Dis. 2016, 62, 1262–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laksmi, P.W.; Harimurti, K.; Setiati, S.; Soejono, C.H.; Aries, W.; Roosheroe, A.G.J.A.M.I. Management of immobilization and its complication for elderly. Acta. Med. Indones. 2008, 40, 233–240. [Google Scholar] [PubMed]
- Faller, N.; Limacher, A.; Méan, M.; Righini, M.; Aschwanden, M.; Beer, J.H.; Frauchiger, B.; Osterwalder, J.; Kucher, N.; Lämmle, B.; et al. Predictors and Causes of Long-Term Mortality in Elderly Patients with Acute Venous Thromboembolism: A Prospective Cohort Study. Am. J. Med. 2017, 130, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M.; Petermann, G.W. 2015 infectious diseases society of america (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adultsa. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Pola, E.; Autore, G.; Formica, V.M.; Pambianco, V.; Colangelo, D.; Cauda, R.; Fantoni, M. New classification for the treatment of pyogenic spondylodiscitis: Validation study on a population of 250 patients with a follow-up of 2 years. Eur. Spine J. 2017, 26, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, H.S.; Kim, J.W.; Kim, S.W.; Oh, J.-K.; Kim, Y.-W.; Park, M.S.; Kim, T.-H. Treatment outcomes in patients with pyogenic vertebral osteomyelitis who have cirrhosis. Sci. Rep. 2019, 9, 15223. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H.; Kim, S.W.; Oh, J.K.; Kim, Y.W.; Kim, T.H. Outcomes of additional instrumentation in elderly patients with pyogenic vertebral osteomyelitis and previous spinal instrumentation. Spine J. 2019, 19, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ryu, H.; Kim, S.W.; Oh, J.K.; Kim, T.H. Prediction of Recurrence in Pyogenic Vertebral Osteomyelitis by Artificial Neural Network Using Time-series Data of C-Reactive Protein: A Retrospective Cohort Study of 704 Patients. Spine 2021, 46, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Xu, C.; Kuo, F.C.; Ghanem, E.; Higuera, C.; Parvizi, J. Risk Factors for Failure and Optimal Treatment of Total Joint Arthroplasty for Septic Arthritis. J. Arthroplast. 2021, 36, 892–896. [Google Scholar] [CrossRef] [PubMed]
| Variables | Category | Whole Cohort | ||||
|---|---|---|---|---|---|---|
| Nonoperative Group | Surgical Group | p-Value 2 | ||||
| Non-Instrumented Group | Instrumented Group | p-Value 1 | ||||
| Number of patients | 194 | 130 | 115 | |||
| Age | Median (Interquartile Ranges) | 80 (80–85) | 80 (79–83) | 81 (79–82) | 0.838 | <0.001 |
| 75 to 79 years | 38 (20) | 44 (34) | 38 (33) | 0.979 | <0.001 | |
| 80 to 84 years | 107 (55) | 69 (53) | 61 (53) | |||
| ≥85 years | 49 (25) | 17 (13) | 16 (14) | |||
| Sex ratio (F:M) | 126:68 | 86:44 | 73:42 | 0.661 | 0.991 | |
| BMI (kg/m2) | 22.6 (20.9–24.6) | 22.4 (21.2–24.5) | 23.9 (21.0–26.3) | 0.170 | 0.093 | |
| Charlson Comorbidity Index score | Median (Interquartile ranges) | 3.0 (2.0–5.0) | 3.0 (1.0–4.0) | 2.0 (1.0–4.0) | 0.041 | 0.006 |
| 0 and 1 | 46 (24) | 37 (28) | 50 (43) | 0.047 | 0.028 | |
| 2 and 3 | 79 (41) | 49 (38) | 36 (31) | |||
| over 4 | 69 (36) | 44 (34) | 29 (25) | |||
| Comorbidities | Cerebrovascular Disease | 31 (16) | 27 (21) | 26 (23) | 0.727 | 0.135 |
| Myocardial Infarction | 48 (25) | 36 (28) | 29 (25) | 0.661 | 0.670 | |
| Congestive Heart Failure | 40 (21) | 22 (17) | 11 (10) | 0.092 | 0.046 | |
| Chronic Obstructive Pulmonary Disease | 24 (12) | 15 (12) | 10 (9) | 0.463 | 0.474 | |
| Liver Cirrhosis | 26 (13) | 17 (13) | 9 (8) | 0.183 | 0.369 | |
| End-Stage Renal Disease | 56 (29) | 41 (32) | 27 (23) | 0.160 | 0.797 | |
| Diabetes | 0.612 | 0.867 | ||||
| Complicated 3 | 92 (47) | 60 (46) | 52 (45) | |||
| Uncomplicated | 26 (13) | 22 (17) | 15 (13) | |||
| Bone Mineral Density (g/cm2) | Spine | 0.808 (0.721–0.919) | 0.854 (0.732–1.010) | 0.892 (0.777–0.981) | 0.317 | 0.001 |
| Femur | 0.639 (0.557–0.741) | 0.645 (0.575–0.743) | 0.700 (0.609–0.757) | 0.101 | 0.082 | |
| American Spinal Injury Association Scale Grade | Grade A, B and C | 9 (5) | 18 (14) | 25 (22) | 0.118 | <0.001 |
| Grade D | 90 (46) | 71 (55) | 49 (43) | |||
| Grade E | 95 (49) | 41 (32) | 41(36) | |||
| Duration of Intravenous Antibiotics (Days) | 52 (45–63) | 44 (42–56) | 52 (45–63) | <0.001 | 0.004 | |
| Hospital Stay (Days) | 60 (53–72) | 52 (47–59) | 54 (47–63) | 0.084 | <0.001 | |
| Follow-Up Period | 605 (387 -1132) | 801 (478–1262) | 1036 (597–1457) | |||
| Variables | Category | Whole Cohort | ||||
|---|---|---|---|---|---|---|
| Nonoperative Group | Surgical Group | p-Value 2 | ||||
| Non-Instrumented Group | Instrumented Group | p-Value 1 | ||||
| Primary Region 3 | Cervical Spine | 17 (9) | 10 (8) | 23 (20) | 0.022 | 0.405 |
| Thoracic spine | 77 (40) | 52 (40) | 48 (42) | |||
| Lumbosacrum | 95 (49) | 64 (49) | 42 (37) | |||
| Multiple | 5 (3) | 4 (3) | 2 (2) | |||
| Presence of abscess | Epidural abscess | 103 (53) | 115 (88) | 111 (97) | 0.019 | <0.001 |
| Posterior to epidural space | 121 (62) | 96 (74) | 91 (79) | 0.332 | 0.002 | |
| Anterior to epidural space | 89 (46) | 81 (62) | 66 (57) | 0.433 | 0.003 | |
| Number of infected vertebral bodies | Within 2 vertebral bodies | 51 (26) | 95 (73) | 77 (67) | 0.296 | 0.418 |
| Over 3 vertebral bodies | 143 (74) | 35 (27) | 38 (33) | |||
| Severity of infection by Pola, et al. | Type A | 4 (2) | 2 (2) | 0 (0) | 0.015 | <0.001 |
| Type B | 86 (44) | 13 (10) | 4 (3) | |||
| Type C | 104 (54) | 115 (88) | 111 (97) | |||
| Causative organism | Staphylococcus aureus | 78 (40) | 52 (40) | 47 (41) | 0.586 | 0.978 |
| Methicillin resistant | 40 (21) | 24 (18) | 24 (21) | |||
| methicillin sensitive | 38 (20) | 28 (22) | 23 (20) | |||
| Other gram-positive bacteria | 23 (12) | 15 (12) | 15 (13) | |||
| Gram-negative bacteria | 70 (36) | 46 (35) | 45 (39) | |||
| Others or unidentified | 23 (12) | 17 (13) | 8 (7) | |||
| White blood cell (×109/L) | Initial | 12,399 (9926–15,556) | 12,679 (10,105–15,436) | 13,060 (10,330–16,799) | 0.155 | 0.380 |
| Erythrocyte sedimentation rate (esr, mm/h) | Initial | 59 (44–70) | 62 (47–71) | 69 (53–75) | 0.008 | 0.002 |
| C-reactive protein (crp, mg/L) | Initial | 71 (57–83) | 69 (58–83) | 75 (67–88) | 0.036 | 0.180 |
| Major medical events after treatment | At least one of the following complications | 60 (31) | 39 (30) | 25 (22) | 0.142 | 0.267 |
| Cardiac event | 15 (8) | 11 (8) | 7 (6) | |||
| Respiratory complication | 38 (20) | 25 (19) | 15 (13) | |||
| Cerebrovascular complication | 5 (3) | 4 (3) | 4 (3) | |||
| pulmonary embolism | 11 (6) | 8 (6) | 4 (3) | |||
| Recurrence | occurrence | 22 (11) | 19 (15) | 11 (10) | 0.229 | 0.771 |
| Interval between initial diagnosis and recurrence (days) | 55 (39–93) | 39 (23–57) | 142 (96–194) | <0.001 | 0.767 | |
| Mortality | 90-day mortality | 15 (8) | 14 (11) | 10 (9) | 0.586 | 0.450 |
| 1-year mortality | 30 (15) | 36 (28) | 20 (17) | 0.055 | 0.053 | |
| Outcome | Category | Group | Odds Ratios/Hazard Ratios | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Recurrence 1 | Non-instrumented group vs. nonoperative group | 0.99 | (0.45–2.20) | 0.983 | |
| Instrumented group vs. nonoperative group | 0.75 | (0.30–1.91) | 0.547 | ||
| Mortality | 90-day mortality 1 | Non-instrumented group vs. nonoperative group | 1.88 | (0.69–5.11) | 0.218 |
| Instrumented group vs. nonoperative group | 1.73 | (0.58–5.20) | 0.330 | ||
| 1-year mortality 1 | Non-instrumented group vs. nonoperative group | 2.67 | (1.27–5.61) | 0.010 | |
| Instrumented group vs. nonoperative group | 1.35 | (0.59–3.07) | 0.477 | ||
| Overall mortality 2 | Non-instrumented group vs. nonoperative group | 1.67 | (1.09–2.57) | 0.019 | |
| Instrumented group vs. nonoperative group | 1.07 | (0.65–1.75) | 0.798 |
| Outcomes | Variables | Category | Multivariable (Backward) | Bootstrap-Adjusted | Bias | ||
|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | |||||
| Recurrence | Comorbidities | Liver cirrhosis | 3.60 | (1.17–11.09) | 0.026 | 3.79 (1.06–12.10) | −5.2 |
| Diabetes, complicated | 5.00 | (1.73–14.50) | 0.003 | 4.38 (1.25–30.63) | 12.5 | ||
| ASIA grade | Grade A, B and C vs. grade E | 4.28 | (1.36–13.52) | 0.013 | 3.94 (1.01–25.30) | 8 | |
| Causative organism | MRSA vs. no MRSA | 4.35 | (1.67–11.35) | 0.003 | 4.02 (1.10–19.87) | 7.5 | |
| One-year mortality | CCI score | Per 1 point | 1.41 | (1.17–1.69) | <0.001 | 1.39 (1.13–1.79) | 1.1 |
| Comorbidities | Liver cirrhosis | 3.01 | (1.04–8.74) | 0.042 | 2.89 (0.93–13.05) | 4.1 | |
| ASIA grade | Grade A, B and C vs. grade E | 7.44 | (2.89–19.16) | <0.001 | 6.76 (2.34–31.63) | 9.1 | |
| Outcomes | Category | Group | Odds Ratios/Hazard Ratios | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Recurrence 1 | Instrumented group vs. non-instrumented group | 1.29 | (0.45–3.73) | 0.638 | |
| Mortality | 90-day mortality 1 | Instrumented group vs. non-instrumented group | 1.43 | (0.45–4.81) | 0.524 |
| 1-year mortality 1 | Instrumented group vs. non-instrumented group | 0.87 | (0.36–2.11) | 0.764 | |
| Overall mortality 2 | Instrumented group vs. non-instrumented group | 1.12 | (0.61–2.08) | 0.713 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, J.; Kim, T.-H. Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis. J. Clin. Med. 2021, 10, 5451. https://doi.org/10.3390/jcm10225451
Lee JH, Kim J, Kim T-H. Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis. Journal of Clinical Medicine. 2021; 10(22):5451. https://doi.org/10.3390/jcm10225451
Chicago/Turabian StyleLee, Jeong Hwan, Jihye Kim, and Tae-Hwan Kim. 2021. "Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis" Journal of Clinical Medicine 10, no. 22: 5451. https://doi.org/10.3390/jcm10225451
APA StyleLee, J. H., Kim, J., & Kim, T.-H. (2021). Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis. Journal of Clinical Medicine, 10(22), 5451. https://doi.org/10.3390/jcm10225451






