Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus
Abstract
:1. Introduction
2. Background on rTMS and Tinnitus
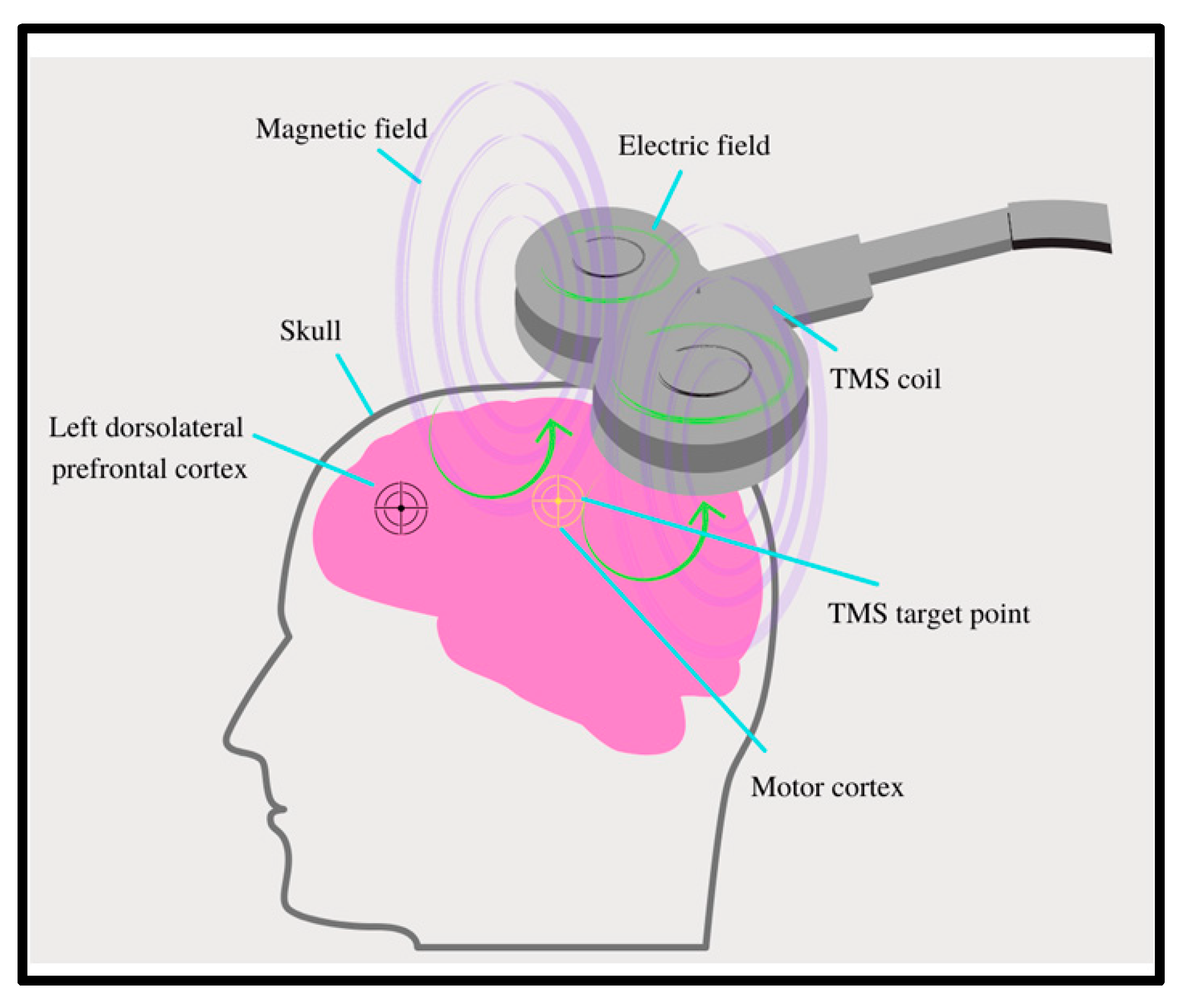
2.1. Technology Overview of rTMS
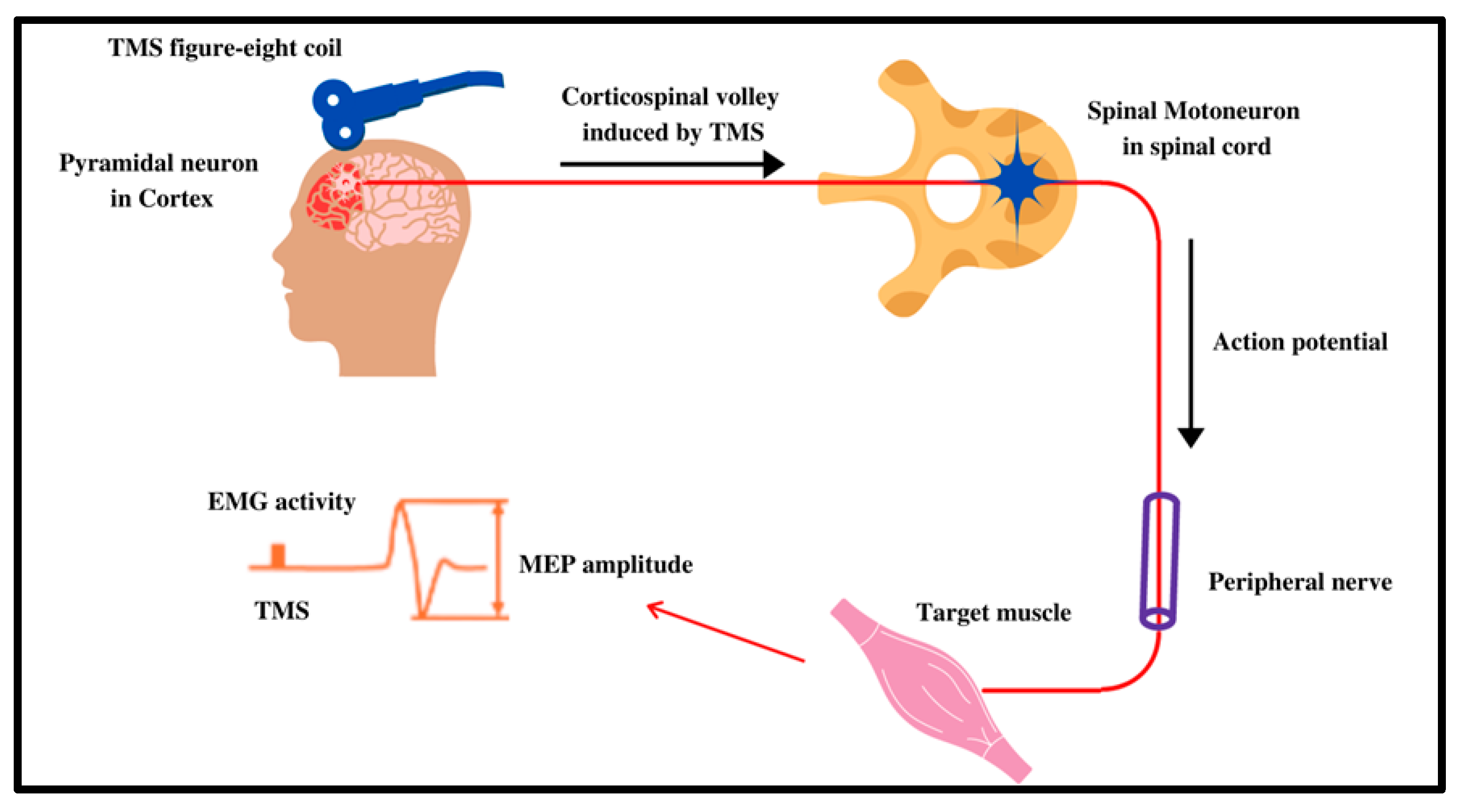
2.2. Proposed Mechanism of Action
2.3. Pathophysiology of Tinnitus
2.4. Questionnaires for Evaluating Tinnitus
3. Protocols for rTMS and Tinnitus
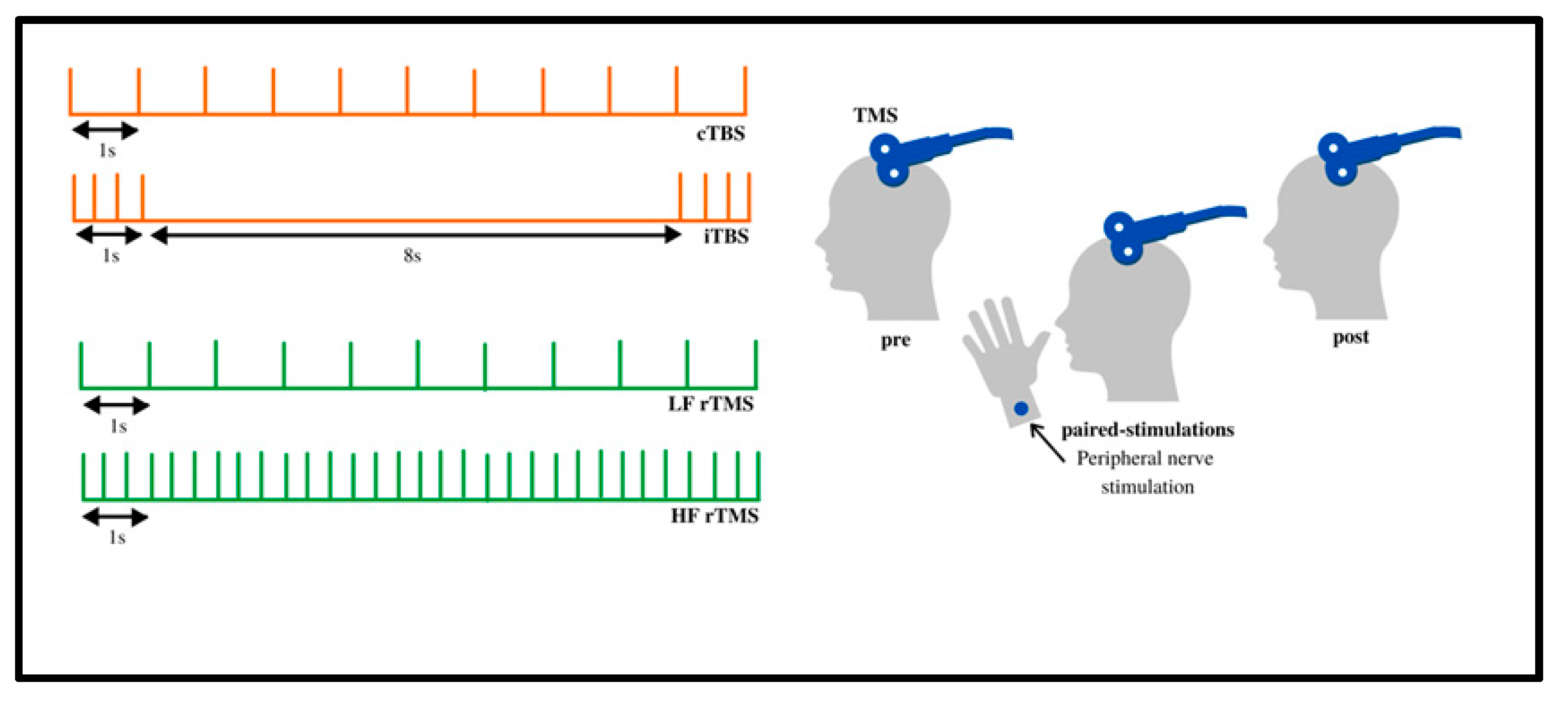
3.1. Frequency, Amount, and Location of Pulses
3.1.1. Frequency
3.1.2. rTMS Pulse Rate
3.1.3. Location of Treatment
3.2. Duration of Treatment and Follow-Up
3.3. Primary Baseline and Outcome Measurement Tools
3.4. Efficacy of rTMS in the Treatment of Tinnitus
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barredo, J.; Berlow, Y. Multimodal Elements of Suicidality Reduction After Transcranial Magnetic Stimulation. Neuromodulation 2021, 24, 930–937. [Google Scholar] [CrossRef]
- Flamez, A.; Wu, G.R. Opposite effects of one session of 1 Hz rTMS on functional connectivity between pre-supplementary motor area and putamen depending on the dyskinesia state in Parkinson’s disease. Clin. Neurophysiol. 2021, 132, 851–856. [Google Scholar] [CrossRef]
- Rachid, F. Reversed Stimulus Parameters in a Left-Handed Patient with Major Depression Treated with Repetitive Transcranial Magnetic Stimulation: A Case Report. J. ECT 2021, 37, e27. [Google Scholar] [CrossRef]
- Mansouriyeh, N.; Mahmoud-Aliloo, M. The Effect of High-frequency Repetitive Transcranial Magnetic Stimulation on Reducing Depression and Anxiety in Methamphetamine Users. Addict. Health 2020, 12, 278–286. [Google Scholar]
- National Institute of Mental Health. Brain Stimulations Therapies. Available online: www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml#part_152879 (accessed on 17 September 2021).
- Wei, W.; Yi, X. The efficacy of repetitive transcranial magnetic stimulation on emotional processing in apathetic patients with Parkinson’s disease: A Placebo-controlled ERP study. J. Affect. Disord. 2021, 282, 776–785. [Google Scholar] [CrossRef]
- Miron, J.P.; Voetterl, H. Optimized repetitive transcranial magnetic stimulation techniques for the treatment of major depression: A proof of concept study. Psychiatry Res. 2021, 298, 113790. [Google Scholar] [CrossRef]
- Zumbansen, A.; Black, S.E. Non-invasive brain stimulation as add-on therapy for subacute post-stroke aphasia: A randomized trial (NORTHSTAR). Eur. Stroke J. 2020, 5, 402–413. [Google Scholar] [CrossRef]
- To, W.T.; Ridder, D.D. Changing Brain Networks Through Non-invasive Neuromodulation. Front. Hum. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef]
- Horton, G.A.; Ibrahim, O. Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Tinnitus: A Preliminary Study of The Influence of Traumatic Brain Injury on Treatment Response. Int. Tinnitus J. 2021, 25, 51–58. [Google Scholar] [CrossRef]
- Poeppl, T.B.; Schecklmann, M. Prediction of response to repetitive transcranial magnetic stimulation in phantom sounds based on individual brain anatomy. Brain Commun. 2021, 3, fcab115. [Google Scholar] [CrossRef]
- Carter, G.; Govindan, R.B. Change in EEG Activity is Associated with a Decrease in Tinnitus Awareness after rTMS. Front. Neurol. Neurosci. Res. 2021, 2, 100010. [Google Scholar]
- Yang, H.; Cheng, G. Efficacy of Repetitive Transcranial Magnetic Stimulation (rTMS) for Tinnitus: A Retrospective Study. Ear Nose Throat J. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Han, B.I.; Lee, H.W. Tinnitus Update. J. Clin. Neurol. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Hu, J.; Cui, J. The Neural Mechanisms of Tinnitus: A Perspective from Functional Magnetic Resonance Imaging. Front. Neurosci. 2021, 15, 621145. [Google Scholar] [CrossRef]
- Zhang, J. Auditory cortex stimulation to suppress tinnitus: Mechanisms and strategies. Hear. Res. 2013, 295, 38–57. [Google Scholar] [CrossRef]
- Salvi, R.; Radziwon, K. Review: Neural Mechanisms of Tinnitus and Hyperacusis in Acute Drug-Induced Ototoxicity. Am. J. Audiol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Rizk, H.G.; Lee, J.A. Drug-Induced Ototoxicity: A Comprehensive Review and Reference Guide. Pharmacotherapy 2020, 40, 1265–1275. [Google Scholar] [CrossRef]
- National Institute of Health. Tinnitus. Available online: https://www.nidcd.nih.gov/health/tinnitus (accessed on 17 September 2021).
- Chari, D.A.; Limb, C.J. Tinnitus. Med. Clin. N. Am. 2018, 102, 1081–1093. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Nazarian, R. The cochlear implant: Historical aspects and future prospects. Anat. Rec. 2012, 295, 1967–1980. [Google Scholar] [CrossRef] [Green Version]
- Oberman, L.; Edwards, D.; Eldaief, M.; Pascual-Leone, A. Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. J. Clin. Neurophysiol. 2011, 28, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.P. Neurophysiology of cortical stimulation. Int. Rev. Neurobiol. 2012, 107, 57–85. [Google Scholar]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Ramirez, A.; Arbuckle, M.R. Synaptic Plasticity: The Role of Learning and Unlearning in Addiction and Beyond. Biol. Psychiatry 2016, 80, e73–e75. [Google Scholar] [CrossRef] [Green Version]
- Noh, N.A.; Fuggetta, G.; Manganotti, P. Theta-burst Transcranial Magnetic Stimulation Alters the Functional Topography of the Cortical Motor Network. Malays. J. Med. Sci. 2015, 22, 36–44. [Google Scholar]
- Bliss, T.V.; Cooke, S.F. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, C.; Xue, S.; Bai, J.; Yu, S.; Li, X.; Wang, H.; Tan, Q. Mechanism of Repetitive Transcranial Magnetic Stimulation for Depression. Shanghai Arch. Psychiatry 2018, 30, 84–92. [Google Scholar]
- Thomson, A.C.; Kenis, G.; Tielens, S.; de Graaf, T.A.; Schuhmann, T.; Rutten, B.P.F.; Sack, A.T. Transcranial Magnetic Stimulation-Induced Plasticity Mechanisms: TMS-Related Gene Expression and Morphology Changes in a Human Neuron-Like Cell Model. Front. Mol. Neurosci. 2020, 13, 528396. [Google Scholar] [CrossRef]
- Oathes, D.J.; Balderston, N.L.; Kording, K.P.; DeLuisi, J.A.; Perez, G.M.; Medaglia, J.D.; Fan, Y.; Duprat, R.J.; Satterthwaite, T.D.; Sheline, Y.I.; et al. Combining transcranial magnetic stimulation with functional magnetic resonance imaging for probing and modulating neural circuits relevant to affective disorders. Wiley Interdiscip. Rev. Cogn. Sci. 2021, 12, 1553. [Google Scholar] [CrossRef]
- Langguth, B. Non-Invasive Neuromodulation for Tinnitus. J. Audiol. Otol. 2020, 24, 113–118. [Google Scholar] [CrossRef]
- Rammo, R.; Ali, R.; Pabaney, A.; Seidman, M.; Schwalb, J. Surgical Neuromodulation of Tinnitus: A Review of Current Therapies and Future Applications. Neuromodulation 2019, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Poeppl, T.B.; Langguth, B.; Lehner, A.; Frodl, T.; Rupprecht, R.; Kreuzer, P.M.; Landgrebe, M.; Schecklmann, M. Brain stimulation-induced neuroplasticity underlying therapeutic response in phantom sounds. Hum. Brain Mapp. 2018, 39, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.B.; Stewart, B.J.; Griest, S.E.; Martin, W.H.; Henry, J.A.; Abrams, H.B.; McArdle, R.; Newman, C.W.; Sandridge, S.A. Assessment of tinnitus: Measurement of treatment outcomes. Prog. Brain Res. 2007, 166, 511–521. [Google Scholar]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, L.; Mertens, G.; Van de Heyning, P.; Vanderveken, O.M.; Topsakal, V.; De Hertogh, W.; Michiels, S.; Van Rompaey, V.; Gilles, A. Sensitivity to change and convergent validity of the Tinnitus Functional Index (TFI) and the Tinnitus Questionnaire (TQ): Clinical and research perspectives. Hear. Res. 2019, 382, 107796. [Google Scholar] [CrossRef] [PubMed]
- Hallam, R.S. TQ: Manual of the Tinnitus Questionnaire; Polpresa Press: London, UK, 2009. [Google Scholar]
- Sweetow, R.W.; Levy, M.C. Tinnitus severity scaling for diagnostic/therapeutic usage. Hear. Instrum. 1990, 41, 20–21. [Google Scholar]
- Raj-Koziak, D.; Gos, E.; Swierniak, W.; Rajchel, J.J.; Karpiesz, L.; Niedzialek, I.; Wlodarczyk, E.; Skarzynski, H.; Skarzynski, P.H. Visual Analogue Scales as a Tool for Initial Assessment of Tinnitus Severity: Psychometric Evaluation in a Clinical Population. Audiol. Neurootol. 2018, 23, 229–237. [Google Scholar] [CrossRef]
- Wang, H.; Li, B.; Wang, M.; Li, M.; Yu, D.; Shi, H.; Yin, S. Factor Analysis of Low-Frequency Repetitive Transcranial Magnetic Stimulation to the Temporoparietal Junction for Tinnitus. Neural Plast. 2016, 2016, 2814056. [Google Scholar] [CrossRef]
- Kan, Y.; Wang, W.; Zhang, S.X.; Ma, H.; Wang, Z.C.; Yang, J.G. Neural metabolic activity in idiopathic tinnitus patients after repetitive transcranial magnetic stimulation. World J. Clin. Cases 2019, 7, 1582–1590. [Google Scholar] [CrossRef]
- Roland, L.T.; Peelle, J.E.; Kallogjeri, D.; Nicklaus, J.; Piccirillo, J.F. The effect of noninvasive brain stimulation on neural connectivity in Tinnitus: A randomized trial. Laryngoscope 2016, 126, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Lehner, A.; Schecklmann, M.; Greenlee, M.W.; Rupprecht, R.; Langguth, B. Triple-site rTMS for the treatment of chronic tinnitus: A randomized controlled trial. Sci. Rep. 2016, 6, 22302. [Google Scholar] [CrossRef] [Green Version]
- Noh, T.S.; Rah, Y.C.; Kyong, J.S.; Kim, J.S.; Park, M.K.; Lee, J.H.; Oh, S.H.; Chung, C.K.; Suh, M.W. Comparison of treatment outcomes between 10 and 20 EEG electrode location system-guided and neuronavigation-guided repetitive transcranial magnetic stimulation in chronic tinnitus patients and target localization in the Asian brain. Acta Otolaryngol. 2017, 137, 945–951. [Google Scholar] [CrossRef]
- Noh, T.S.; Kyong, J.S.; Chang, M.Y.; Park, M.K.; Lee, J.H.; Oh, S.H.; Kim, J.S.; Chung, C.K.; Suh, M.W. Comparison of Treatment Outcomes Following Either Prefrontal Cortical-only or Dual-site Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus Patients: A Double-blind Randomized Study. Otol. Neurotol. 2017, 38, 296–303. [Google Scholar]
- James, G.A.; Thostenson, J.D.; Brown, G.; Carter, G.; Hayes, H.; Tripathi, S.P.; Dobry, D.J.; Govindan, R.B.; Dornhoffer, J.L.; Williams, D.K.; et al. Neural activity during attentional conflict predicts reduction in tinnitus perception following rTMS. Brain Stimul. 2017, 10, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Cacace, A.T.; Hu, J.; Romero, S.; Xuan, Y.; Burkard, R.F.; Tyler, R.S. Glutamate is down-regulated and tinnitus loudness-levels decreased following rTMS over auditory cortex of the left hemisphere: A prospective randomized single-blinded sham-controlled cross-over study. Hear. Res. 2018, 358, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Landgrebe, M.; Hajak, G.; Wolf, S.; Padberg, F.; Klupp, P.; Fallgatter, A.J.; Polak, T.; Höppner, J.; Haker, R.; Cordes, J.; et al. 1-Hz rTMS in the treatment of tinnitus: A sham-controlled, randomized multicenter trial. Brain Stimul. 2017, 10, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Sahlsten, H.; Virtanen, J.; Joutsa, J.; Niinivirta-Joutsa, K.; Löyttyniemi, E.; Johansson, R.; Paavola, J.; Taiminen, T.; Sjösten, N.; Salonen, J.; et al. Electric field-navigated transcranial magnetic stimulation for chronic tinnitus: A randomized, placebo-controlled study. Int. J. Audiol. 2017, 56, 692–700. [Google Scholar] [CrossRef]
- Ciminelli, P.; Machado, S.; Palmeira, M.; Coutinho, E.S.; Sender, D.; Nardi, A.E. Dorsomedial Prefrontal Cortex Repetitive Transcranial Magnetic Stimulation for Tinnitus: Promising Results of a Blinded, Randomized, Sham-Controlled Study. Ear Hear. 2020, 42, 12–19. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Poeppl, T.B.; Vielsmeier, V.; Schecklmann, M.; Langguth, B.; Lehner, A. The more the merrier? Preliminary results regarding treatment duration and stimulation frequency of multisite repetitive transcranial magnetic stimulation in chronic tinnitus. Prog. Brain Res. 2021, 262, 287–307. [Google Scholar]
- Schoisswohl, S.; Agrawal, K.; Simoes, J.; Neff, P.; Schlee, W.; Langguth, B. RTMS parameters in tinnitus trials: A systematic review. Sci. Rep. 2019, 9, 12190. [Google Scholar] [CrossRef]
- Lefebvre-Demers, M.; Doyon, N.; Fecteau, S. Non-invasive neuromodulation for tinnitus: A meta-analysis and modeling studies. Brain Stimul. 2021, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Chen, C.; Wang, T.; Gao, C.; Wang, Y.; Guan, X. Low-frequency repetitive transcranial magnetic stimulation for the treatment of chronic tinnitus: A systematic review and meta-analysis of randomized controlled trials. BioMed Res. Int. 2020, 2020, 3141278. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, H.; Cheng, G.; Huang, L.; Zhang, T.; Jia, H. Repetitive transcranial magnetic stimulation on chronic tinnitus: A systematic review and meta-analysis. BMC Psychiatry 2020, 20, 547. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Subject Number | Primary Baseline Evaluation | rTMS Protocol (Session Number, Frequency, Amount of Stimuli) | Location of Treatment | Primary Evaluation of Outcome | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Wang et al. [41] | 2016 | -289 patients with chronic tinnitus -30 healthy control | -Tinnitus loudness determined by visual analog scale (VAS) -Hearing level with audiometer -Tinnitus loudness evaluated with TinniTest audiometer | -Underwent repetitive magnetic transcranial stimulation (rTMS) over the left temporoparietal cortex region. -Stimuli consisted of 1000 stimuli at 1 hertz (Hz) daily and 110% of the motor cortex threshold for 5 consecutive days per week for 2 weeks (10 sessions total). -Control received same treatment | -Left temporoparietal cortex | -VAS score after last treatment | -rTMS showed an effect in 138 of the patients (47.8%) and no effect in 151 patients (52.2%) in the active group. -VAS average prior was 5.5 and 2.7 after -Significant tinnitus suppression found in patients with shorter tinnitus duration, normal hearing, and without sleep disturbance. | -rTMS resulted in a significant reduction in tinnitus loudness -Study states imaging would help determine the best site of treatment |
| Poeppl et al. [34] | 2018 | -60 patients with chronic tinnitus -0 control | -MRI immediately before treatment -Tinnitus Questionnaire (TQ) | -Underwent 10 consecutive days with 10 sessions -Patients received rTMS of the left DLPFC (40 trains with 50 stimuli; 25 s intertrain interval; 20 Hz; 110% resting motor threshold (RMT)), followed by low-frequency rTMS (2000 Stimuli; 1 Hz; 110% RMT) of the left temporal cortex. | -Left dorsolateral prefrontal cortex (DLPFC) and left temporal cortex (TC) | -Magnetic resonance imaging (MRI) after last treatment -Responders classified as scoring 5 points fewer on tinnitus questionnaire | -Assessed for longitudinal gray matter changes and structural connectivity -Longitudinal mesoscopic gray matter changes of DLPFC, left operculo-insular, and right inferior temporal Cortex (ITC) in responders (n = 22) but not in non-responders (n = 38) -Increased connectivity in DLPFC–insula and insula–ITC in responders. Weak DLPFC–insula connectivity and no insula–ITC connectivity in non-responders. | -Results support the role of non-auditory brain regions in tinnitus and as possible therapeutic targets in rTMS. |
| Kan et al. [42] | 2019 | -11 patients with idiopathic tinnitus -11 healthy controls | -Tinnitus handicap inventory (THI) and VAS -Positron emission tomography (PET) scans before treatment for regions of increased activity in idiopathic tinnitus compared to controls | -1000 TMS pulses at a frequency of 1 Hz for a total of 30 min for 10 consecutive days, once a day | -Left temporoparietal cortex | -Tinnitus handicap inventory (THI) score -VAS score -PET scan -All after last treatment | -No significant statistical difference before and after treatment regarding THI score (t = 1.019, p = 0.342 > 0.05) and VAS (t = 0.00, p = 1.0 > 0.05). -Posttreatment PET scan showed increased activities in the right parahippocampal gyrus, right superior temporal gyrus, right superior frontal gyrus, anterior insula, left inferior parietal lobule, and left precentral gyrus. -Decreased activities were noted in the left postcentral gyrus and left inferior temporal gyrus (ITG) | -Noted limitations by small sample size -Left temporoparietal cortex alone may not be sufficient |
| Yang et al. [13] | 2021 | -199 patients with tinnitus identified in a retrospective review | -THI and VAS | -Each patient underwent 10 sessions, 5 sessions a week for 2 weeks -2000 stimuli per session of 1 Hz | -Left temporal cortex and left prefrontal cortex | -At 3-month follow-up THI and VAS reevaluated. -A reduction in THI score by more than 6 points and VAS by 1 or more from the baseline result was considered effective | -62.3% of all patients responded based on THI scores and 66.3% based on VAS score. -Patients with shorter duration of illness (1 week) responded the best to treatment with a rate of 82.8% versus 57.6%, 53.5%, and 67.2% for patients of 1-week to 1-month, 1-month to 1-year, and over 1-year duration | -rTMS is effective in treating tinnitus, but the efficacy is dependent on the duration of symptoms prior to treatment |
| Author | Year | Subjects per Group and Blinding | Primary Baseline Evaluation | rTMS Protocol Group 1 (Session Number, Frequency, Amount of Stimuli) | Protocol Group 2 | Primary Evaluation of Outcomes | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Roland et al. [43] | 2016 | -Group 1 (experimental): 16 patients with nonpulsatile tinnitus -Group 2 (Sham): 14 patients with nonpulsatile tinnitus -Double-blinded | -Resting state functional connectivity MRI (rs-fcMRI) -THI | -Group 1: Active treatment was delivered at 1 Hz at 110% of RMT at the temporoparietal junction for 42.5 min (2500 stimuli) with interval stimulation for 2 or 4 weeks. | -Group 2 (sham): same protocol with placebo rTMS | -rs-fcMRI following treatment for 2 or 4 weeks -THI following treatment | -No statistically significant changes found between pre and post intervention in both the rs-fcMRI and THI | -Concluded both a lack of symptom change and neural connectivity changes -Suggest they may not have had sufficient stimulation to the area of interest or should consider non-auditory brain regions associated with tinnitus |
| Lehner et al. [44] | 2016 | -Group 1: 24 patients with tinnitus (Single site) -Group 2: 25 patients with tinnitus (triple site) -Group 3: 25 patients with tinnitus (placebo) -Double-blinded | -8 various tinnitus questionnaires | -Group 1 (single site): 3000 pulses/day of the left temporoparietal cortex with low-frequency (1 Hz) rTMS of the left temporoparietal cortex -Group 2 (triple site): 1000 pulses/day of high-frequency 20 Hz stimulation of the left DLPFC, followed by 1000 pulses/day of low-frequency (1 Hz) to both the left and right temporoparietal cortex (3000 pulses total) -Ten sessions total for each group | Group 3 (placebo): sham coil was localized at the auditory cortex by using a PET-guided neuronavigation system. | -8 tinnitus questionnaires on the last day of treatment (day 12), day 90 and day 180 following treatment | -Both the single site and triple site showed statistically significant reductions in tinnitus severity, but the difference between the two is not significant besides at day 90. | -Study did not find significant differences between one or multi-site rTMS treatment -More work needed on exact protocols for a more effective and individualized treatment |
| Noh et al. [45] | 2017 | -Group 1: 9 patients with tinnitus -Group 2: 13 patients with tinnitus -Blinding not possible | -THI score -VAS score | -Group 1: Auditory cortex (AC) and frontal cortex (FC) determined by 10–20 EEG system -rTMS was administered at a frequency of 1 Hz with an intensity of 110% RMT -40 s on and 20 s off -Total of 12,000 pulses: 2000 pulses over the AC, and 1000 pulses over the FC daily for 4 days. | -Group 2: coil navigated to the primary AC and FC by a MRI neuronavigation system -Same rTMS treatment as group 1 | -THI weeks 1, 4, and 8 after baseline -VAS at weeks 1, 4, 8, and 12 after baseline | -Both groups had a significant reduction in THI scores -Group 1 effect lasted 8 weeks and group 2 effect lasted 4 weeks, but the differences were not statistically different -VAS score reduction not statistically significant in group 1 but statistically significant in group 2 up to 12 weeks post treatment -ΔVAS between the groups was not statistically significant | -Localizing technique for treatment target not a crucial factor in the rTMS outcome of the same locations |
| Noh et al. [46] | 2017 | -Group 1: 9 patients with chronic tinnitus (dual-site) -Group 2: 8 patients with chronic tinnitus (single-site) -Double blinded | -THI score -VAS score -State-Trait Anxiety Inventory (STAI) -Beck’s Depression Inventory (BDI) -Pittsburgh Sleep Quality Index (PSQI) | Group 1: Low frequency (1 Hz) treatments with 2000 pulses applied to the AC and 1000 pulses applied to the DLPFC for 4 days (total of 12,000 pulses) | Group 2: Low frequency (1 Hz) treatments with 3000 pulses applied to the DLPFC for 4 days (total of 12,000 pulses). | -THI, VAS, STAI, PSQI at weeks 1, 2, 4, and 12 after treatment | -Group 1 showed significant reductions in THI and VAS scores at all weeks of evaluation, whereas group 2 did not -Group 1 showed significant improvements in STAI at 12 weeks, and PSQI scores at 4 weeks. -Group 2 showed a significant improvement only in STAI at 12 weeks. | -Targeting both the AC and DLPFC had better outcomes in all areas of evaluation in comparison to just targeting the DLPFC -Non-auditory cortex stimulation only is not sufficient to impact tinnitus |
| James et al. [47] | 2017 | -12 total participants in a crossover study -Half started at 1 Hz, the other half at 10 Hz, with a sham in between crossover -Double-blinded | -fMRI -Visual Analog Rating (VAR) | -Both groups received: Sham, 1 Hz, and 10 Hz for four sessions per arm, 1800 pulses per session, delivered at 110% of RMT over the posterior superior temporal gyrus (STG) | -Groups crossed over with a 21-day washout period | -fMRI after final treatment -VAR after final treatment | -Both 1 Hz and 10 Hz rTMS stimulation showed changes in tinnitus awareness from baseline compared to sham -All three measures of the VAR (awareness, loudness, and annoyance) were improved with 1 Hz. -Higher DLPFC activity at baseline may predict a poorer response to rTMS | -Using 1 Hz and 10 Hz can lead to similar results, even though they lead to inverse effects on neural excitability -The role of DLPFC plays a role in tinnitus and rTMS responsiveness |
| Cacace et al. [48] | 2017 | -25 total participants with chronic tinnitus -Single-blinded crossover | -THI for inclusion -Audiogram -Tinnitus Handicap Questionnaire (THQ) -Metabolite levels using magnetic resonance spectroscopy (MRS) | -Active rTMS: 1 Hz and at a power setting of 110% of RMT over the left AC -20-min sessions with a total of 1200 stimuli -Participants received 5 days of active rTMS and then 5 days of sham rTMS stimulation, sequentially | -Sham treatment with same time frame | -Audiogram at day 5 -THQ at day 5 -MRS at day 5 | -Significant decrease in the loudness of tinnitus -Significant reduction in THQ score -Down regulation in the glutamate (excitatory) seen in the left and not the right hemisphere | -Perceptual, psychoacoustic, and neurochemical analysis of rTMS treatment showed improvement in tinnitus |
| Landgrebe et al. [49] | 2017 | -163 patients with chronic tinnitus -Group 1: 75 patients (experimental) -Group 2: 78 patients (sham) -Patient and rater blinded | -Tinnitus Questionnaire (TQ) -THI -Tinnitus Severity Scale (TSS) | -Group 1: 10 sessions active 1-Hz-rTMS (2000 stimuli, 110% RMT) to the left temporal cortex (AC). | -Group 2: Sham rTMS with the same time frame | -TQ, THI, TSS at day 12 | -No statistically significant difference in outcome measures between the active and the sham group | -No effect found in the largest trial testing rTMS of the left AC alone -Larger trials of other protocols should be carried out |
| Sahlsten et al. [50] | 2017 | -39 patients with chronic tinnitus -Group 1: 19 patients (experimental) -Group 2: 20 patients (placebo) -Single-blinded | -THI -VAS -Audiogram | -Group 1: 10 sessions over 2 weeks of 4000 pulses at 1 Hz to the left superior temporal gyrus (STG) at 100% of RMT | -Group 2: Placebo rTMS | -THI, VAS after 10 days, at 1 month and at 3 months post treatment | -Significant reduction in THI score in both groups but not between groups -Significant decrease in mean intensity, annoyance and distress VAS scores at 3 months post-treatment but not significant over time -No changes in hearing in both groups | -Improvement in both VAS and THI in the whole study group but not between groups -May be attributed to too many pulses or placebo effect -Best protocol of rTMS remains uncertain |
| Ciminelli et al. [51] | 2020 | -Group 1 (Experimental): 15 patients with tinnitus -Group 2 (Sham): 14 patients with tinnitus -Single-blinded | -THI score -VAS score -Tinnitus loudness | -Group 1 (experimental group): Each session of 10 Hz stimulation applied 3000 pulses to each DMPFC for 15 min each side (6000 pulses total). -5 s on and 10 s off were used, for a total time of 30 min -Treatment was 5 times a week for 4 weeks (20 total sessions) | -Group 2 (sham) received the same protocol but with a placebo coil -Coil produced the same sound and sensation | -THI and VAS score at weeks 1, 2, and 4 of treatment and 16 weeks after baseline -Tinnitus loudness following treatment | -A significant difference of 11.53 in THI (95% confidence interval [CI]: −23.12 to 0.06; p = 0.05) -VAS difference of 0.80 not statistically significant (95% CI, −2.21 to 0.61) -Tinnitus loudness score reduction of 4.46 dB was borderline significant (95% CI: −9.60 to 0.68 dB; p = 0.09). | -Results show a benefit in 2/3 parameters used to evaluate tinnitus |
| Kreuzer et al. [52] | 2021 | -Exploratory open label study -Randomized, parallel-group design with 80 patients -32 received “standard triple protocol” (Group 1) -38 received “high-frequency triple protocol” (Group 2) | -Primary baseline: TQ | -“Standard triple” protocol: 20 Hz stimulation of the DLPFC followed by 1 Hz to the left and right temporoparietal cortex with 1000 stimuli for 4 weeks - Total of 3000 stimuli per session -6 patients were treated for only 2 weeks | -“High-frequency triple” protocol: 20 Hz to the left DLPFC and the left and right temporoparietal junction area with 1000 stimuli for 4 weeks -Total of 3000 stimuli per session -5 patients were treated for only 2 weeks | -Primary outcome: TQ assessed at baseline, week 2, week 4, week 12 | -Change in TQ from baseline to week 12 was significant (P = 0.016). -No significant interaction effect between measurement time point (2 vs. 4 weeks) and group (standard vs. high-frequency) | -Due to the pilot nature of the study, clinical relevance remains unknown -4 weeks is a feasible treatment time, but not superior to 2 weeks -High frequency not superior or inferior to the standard therapy |
| Carter et al. [12] | 2021 | -Double-blinded, sham-controlled -19 patients in crossover study -All patients received Sham (Group 1) -All patients received treatment (Group 2) | -Electro-encephalography (EEG) -VAS and line mark (LM) rating of loudness, annoyance, awareness -THQ | -Group 1: All participants received sham rTMS first -Three 4-day courses of participants received 1800 pulses at a 110% motor threshold targeted over the posterior, superior temporal gyrus. | -Group 2: Participants were randomized to either the 1 Hz and 10 Hz and then crossed over to second frequency after completing the first -Three 4-day courses of rTMS participants received 1800 pulses targeted over the posterior, superior temporal gyrus | -VAR/LM and EEG evaluation at baseline, the end of each treatment week and 2 months following treatment | -No significant change in VAS compared to before and immediately following treatment, during sham, or active 10-Hz treatment -1-Hz treatment led to a significant decrease in both the LM and VAS awareness ratings at days 1–3 with loudness (p = 0.0447), annoyance (p = 0.0195), and awareness (p = 0.0430) -No changes in any EEG frequency band between baseline and sham -EEG after 1 Hz: significant increase in beta and delta coherence -EEG after 10 Hz: increase in theta and beta coherence | -No immediate effect of rTMS on tinnitus during a single rTMS session -1 Hz was associated with a decrease in tinnitus awareness and was associated with an increase in beta coherence -EEG changes noted in treatment responders but absent in non-responders and sham treatment -Beta coherence is a possible biomarker of the rTMS effect |
| Author | Year | Number of Studies Analyzed and Years of Interest | Location Target(s) | Neuromodulation Frequencies | Main Outcome Assessment | Heterogeneity (I2 Analysis) | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Schoisswoh et al. [53] | 2019 | -55 significant study arms from 2005 to 2017 -18 insignificant study arms from 2007 to 2017 -Randomized controlled trials | -Temporal cortex (n = 32,9) -Temporoparietal cortex (n = 23, 9) -Prefrontal in addition to AC (n = 9,7) | -Inhibitory: 1 Hz, cTBS (n = 49, 18) -Excitatory: 10 Hz, 25 Hz (n = 6, 0) | -Chi-squared analysis of reported significant and not significant results of study arms | -Not applicable, only a systematic review performed | -Higher efficacy in active rTMS compared to sham rTMS -Lower stimulation intensity associated with significance -Lower number of pulses increased significance -Adding prefrontal cortical areas did not contribute to significance | -Meta-analysis would have given less of a dichotomized result -There are many factors that go into rTMS efficacy in treating tinnitus -The prefrontal cortex may not be significant due to the addition of more pulses -rTMS protocols need to be more standardized for a definitive analysis |
| Lefebvre-Demers et al. [54] | 2020 | -28 studies from 2004 to 2019 -Randomized controlled trials | -Auditory cortex (n = 16) -Temporoparietal area (n = 17) -DLPFC and left AC (n = 3) - DLPFC and both AC (n = 1) -Frontal cortex (n = 1) | -1 Hz (n =20) -10 Hz (n =1), -50 Hz cTBS (n = 4) -27,12 MHz (n 1⁄4 1) -≥1 frequency (20 Hz and 1 Hz, n = 1; 25 Hz and 1 Hz, n = 1) | -THI (n = 20) -Tinnitus Questionnaire (TQ) (n = 6) -Tinnitus Functional Index (TFI) (n = 1) -Tinnitus Severity Index (TSI) (n = 1) | -moderate total heterogeneity (54.9%) | -Sample size: 34 ± 29 participants -Tinnitus outcomes: Pre- to post-rTMS Hedges g-value of 0.45 (CI = 0.66; 0.24; p < 0.0001), showing a moderate effect -Active rTMS showed a statistically significant mean change in questionnaire scores of 7.60 -Location: rTMS targeting the AC significantly reduced symptoms compared to other sites | -rTMS is an effective treatment option for tinnitus based on the effect on standardized questionnaires |
| Dong et al. [55] | 2020 | -10 studies from 2010 to 2019 -Randomized controlled trials | -Temporal cortex/auditory cortex only (n = 7) -Temporoparietal cortex only (n = 2) -Temporal with the frontal regions (n = 1) | -1 Hz with 100% or 110% RMT with varying pulses of 1000, 1020, 1500, 2000, 3000, and 4000) | -THI only (n = 4) -TQ only (n = 1) -VAS only (n = 1) -THI and TQ (n = 1) -THI and VAS (n = 1) -THI, TQ, and VAS (n = 2) | -No heterogeneity | -A pooled analysis showed that active rTMS had no significant effect on THI scores compared with sham in the short term, medium term, or long term -A pooled analysis showed no significant effect of active rTMS on the loudness assessed by VAS in the short term, medium term, or long term -A pooled analysis showed no significant improvement of the severity assessed by TQ in the short term, medium term, or long term | -The review showed no significant improvement of tinnitus symptoms following rTMS treatment compared to sham -Inconsistent with previous studies and may be limited to a small sample size |
| Liang et al. [56] | 2020 | -29 studies from 2010 to 2019 -Randomized controlled trials | -Auditory cortex (n = 27) -Motor cortex (n = 1) -Not specified (n = 1) | -1 Hz most frequently used (93.1%) | -THI and TQ scores at 1 week, 2 weeks, 1 month, and 6 months post treatment | -0% at 1 week -0% at 1 month -21% at 6 months | -Significant difference in THI scores at 1 week, 1 month, and 6 months compared to sham -Significant difference in TQ scores 1-week post treatment compared to sham | -Efficacy of active rTMS compared to sham proven in the analysis -More studies needed for further confirmation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denton, A.J.; Finberg, A.; Ashman, P.E.; Bencie, N.B.; Scaglione, T.; Kuzbyt, B.; Telischi, F.F.; Mittal, R.; Eshraghi, A.A. Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus. J. Clin. Med. 2021, 10, 5422. https://doi.org/10.3390/jcm10225422
Denton AJ, Finberg A, Ashman PE, Bencie NB, Scaglione T, Kuzbyt B, Telischi FF, Mittal R, Eshraghi AA. Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus. Journal of Clinical Medicine. 2021; 10(22):5422. https://doi.org/10.3390/jcm10225422
Chicago/Turabian StyleDenton, Alexa J., Ariel Finberg, Peter E. Ashman, Nathalie B. Bencie, Tricia Scaglione, Brianna Kuzbyt, Fred F. Telischi, Rahul Mittal, and Adrien A. Eshraghi. 2021. "Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus" Journal of Clinical Medicine 10, no. 22: 5422. https://doi.org/10.3390/jcm10225422
APA StyleDenton, A. J., Finberg, A., Ashman, P. E., Bencie, N. B., Scaglione, T., Kuzbyt, B., Telischi, F. F., Mittal, R., & Eshraghi, A. A. (2021). Implications of Transcranial Magnetic Stimulation as a Treatment Modality for Tinnitus. Journal of Clinical Medicine, 10(22), 5422. https://doi.org/10.3390/jcm10225422







