Preoperative Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Mean Platelet Volume as Predictors of 1-Year Mortality in Patients Undergoing an Open Repair of Abdominal Aortic Aneurysms: A Retrospective Study
Abstract
:1. Introduction
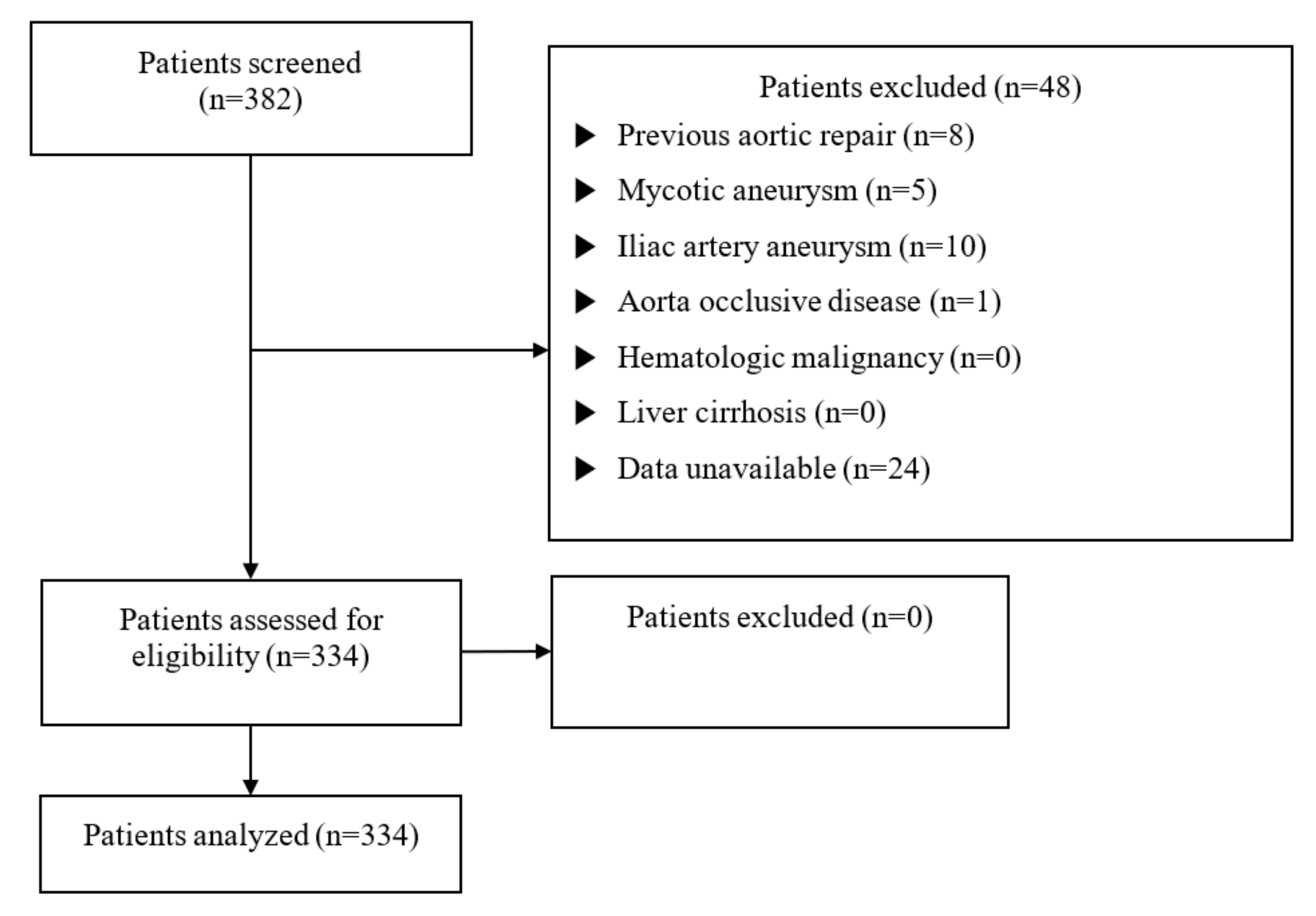
2. Materials & Methods
2.1. Study Population
2.2. Demographic and Clinical Data
2.3. Study Endpoints
2.4. Statistical Analyses
3. Results
3.1. Baseline and Perioperative Characteristics
3.2. Independent Prognostic Significance of NLR, MPV, and PLR in Predicting 1-Year Mortality after AAA Open Repair
3.3. Baseline and Perioperative Characteristics Depending on the Presence of Rupture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef]
- Wieker, C.M.; Spazier, M.; Böckler, D. Indications for and outcome of open AAA repair in the endovascular era. J. Cardiovasc. Surg. 2016, 57, 185–190. [Google Scholar]
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneu-rysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef]
- Santo, A.H.; Puech-Leão, P.; Krutman, M. Trends in abdominal aortic aneurysm-related mortality in Brazil, 2000–2016: A multiple-cause-of-death study. Clinics 2021, 76, e2388. [Google Scholar] [CrossRef]
- Lareyre, F.; Carboni, J.; Chikande, J.; Massiot, N.; Voury-Pons, A.; Umbdenstock, E.; Jean-Baptiste, E.; Hassen-Khodja, R.; Raffort, J. Association of Platelet to Lymphocyte Ratio and Risk of 30-Day Postoperative Complications in Patients Undergoing Abdominal Aortic Surgical Repair. Vasc. Endovasc. Surg. 2019, 53, 5–11. [Google Scholar] [CrossRef]
- Appleton, N.D.; Bailey, D.M.; Morris-Stiff, G.; Lewis, M.H. Neutrophil to Lymphocyte Ratio Predicts Perioperative Mortality Following Open Elective Repair of Abdominal Aortic Aneurysms. Vasc. Endovasc. Surg. 2014, 48, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Aurelian, S.V.; Adrian, M.; Andercou, O.; Bruno, S.; Alexandru, O.; Catalin, T.; Dan, B. Neutrophil-to-Lymphocyte Ratio: A Comparative Study of Rupture to Nonruptured Infrarenal Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2019, 58, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy 2001, 102, 5–14. [Google Scholar]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Kordzadeh, A.; Malietzis, G.; Browne, T.; Prionidis, I.; Panayiotopoulos, Y.P. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): A retrospective cohort study. Int. J. Surg. 2015, 15, 45–48. [Google Scholar] [CrossRef]
- Bhutta, H.; Agha, R.; Wong, J.; Tang, T.Y.; Wilson, Y.G.; Walsh, S.R. Neutrophil-Lymphocyte Ratio Predicts Medium-Term Survival Following Elective Major Vascular Surgery: A Cross-Sectional Study. Vasc. Endovasc. Surg. 2011, 45, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Becker, R.C.; Berger, P.B.; Bhatt, D.L.; Eikelboom, J.W.; Konkle, B.; Mohler, E.R.; Reilly, M.P.; Berger, J.S. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 148–156. [Google Scholar] [CrossRef]
- Balta, S.; Ozturk, C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015, 26, 680–681. [Google Scholar] [CrossRef]
- Milovanovic Alempijevic, T.; Stojkovic Lalosevic, M.; Dumic, I.; Jocic, N.; Pavlovic Markovic, A.; Dragasevic, S.; Jovicic, I.; Lukic, S.; Popovic, D.; Milosavljevic, T. Diagnostic Accuracy of Platelet Count and Platelet Indices in Noninvasive Assess-ment of Fibrosis in Nonalcoholic Fatty Liver Disease Patients. Can. J. Gastroenterol. Hepatol. 2017, 2017, 6070135. [Google Scholar] [CrossRef] [Green Version]
- Bath, J.; Smith, J.B.; Kruse, R.L.; Vogel, T.R. Association of neutrophil-to-lymphocyte ratio with outcomes after elective ab-dominal aortic aneurysm repair. J. Vasc. Nurs. 2019, 37, 213–220. [Google Scholar] [CrossRef]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutro-phil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef]
- Siminiak, T.; Flores, N.A.; Sheridan, D.J. Neutrophil interactions with endothelium and platelets: Possible role in the devel-opment of cardiovascular injury. Eur. Heart J. 1995, 16, 160–170. [Google Scholar] [CrossRef]
- Pircher, J.; Engelmann, B.; Massberg, S.; Schulz, C. Platelet–Neutrophil Crosstalk in Atherothrombosis. Thromb. Haemost. 2019, 119, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Lareyre, F.; Raffort, J.; Le, D.; Chan, H.L.; Le Houerou, T.; Cochennec, F.; Touma, J.; Desgranges, P. High Neutrophil to Lymphocyte Ratio Is Associated with Symptomatic and Ruptured Thoracic Aortic Aneurysm. Angiology 2018, 69, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.H.; Croal, B.L.; Cuthbertson, B.H.; Small, G.R.; Ifezulike, A.I.; Gibson, G.; Jeffrey, R.R.; Buchan, K.G.; El-Shafei, H.; Hillis, G.S. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am. Heart J. 2007, 154, 995–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedel, C.; Selvi, F. Association of Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios with In-Hospital Mortality in Patients with Type A Acute Aortic Dissection. Braz. J. Cardiovasc. Surg. 2019, 34, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Z.; Chen, Q.-J.; Sun, H.-P.; Zeng, R.; Zeng, Z.; Gao, X.-M.; Ma, Y.-T.; Yang, Y.-N. Mean platelet volume to platelet count ratio predicts in-hospital complications and long-term mortality in type A acute aortic dissection. Blood Coagul. Fibrinolysis 2016, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Patrono, C. Platelet Activation and Atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef]
- ahin, D.Y.; Gür, M.; Elbasan, Z.; Yıldırım, A.; Akıllı, R.E.; Koyunsever, N.Y.; Özaltun, B.; Gözübüyük, G.; Kıvrak, A.; Çaylı, M. Mean platelet volume associated with aortic distensibility, chronic inflammation, and diabetes in patients with stable coronary artery disease. Clin. Appl. Thromb. Hemost. 2014, 20, 416–421. [Google Scholar]
- Kurtul, A.; Ornek, E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Erhart, P.; Cakmak, S.; Grond-Ginsbach, C.; Hakimi, M.; Böckler, D.; Dihlmann, S. Inflammasome activity in leucocytes decreases with abdominal aortic aneurysm progression. Int. J. Mol. Med. 2019, 44, 1299–1308. [Google Scholar] [CrossRef] [Green Version]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert. Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [Green Version]
- Dale, M.A.; Ruhlman, M.K.; Baxter, B.T. Inflammatory cell phenotypes in aaas: Their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1746–1755. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Bai, S.; Ao, Q.; Wang, X.; Tian, X.; Li, X.; Tong, H.; Hou, W.; Fan, J. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. J. Immunol. Res. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2020, 11, 3758. [Google Scholar] [CrossRef]
- Dawson, J.; Cockerill, G.; Choke, E.; Loftus, I.; Thompson, M.M. Aortic Aneurysms as a Source of Circulating Interleukin-6. Ann. N. Y. Acad. Sci. 2006, 1085, 320–323. [Google Scholar] [CrossRef]
- Dawson, J.; Cockerill, G.W.; Choke, E.; Belli, A.-M.; Loftus, I.; Thompson, M.M. Aortic aneurysms secrete interleukin-6 into the circulation. J. Vasc. Surg. 2007, 45, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Liu, C.-L.; Lv, B.-J.; Zhang, J.-Y.; Cheng, L.; Cheng, X.; Lindholt, J.S.; Rasmussen, L.M.; Shi, G.-P. Plasma cytokine levels and risks of abdominal aortic aneurysms: A population-based prospective cohort study. Ann. Med. 2015, 47, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega de Céniga, M.; Esteban, M.; Quintana, J.M.; Barba, A.; Estallo, L.; de la Fuente, N.; Viviens, B.; Martin-Ventura, J.L. Search for Serum Biomarkers Associated with Abdominal Aortic Aneurysm Growth—A Pilot Study. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 297–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainas, T.; Lubbers, T.; Stassen, F.R.; Herngreen, S.B.; van Dieijen-Visser, M.P.; Bruggeman, C.A.; Kitslaar, P.J.; Schurink, G.W. Serum c-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation 2003, 107, 1103–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juvonen, J.; Surcel, H.M.; Satta, J.; Teppo, A.M.; Bloigu, A.; Syrjälä, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, F.A.; Buurman, W.A.; Wodzig, W.K.; Schurink, G.W. Biomarkers of abdominal aortic aneurysm progression. Part 2: Inflammation. Nat. Rev. Cardiol. 2009, 6, 543–552. [Google Scholar] [CrossRef]
| Survivor (n = 305) | Non-Survivor (n = 29) | p-Value | |
|---|---|---|---|
| Female sex | 61 (20.0%) | 2 (6.9%) | 0.085 |
| Age (years) | 71.69 ± 9.42 | 73.28 ± 8.54 | 0.382 |
| Height (cm) | 165.35 ± 8.25 | 166.04 ± 8.11 | 0.389 |
| Weight (kg) | 64.51 ± 11.19 | 64.56 ± 13.30 | 0.985 |
| BMI | 23.51 ± 3.13 | 23.42 ± 4.52 | 0.925 |
| Smoking | 113 (37.0%) | 11 (37.9%) | 0.925 |
| HTN | 214 (70.4%) | 17 (58.6%) | 0.189 |
| DM | 54 (17.7%) | 5 (17.2%) | 0.950 |
| CVA | 43 (14.1%) | 5 (17.2%) | 0.586 |
| CAOD | 72 (23.7%) | 8 (27.6%) | 0.638 |
| COPD | 7 (2.3%) | 1 (3.4%) | 0.520 |
| CRF | 26 (8.5%) | 1 (3.4%) | 0.491 |
| ESRD | 3 (1.0%) | 0 | 1.000 |
| Rupture | 81 (26.6%) | 17 (58.6%) | <0.001 * |
| Emergency | 167 (54.8%) | 26 (89.7%) | <0.001 * |
| Preop steriod | 5 (1.6%) | 0 | 1.000 |
| Preop WBC (/μL) | 9661.15 ± 4531.01 | 14,328.62 ± 6821.57 | 0.001 * |
| Preop neutrophil (/μL) | 7089.71 ± 4543.53 | 11,833.71 ± 6670.75 | <0.001 * |
| Preop lymphocyte (/μL) | 1712.39 ± 814.84 | 1483.16 ± 689.47 | <0.001 * |
| Preop Hb (g/dL) | 12.07 ± 2.61 | 10.39 ± 2.66 | 0.001 * |
| Preop Hct (%) | 36.25 ± 7.54 | 31.39 ± 7.81 | 0.001 * |
| Preop PLT (/μL) | 204,328.31 ± 72993.61 | 187,206.90 ± 107334.38 | 0.407 |
| Preop NLR | 5.76 ± 6.44 | 10.53 ± 7.60 | 0.003 * |
| Preop MPV (fL) | 9.38 ± 1.20 | 9.11 ± 1.39 | 0.267 |
| Preop PLR | 145.35 ± 91.11 | 154.20 ± 113.19 | 0.626 |
| Preop PT (INR) | 1.11 ± 0.24 | 1.47 ± 0.54 | 0.002 * |
| Preop procalcitonin (ng/mL) | 0.22 ± 0.73 | 0.45 ± 0.41 | 0.402 |
| Preop CRP (mg/L) | 21.87 ± 43.82 | 35.84 ± 47.59 | 0.234 |
| Preop BNP (pg/mL) | 166.52 ± 300.09 | 234.42 ± 264.01 | 0.488 |
| Preop BUN (mg/dL) | 20.06 ± 9.69 | 24.32 ± 10.80 | 0.028 * |
| Preop Cr (mg/dL) | 1.17 ± 0.82 | 1.32 ± 0.49 | 0.323 |
| Preop eGFR (mL/min/1.73 m2) | 72.89 ± 22.87 | 59.00 ± 24.87 | 0.024 * |
| First Tertile (n = 111) | Second Tertile (n = 111) | Third Tertile (n = 112) | p-Value | |
|---|---|---|---|---|
| Female sex | 23 (20.7%) | 19 (17.1%) | 21 (18.8%) | 0.790 |
| Age (years) | 69.08 ± 8.82 | 73.07 ± 9.55 | 73.30 ± 9.12 | 0.001 * |
| Height (cm) | 164.85 ± 9.22 | 164.99 ± 7.78 | 166.38 ± 7.56 | 0.321 |
| Weight (kg) | 65.85 ± 12.04 | 63.72 ± 11.42 | 63.94 ± 10.48 | 0.309 |
| BMI | 24.12 ± 3.32 | 23.37 ± 3.28 | 22.99 ± 3.05 | 0.032 |
| Smoking | 42 (37.8%) | 41 (36.9%) | 41 (36.6%) | 0.981 |
| HTN | 84 (76.4%) | 68 (61.3%) | 79 (70.5%) | 0.049 * |
| DM | 23 (20.7%) | 16 (14.4%) | 20 (17.9%) | 0.467 |
| CVA | 18 (16.2%) | 13 (11.7%) | 17 (15.2%) | 0.605 |
| CAOD | 27 (24.3%) | 32 (29.1%) | 21 (18.8%) | 0.196 |
| COPD | 1 (0.9%) | 5 (4.5%) | 2 (1.8%) | 0.236 |
| CRF | 8 (7.2%) | 8 (7.2%) | 11 (9.8%) | 0.710 |
| ESRD | 0 | 1 (0.9%) | 2 (1.8%) | 0.776 |
| Rupture | 4 (3.6%) | 24 (21.6%) | 70 (62.5%) | <0.001 * |
| Emergency | 28 (25.2%) | 58 (52.3%) | 107 (95.5%) | <0.001 * |
| Preop steroid | 1 (0.9%) | 3 (2.7%) | 1 (0.9%) | 0.542 |
| Amount of crystalloid (mL) | 2875.94 ± 1796.88 | 2675.95 ± 1675.66 | 3182.89 ± 1592.65 | 0.089 |
| Amount of colloid (mL) | 442.59 ± 591.56 | 503.18 ± 615.34 | 554.09 ± 772.70 | 0.465 |
| Urine output (mL) | 443.70 ± 364.54 | 427.06 ± 362.91 | 458.11 ± 458.75 | 0.851 |
| Cell saver (mL) | 443.70 ± 364.54 | 427.06 ± 362.91 | 458.11 ± 458.75 | 0.505 |
| Bleeding (mL) | 974.84 ± 881.08 | 1121.82 ± 1345.87 | 1356.09 ± 1679.08 | 0.156 |
| pRBC transfusion (pack) | 1.07 ± 2.01 | 1.50 ± 2.54 | 3.34 ± 4.26 | <0.001 * |
| FFP (pack) | 0.77 ± 1.71 | 1.02 ± 2.06 | 2.27 ± 3.10 | <0.001 * |
| PLTconc (pack) | 0.76 ± 2.68 | 0.77 ± 2.87 | 1.96 ± 4.99 | 0.062 |
| Operative time (min) | 140.22 ± 74.89 | 135.56 ± 56.73 | 148.06 ± 65.23 | 0.361 |
| Anesthesia time (min) | 218.77 ± 84.55 | 203.53 ± 62.35 | 198.84 ± 71.67 | 0.108 |
| ACC time (min) | 42.03 ± 19.80 | 36.27 ± 17.21 | 42.59 ± 22.08 | 0.066 |
| First Tertile (n = 111) | Second Tertile (n = 111) | Third Tertile (n = 112) | p-Value | |
|---|---|---|---|---|
| HOD (days) | 14.02 ± 13.97 | 20.29 ± 21.72 | 19.65 ± 15.31 | 0.005 * |
| ICU (days) | 6.04 ± 61.20 | 3.49 ± 5.64 | 6.43 ± 9.25 | 0.805 |
| Reintubation | 7 (6.4%) | 11 (10.3%) | 22 (20.0%) | 0.007 * |
| MV > 24 h | 7 (6.3%) | 12 (10.8%) | 34 (30.4%) | <0.001 * |
| ICU readmission | 7 (6.3%) | 13 (11.7%) | 9 (8.0%) | 0.344 |
| Reopen for bleeding | 1 (0.9%) | 2 (1.8%) | 8 (7.1%) | 0.046 * |
| CVA | 2 (1.8%) | 1 (0.9%) | 2 (1.8%) | 1.000 |
| New RRT | 2 (1.8%) | 6 (5.4%) | 4 (3.6%) | 0.358 |
| Pulmonary Cx | 8 (7.2%) | 22 (19.8%) | 27 (24.1%) | 0.002 * |
| Infection | 4 (3.6%) | 8 (7.2%) | 17 (15.2%) | 0.007 * |
| Wound infection | 0 | 1 (0.9%) | 2 (1.8%) | 0.776 |
| AKI | 50 (45.0%) | 45 (40.9%) | 55 (50.0%) | 0.399 |
| MI | 1 (0.9%) | 3 (2.7%) | 3 (2.7%) | 0.707 |
| In-hospital mortality | 3 (2.7%) | 10 (9.0%) | 15 (13.4%) | 0.015 * |
| 1-month mortality | 2 (1.8%) | 7 (6.3%) | 12 (10.7%) | 0.023 * |
| 1-year mortality | 3 (2.7%) | 10 (9.0%) | 16 (14.3%) | 0.009 * |
| Univariate OR (CI) | p-Value | Multivariate OR (CI) | p-Value | |
|---|---|---|---|---|
| All Patients | ||||
| Age | 1.027 (0.979–1.076) | 0.277 | ||
| Rupture | 2.859 (1.131–7.225) | 0.026 | 2.706 (1.097–6.673) | 0.031 * |
| Smoking | 1.255 (0.524–3.005) | 0.610 | ||
| HTN | 0.619 (0.258–1.483) | 0.282 | ||
| DM | 1.096 (0.369–3.258) | 0.869 | ||
| CAOD | 1.483 (0.590–3.724) | 0.402 | ||
| CVA | 1.356 (0.436–4.214) | 0.599 | ||
| CKD | 0.430 (0.050–3.663) | 0.440 | ||
| NLR | 1.077 (1.007–1.152) | 0.030 | 1.085 (1.016–1.159) | 0.015 * |
| PLR | 0.996 (0.990–1.002) | 0.181 | 0.995 (0.989–1.001) | 0.103 |
| MPV | 0.710 (0.496–1.016) | 0.061 | 0.716 (0.504–1.019) | 0.063 |
| Non-rupture | ||||
| Age | 1.010 (0.941–1.085) | 0.782 | ||
| Smoking | 0.886 (0.229–3.428) | 0.860 | ||
| HTN | 0.408 (0.113–1.465) | 0.169 | 0.403 (0.125–1.295) | 0.127 |
| DM | 0.623 (0.071–5.440) | 0.668 | ||
| CAOD | 1.252 (0.310–5.052) | 0.752 | ||
| CVA | 1.875 (0.360–9.756) | 0.455 | ||
| CKD | 1.863 (0.201–17.258) | 0.584 | ||
| NLR | 1.040 (0.954–1.133) | 0.376 | ||
| PLR | 1.003 (0.996–1.009) | 0.440 | ||
| MPV | 0.773 (0.440–1.357) | 0.730 | ||
| Rupture | ||||
| Age | 1.025 (0.955–1.101) | 0.495 | ||
| Smoking | 0.852 (0.528–6.494) | 0.336 | ||
| HTN | 0.888 (0.244–3.225) | 0.857 | ||
| DM | 1.568 (0.368–6.676) | 0.543 | ||
| CAOD | 1.791 (0.474–6.767) | 0.390 | ||
| CVA | 0.890 (0.162–4.887) | 0.893 | ||
| CKD | 0.000 (0.000) | 0.999 | ||
| NLR | 1.139 (1.020–1.271) | 0.020 | 1.144 (1.031–1.271) | 0.012 * |
| PLR | 0.988 (0.977–1.000) | 0.047 | 0.986 (0.975–0.998) | 0.017 * |
| MPV | 0.617 (0.367–1.037) | 0.069 | 0.616 (0.374–1.013) | 0.056 |
| Non-Rupture (n = 236) | Rupture (n = 98) | p-Value | |
|---|---|---|---|
| Preop WBC (/μL) | 8249.36 ± 2931.44 | 14,442.14 ± 5960.32 | <0.001 * |
| Preop neutrophil (/μL) | 5569.29 ± 2947.05 | 12,154.97 ± 5628.89 | <0.001 * |
| Preop lymphocyte (/μL) | 1824.74 ± 822.26 | 1374.01 ± 670.37 | <0.001 * |
| Preop NLR | 4.10 ± 4.75 | 11.17 ± 7.90 | <0.001 * |
| Preop Hb (g/dL) | 12.85 ± 2.13 | 9.70 ± 2.47 | <0.001 * |
| Preop Hct (%) | 38.47 ± 6.13 | 29.47 ± 7.30 | <0.001 * |
| Preop PLT (/μL) | 217,017.52 ± 71,052.99 | 168,704.08 ± 78,781.86 | <0.001 * |
| Preop MPV (fL) | 9.26 ± 1.19 | 9.59 ± 1.24 | 0.023 * |
| Preop PLR | 142.22 ± 84.14 | 155.52 ± 111.62 | 0.291 |
| Preop PT (INR) | 1.06 ± 0.16 | 1.35 ± 0.42 | <0.001 * |
| Preop procalcitonin (ng/mL) | 0.18 ± 0.84 | 0.32 ± 0.39 | 0.245 |
| Preop CRP (mg/dL) | 19.34 ± 35.91 | 30.08 ± 57.75 | 0.130 |
| Preop BNP (pg/mL) | 201.50 ± 355.12 | 130.09 ± 189.65 | 0.149 |
| Preop lactate (mmol/L) | 0.97 ± 0.41 | 4.45 ± 2.66 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.E.; Yoon, H.J.; Nam, S.B.; Song, S.W.; Lee, G.; Ham, S.Y. Preoperative Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Mean Platelet Volume as Predictors of 1-Year Mortality in Patients Undergoing an Open Repair of Abdominal Aortic Aneurysms: A Retrospective Study. J. Clin. Med. 2021, 10, 5410. https://doi.org/10.3390/jcm10225410
Ko DE, Yoon HJ, Nam SB, Song SW, Lee G, Ham SY. Preoperative Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Mean Platelet Volume as Predictors of 1-Year Mortality in Patients Undergoing an Open Repair of Abdominal Aortic Aneurysms: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(22):5410. https://doi.org/10.3390/jcm10225410
Chicago/Turabian StyleKo, Da Eun, Hei Jin Yoon, Sang Beom Nam, Suk Won Song, Gisong Lee, and Sung Yeon Ham. 2021. "Preoperative Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Mean Platelet Volume as Predictors of 1-Year Mortality in Patients Undergoing an Open Repair of Abdominal Aortic Aneurysms: A Retrospective Study" Journal of Clinical Medicine 10, no. 22: 5410. https://doi.org/10.3390/jcm10225410
APA StyleKo, D. E., Yoon, H. J., Nam, S. B., Song, S. W., Lee, G., & Ham, S. Y. (2021). Preoperative Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Mean Platelet Volume as Predictors of 1-Year Mortality in Patients Undergoing an Open Repair of Abdominal Aortic Aneurysms: A Retrospective Study. Journal of Clinical Medicine, 10(22), 5410. https://doi.org/10.3390/jcm10225410






