Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Procedures
2.3. Statistics
3. Results
Study Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute for Health and Clinical Excellence (NICE). The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care; National Institute for Health and Clinical Excellence (NICE): London, UK, 2015. [Google Scholar]
- Jędrzejczak, J.; Majkowska-Zwolińska, B.; Ryglewicz, D. Recommendations of the Polish Society of Epileptology regarding the treatment of epileptic seizures in adults. J. Epileptol. 2019, 27, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; Peltola, J.; Roulet, P.E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, J.G.; Riche, W. Antiepileptic Drugs. Updated 2009. Available online: http:/emedicine.medscape.com//article/1187334-overview (accessed on 20 October 2021).
- Mittag, N.; Meister, S.; Berg, A.M.; Walther, U.I. A Case Report of a Carbamazepine Overdose with Focus on Pharmacokinetic Aspects. Pharmacopsychiatry 2016, 49, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, M.; Pineschi, R.; Bonanni, P.; Pellegri, A.; Clementi, E. Precipitation of Carbamazepine Controlled Seizures Due to Low-Dose Risperidone in a Child: A Conspiracy to Unbalance Blood Electrolytes. J. Clin. Psychopharmacol. 2016, 36, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.A.; Raehl, C.L. Clinical and economic outcomes of pharmacist-managed antiepileptic drug therapy. Pharmacotherapy 2006, 26, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parton, M.; Cockerell, C.O. Epilepsytheaetiology and pathogenesis. Hosp. Pharmacist. 2003, 10, 288–295. [Google Scholar]
- Nadkarni, S.; LaJoie, J.; Devinsky, O. Current treatments of epilepsy. Neurology 2005, 64 (Suppl. 3), S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Stolarek, W.; Kasprzak, M.; Krzyżanowski, M.; Szadujkis-Szadurska, K.; Wiciński, M.; Grześk, E. Therapeutic drug monitoring of digoxin-20 years of experience. Pharmacol. Rep. 2018, 70, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Janiszewska, E.; Malinowski, B.; Kubica, A.; Wiciński, M. Adherence in patients with atrial fibrillation treated with dabigatran. Kardiol. Pol. 2018, 76, 1562–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakya, G.; Malla, S.; Shakya, K.N.; Shrestha, R. Therapeutic Drug Monitoring of Antiepileptic Drugs. JNMA 2008, 47, 94–97. [Google Scholar] [CrossRef]
- Langan, Y.; Sander, J.W.A.S. Sudden Unexpected Death in Patients with Epilepsy Definition, Epidemiology and Therapeutic Implications. CNS Drugs 2000, 13, 337–350. [Google Scholar] [CrossRef]
- Gierbolini, J.; Giarratano, M.; Benbadis, S.R. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy-a comparative review. Expert Opin. Pharmacother. 2016, 17, 885–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, U.; Elsner, H.; May, T.W.; Schimichowski, B.; Thorbecke, R. Postictal serum levels of antiepileptic drugs for detection of noncompliance. Epilepsy Behav. 2003, 4, 487–495. [Google Scholar] [CrossRef]
- Shaikh, A.S.; Guo, R. Measurement and Comparison of Carbamazepine Plasma Levels for Assessment of Compliance, Safety and Toxicity. Ind. J. Pharm. Sci. 2017, 79, 1025–1029. [Google Scholar] [CrossRef] [Green Version]
- Burianová, I.; Bořecká, K. Clin Biochem. Routine therapeutic monitoring of the active metabolite of carbamazepine: Is it really necessary? Clin. Biochem. 2015, 48, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Woźniak-Wiśniewska, A.; Błażejewski, J.; Górny, B.; Wołowiec, Ł.; Rogowicz, D.; Nowaczyk, A. The Interactions of Nintedanib and Oral Anticoagulants-Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 22, 282. [Google Scholar] [CrossRef] [PubMed]
| Study Feature (n = 710) | Property Value |
|---|---|
| Age | 19.0 (11.0–31.0) |
| 3–17 (years) | 332 (46.8%) |
| ≥18 (years) | 378 (53.2%) |
| Height (cm) | 168.0 (160.0–175.0) |
| Body weight (kg) | 59.0 (35.0–70.0) |
| BMI (kg/m2) | 22.49 (19.59–25.35) |
| Sex | |
| Men | 339 (47.7%) |
| Women | 371 (52.3%) |
| The main reason for hospitalization | |
| Epilepsy | 672 (94.6%) |
| Suspected drug overdose | 23 (3.2%) |
| Suicide attempt | 8 (1.1%) |
| Not specified | 7 (1.0%) |
| The daily dose of carbamazepine (mg) * | 600.0 (400.0–800.0) |
| The daily dose of carbamazepine per kilogram of body weight (mg) * | 12.00 (9.09–15.79) |
| The number of doses per day | |
| 1 | 22 (3.1%) |
| 2 | 469 (66.1%) |
| ≥3 | 191 (26.9%) |
| No data (including patients with suicide attempt) | 28 (3.9%) |
| Carbamazepine concentration (µg/mL) Time period from the beginning of treatment with carbamazepine (days) | 5.58 (4.01–7.33) |
| 180.0 (60.0–700.0) | |
| Carbamazepine below the therapeutic level (<4 µg/mL) | 177 (24.9%) |
| The therapeutic level of carbamazepine (4–12 µg/mL) | 504 (71.0%) |
| Carbamazepine above therapeutic level (≥12µg/mL) | 29 (4.1%) |
| Study Feature | Carbamazepine Concentration (µg/mL) | p |
|---|---|---|
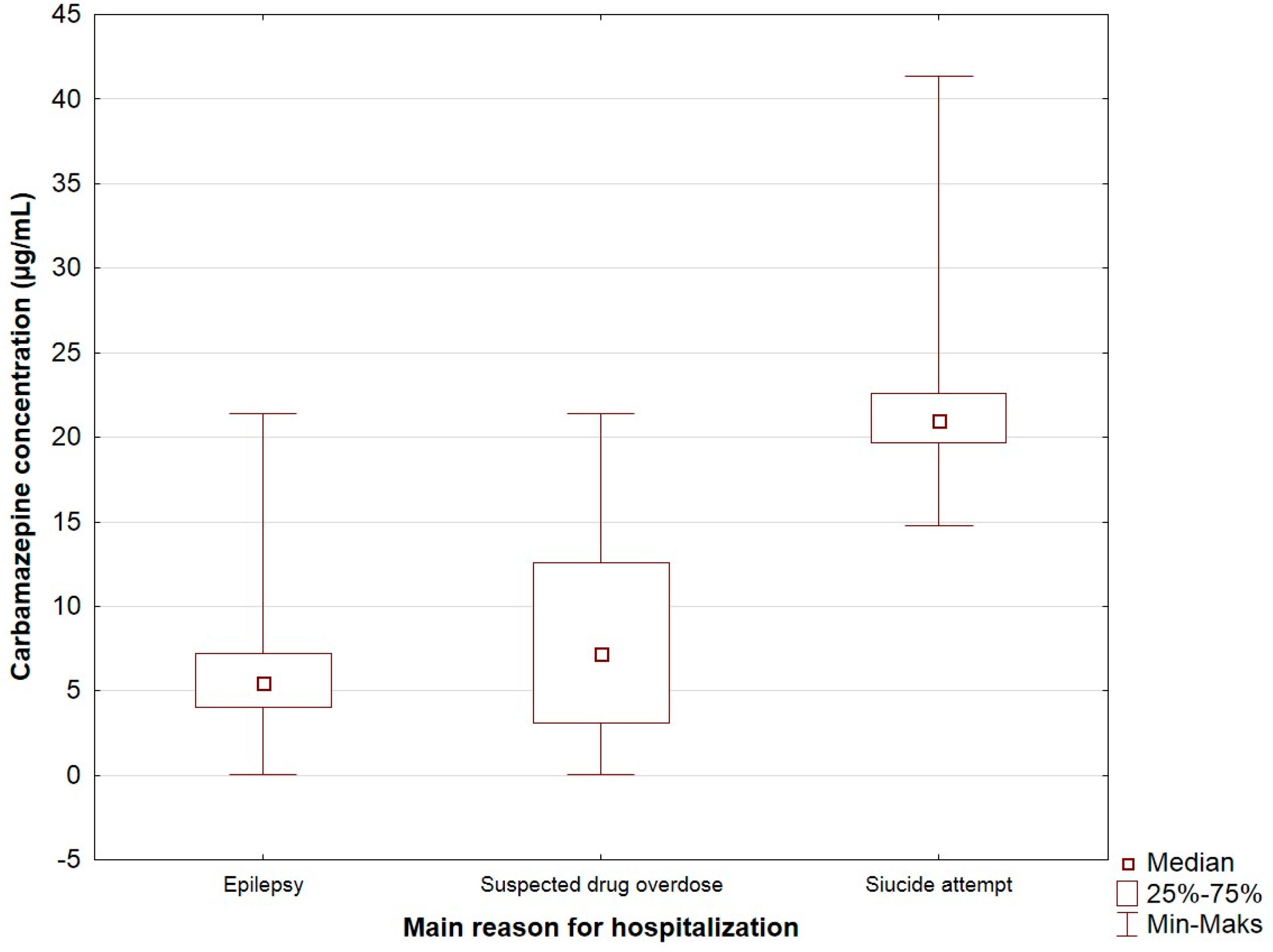
| The main reason for hospitalization (n = 703) | <0.0001 | |
| Epilepsy | 5.52 (4.01–7.22) | |
| Suspected drug overdose | 7.24 (3.08–12.61) | |
| Suicide attempt | 21.0 (19.67–22.63) | |
| Sex (n = 710) | 0.408 | |
| Women | 5.52 (4.01–7.24) | |
| Men | 5.63 (3.88–7.40) | |
| Age (years) (n = 710) | 0.347 | |
| 3–17 | 5.50 (4.07–7.14) | |
| ≥18 | 5.76 (3.92–7.54) | |
| The number of doses per day (n = 682) | 0.2830 | |
| 1 | 5.03 (3.0–6.48) | |
| 2 | 5.70 (3.98–7.26) | |
| ≥3 | 5.36 (4.14–7.23) |
| Study Feature (n = 682) | The Daily Dose of Carbamazepine (mg) | p | The Daily Dose of Carbamazepine Per Kilogram of Body Weight (mg) | p |
|---|---|---|---|---|
| Sex | 0.3593 | 0.0891 | ||
| Men | 600 (400–800) | 11.43 (8.57–15.38) | ||
| Women | 600 (400–800) | 12.1 (9.52–16.0) | ||
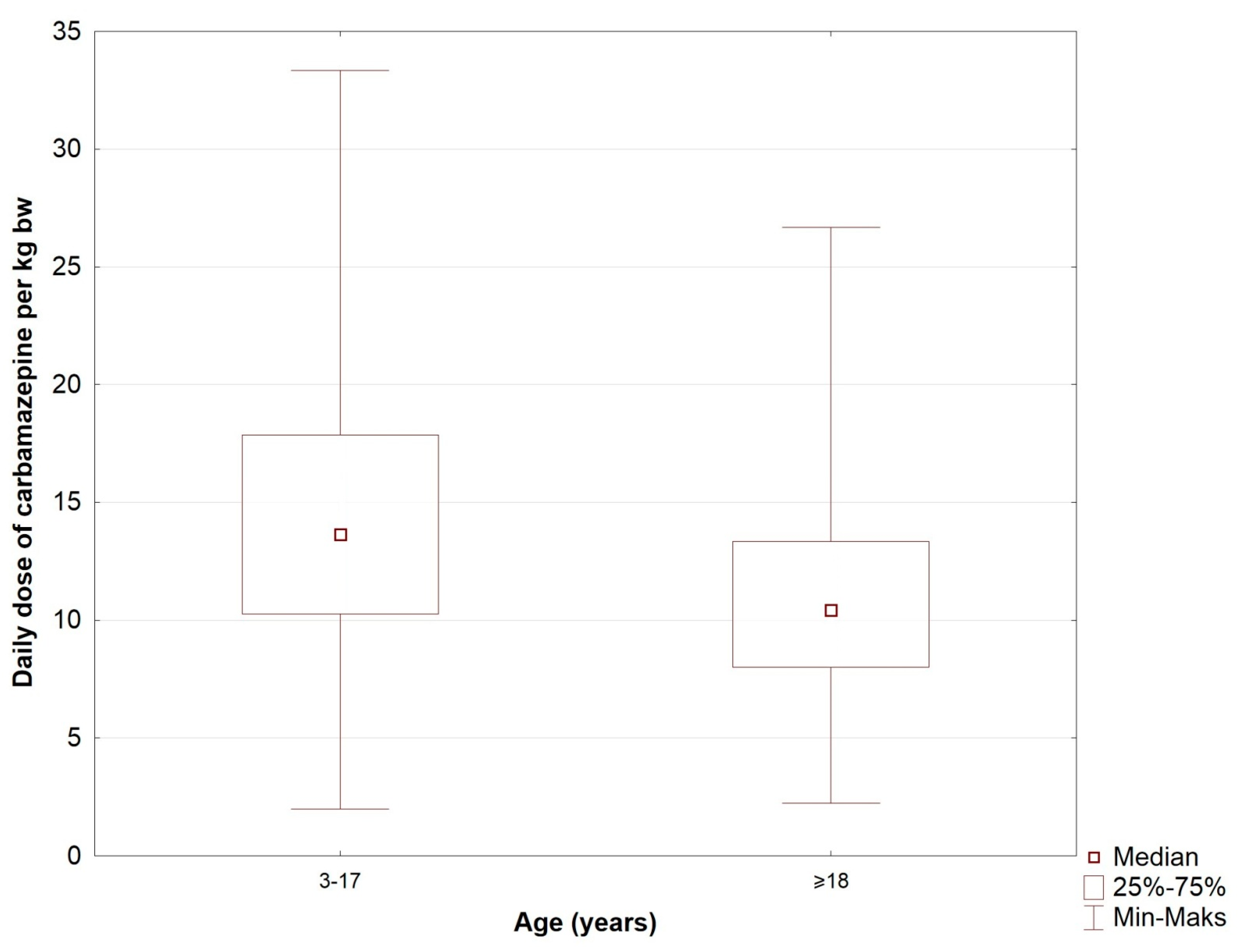
| Age | <0.00001 | <0.00001 | ||
| 3–17(years) | 450 (300–600) | 13.64 (10.26–17.86) | ||
| ≥18 (years) | 800 (600–900) | 10.43 (8.00–13.33) | ||
| Carbamazepine below the therapeutic level (<4 µg/mL) | 400 (300–600) | <0.00001 | 9.92 (6.78–12.50) | <0.00001 |
| The therapeutic level of carbamazepine (4–12 µg/mL) | 600 (450–800) | 12.86 (9.68–16.67) | ||
| Carbamazepine above the therapeutic level (≥12µg/mL) | 850 (600–1000) | 12.50 (10.0–18.46) |
| Study Feature | Sex | p | Age (years) | p | ||
|---|---|---|---|---|---|---|
| Men | Women | 3–17 | ≥18 | |||
| The main reason for hospitalization (n = 703) | ||||||
| Epilepsy | 320 (47.6%) | 352 (52.4%) | 0.056 | 325 (48.4%) | 347 (51.6%) | 0.003 |
| Suspected drug overdose | 9 (39.1%) | 14 (60.9%) | 6 (26.1%) | 17 (73.9%) | ||
| Suicide attempt | 7 (87.5%) | 1 (12.5%) | 0 (0.00%) | 8 (100%) | ||
| The number of doses per day (n = 682) | ||||||
| 1 | 14 (63.6%) | 8 (36.4%) | 0.139 | 13 (4.0%) | 9 (2.5%) | 0.0100 |
| 2 | 213 (45.4%) | 256 (54.6%) | 236 (73.3%) | 233 (64.7%) | ||
| ≥3 | 97 (50.8%) | 94 (49.2%) | 73 (22.7%) | 118 (32.8%) | ||
| Carbamazepine concentration (µg/mL) | ||||||
| Carbamazepine below the therapeutic level (<4 µg/mL) | 86(25.4%) | 91(24.5%) | 0.4502 | 80(24.1%) | 97(25.7%) | 0.0082 |
| The therapeutic level of carbamazepine (4–12 µg/mL) | 236(69.6%) | 268(72.2%) | 244(73.5%) | 260(68.8%) | ||
| Carbamazepine above the therapeutic level (≥12 µg/mL) | 17(5.0%) | 12(3.2%) | 8.0(2.4%) | 21.0(5.6%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grześk, G.; Stolarek, W.; Kasprzak, M.; Grześk, E.; Rogowicz, D.; Wiciński, M.; Krzyżanowski, M. Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. J. Clin. Med. 2021, 10, 5396. https://doi.org/10.3390/jcm10225396
Grześk G, Stolarek W, Kasprzak M, Grześk E, Rogowicz D, Wiciński M, Krzyżanowski M. Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. Journal of Clinical Medicine. 2021; 10(22):5396. https://doi.org/10.3390/jcm10225396
Chicago/Turabian StyleGrześk, Grzegorz, Wioleta Stolarek, Michał Kasprzak, Elżbieta Grześk, Daniel Rogowicz, Michał Wiciński, and Marek Krzyżanowski. 2021. "Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study" Journal of Clinical Medicine 10, no. 22: 5396. https://doi.org/10.3390/jcm10225396
APA StyleGrześk, G., Stolarek, W., Kasprzak, M., Grześk, E., Rogowicz, D., Wiciński, M., & Krzyżanowski, M. (2021). Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. Journal of Clinical Medicine, 10(22), 5396. https://doi.org/10.3390/jcm10225396






