Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Device Implantation
2.3. Data Collection
2.4. Determination of Risk Factors
2.5. Assessment of ALBI Score before CRT
2.6. Reassessment of ALBI Score 6 Months after CRT
2.7. Identification of Adverse Events during the Follow-Up
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Laboratory and Echocardiographic Data
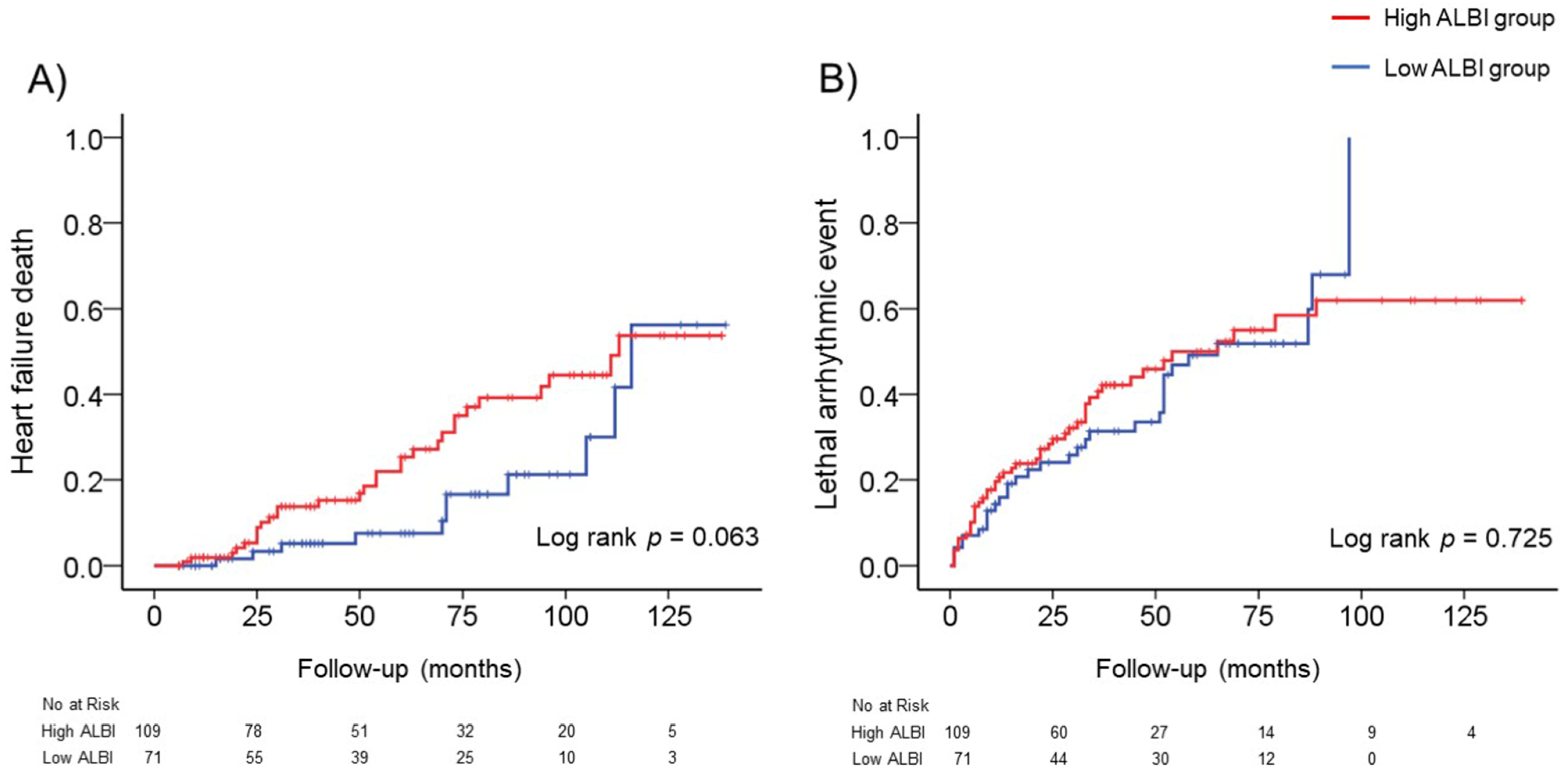
3.3. ALBI Score at Baseline and Adverse Events
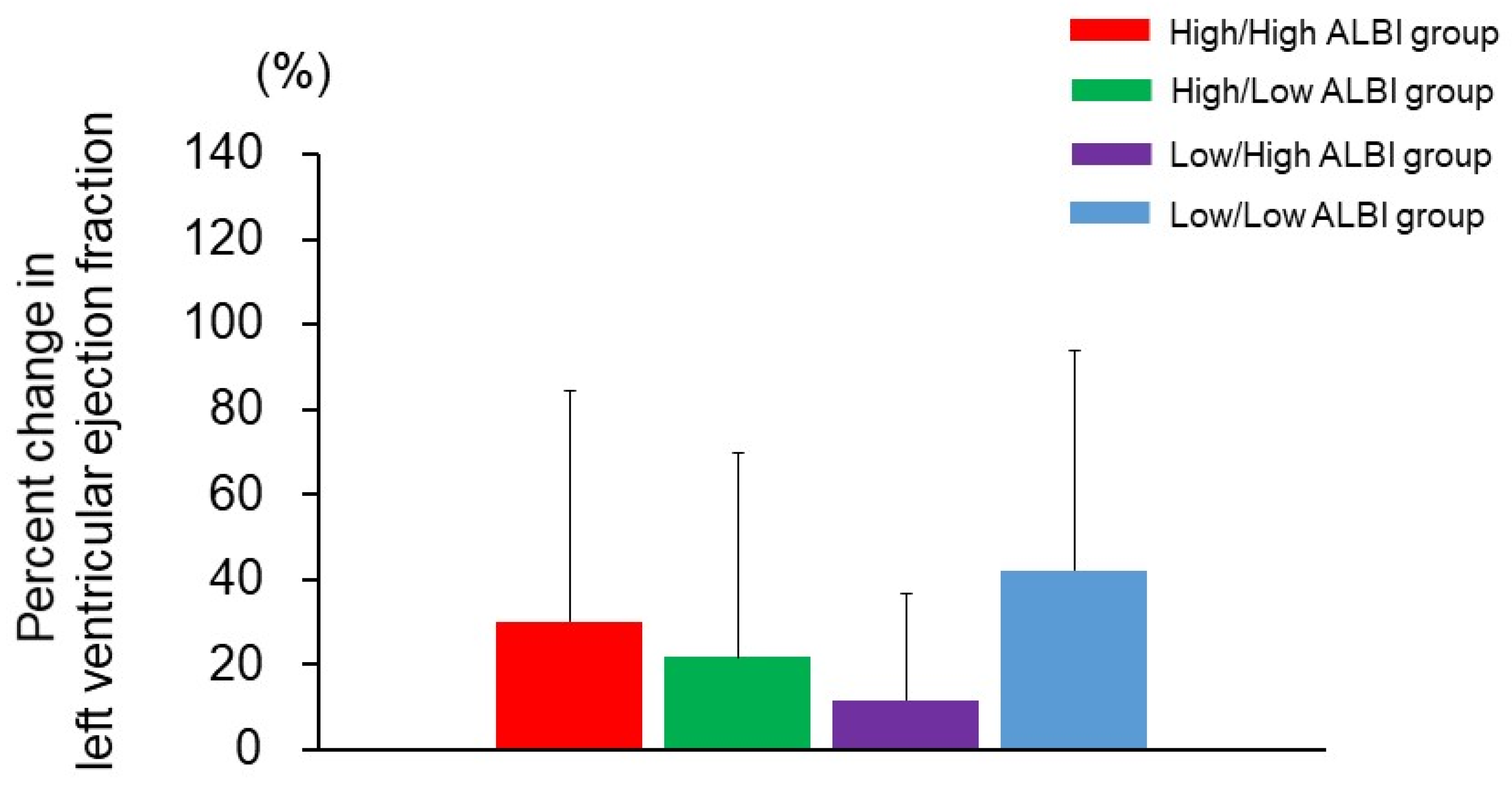
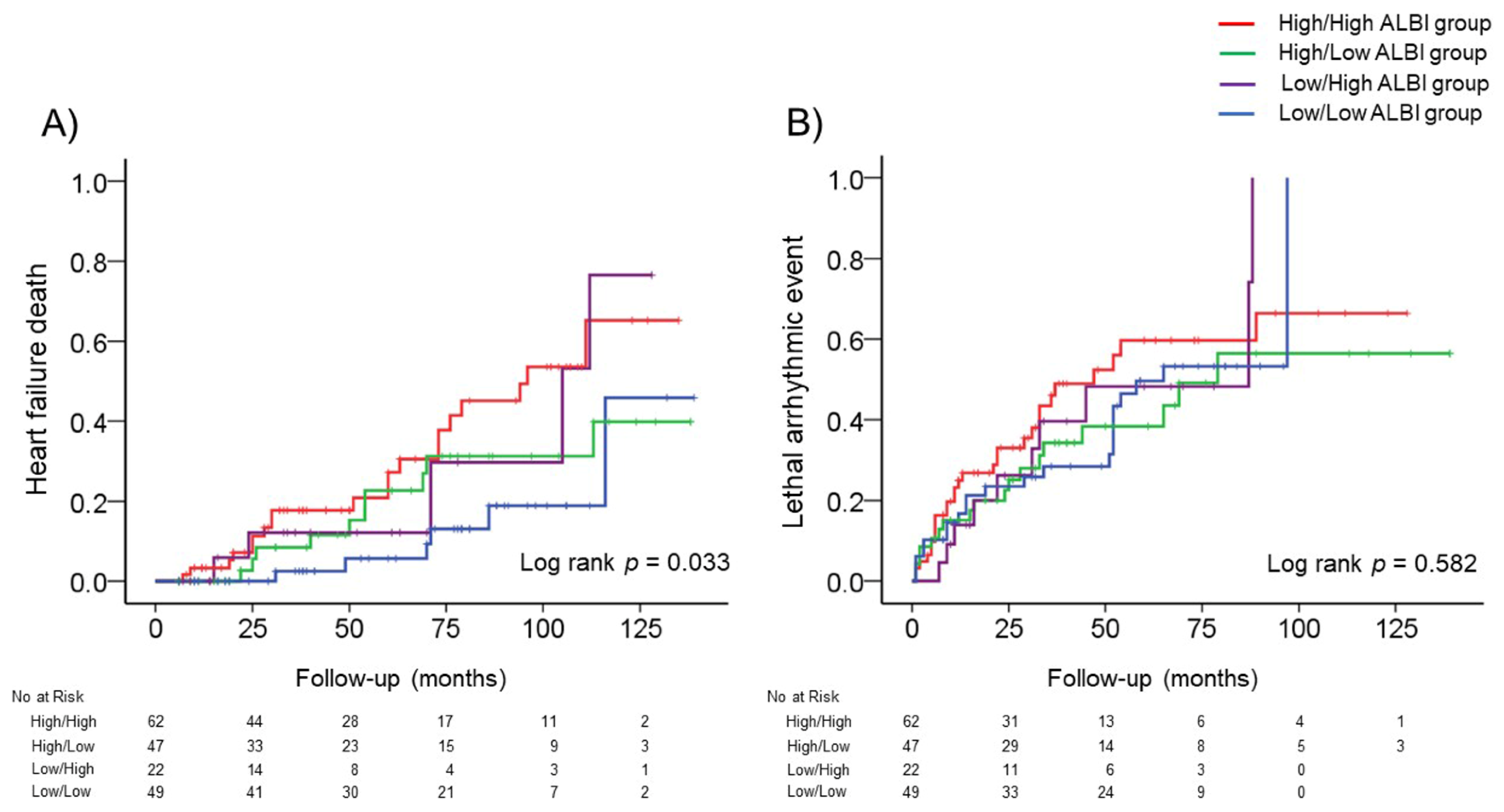
3.4. Changes in the ALBI Score after CRT and Adverse Events
4. Discussion
4.1. Assessment of ALBI Score in HF Patients before CRT
4.2. Reassessment of ALBI Score in HF Patients after CRT
4.3. Clinical Implication
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleeker, G.B.; Bax, J.J.; Fung, J.W.; van der Wall, E.E.; Zhang, Q.; Schalij, M.J.; Chan, J.Y.; Yu, C.M. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am. J. Cardiol. 2006, 97, 260–263. [Google Scholar] [CrossRef]
- Sogaard, P.; Egeblad, H.; Pedersen, A.K.; Kim, W.Y.; Kristensen, B.O.; Hansen, P.S.; Mortensen, P.T. Sequential versus simultaneous biventricular resynchronization for severe heart failure: Evaluation by tissue Doppler imaging. Circulation 2002, 106, 2078–2084. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.A.; Felker, G.M.; Pocock, S.; Mcmurray, J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B.; et al. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef]
- Van Deursen, V.M.; Damman, K.; Hillege, H.L.; van Beek, A.P.; van Veldhuisen, D.J.; Voors, A.A. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J. Card. Fail. 2010, 16, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Matsue, Y.; Kagiyama, N.; Yamaguchi, T.; Kuroda, S.; Okumura, T.; Kida, K.; Mizuno, A.; Oishi, S.; Inuzuka, Y.; Akiyama, E.; et al. Clinical and Prognostic Values of ALBI Score in Patients with Acute Heart Failure. Heart Lung Circ. 2020, 29, 1328–1337. [Google Scholar] [CrossRef]
- Vardas, P.E.; Auricchio, A.; Blanc, J.J.; Daubert, J.C.; Drexler, H.; Ector, H.; Gasparini, M.; Linde, C.; Morgado, F.B.; Oto, A.; et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur. Heart J. 2007, 28, 2256–2295. [Google Scholar] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S.; Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yoshihisa, A.; Sato, Y.; Sato, T.; Kamioka, M.; Kaneshiro, T.; Oikawa, M.; Kobayashi, A.; Suzuki, H.; Ishida, T.; et al. Utility of heart rate turbulence and T-wave alternans to assess risk for readmission and cardiac death in hospitalized heart failure patients. J. Cardiovasc. Electrophysiol. 2018, 29, 1257–1264. [Google Scholar] [CrossRef]
- Ho, K.K.; Anderson, K.M.; Kannel, W.B.; Grossman, W.; Levy, D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993, 88, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef] [PubMed]
- Giallourakis, C.C.; Rosenberg, P.M.; Friedman, L.S. The liver in heart failure. Clin. Liver Dis. 2002, 6, 947–967. [Google Scholar] [CrossRef]
- Poelzl, G.; Ess, M.; Mussner-Seeber, C.; Pachinger, O.; Frick, M.; Ulmer, H. Liver dysfunction in chronic heart failure: Prevalence, characteristics and prognostic significance. Eur. J. Clin. Investig. 2012, 42, 153–163. [Google Scholar] [CrossRef]
- Correale, M.; Tarantino, N.; Petrucci, R.; Tricarico, L.; Laonigro, I.; Di Biase, M.; Brunetti, N.D. Liver disease and heart failure: Back and forth. Eur. J. Intern. Med. 2018, 48, 25–34. [Google Scholar] [CrossRef]
- Yamada, T.; Haruki, S.; Minami, Y.; Numata, M.; Hagiwara, N. The C-reactive protein to prealbumin ratio on admission and its relationship with outcome in patients hospitalized for acute heart failure. J. Cardiol. 2021, 78, 308–313. [Google Scholar] [CrossRef]
- Lellouche, N.; De Diego, C.; Cesario, D.A.; Vaseghi, M.; Horowitz, B.N.; Mahajan, A.; Wiener, I.; Boyle, N.G.; Fonarow, G.C.; Shivkumar, K. Usefulness of preimplantation B-type natriuretic peptide level for predicting response to cardiac resynchronization therapy. Am. J. Cardiol. 2007, 99, 242–246. [Google Scholar] [CrossRef]
- Anand, I.; McMurray, J.J.; Whitmore, J.; Warren, M.; Pham, A.; McCamish, M.A.; Burton, P.B. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004, 110, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R.; et al. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Chau, E.; Sanderson, J.E.; Fan, K.; Tang, M.O.; Fung, W.H.; Lin, H.; Kong, S.L.; Lam, Y.M.; Hill, M.R.; et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002, 105, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, H.; Shishido, K.; Tobita, K.; Moriyama, N.; Murakami, M.; Saito, S. Impact of age on mid-term clinical outcomes and left ventricular reverse remodeling after cardiac resynchronization therapy. J. Cardiol. 2021, 77, 254–262. [Google Scholar] [CrossRef] [PubMed]
| High ALBI Group (n = 109) | Low ALBI Group (n = 71) | p Value | |
|---|---|---|---|
| Age | 67.2 ± 11.5 | 66.7 ± 10.9 | 0.770 |
| Male (n, %) | 77 (70.6%) | 50 (70.4%) | 0.975 |
| Body mass index, kg/cm2 | 22.2 ± 3.6 | 22.5 ± 3.6 | 0.529 |
| Systolic blood pressure, mmHg | 102.0 (94.0–112.0) | 109.0 (96.0–118.0) | 0.030 |
| Diastolic blood pressure, mmHg | 60.0 (56.0–68.0) | 63.0 (59.0–70.0) | 0.068 |
| Heart rate, bpm | 65.9 ± 15.4 | 63.3 ± 14.9 | 0.266 |
| NYHA class III/IV (n, %) | 46 (42.2%) | 17 (23.9%) | 0.012 |
| Ischemic etiology (n, %) | 26 (23.8%) | 10 (14.0%) | 0.109 |
| Hypertension (n, %) | 68 (62.3%) | 41 (57.7%) | 0.534 |
| Diabetes (n, %) | 59 (54.1%) | 32 (45.0%) | 0.235 |
| Chronic kidney disease (n, %) | 76 (69.7%) | 52 (73.2%) | 0.611 |
| Atrial fibrillation (n, %) | 30 (27.5%) | 13 (18.3%) | 0.157 |
| QRS duration >150 ms (n, %) | 70 (64.2%) | 48 (67.6%) | 0.640 |
| LBBB morphology (n, %) | 60 (55.0%) | 44 (61.9%) | 0.358 |
| Implantation for secondary prevention (n, %) | 39 (35.7%) | 24 (33.8%) | 0.786 |
| Atrioventricular block requiring a pacemaker (n, %) | 24 (22.4%) | 12 (16.9%) | 0.369 |
| Medication | |||
| β blockers (n, %) | 109 (100.0%) | 68 (95.7%) | 0.060 |
| ACE-Inhibitors/ARBs (n, %) | 91 (83.4%) | 59 (83.0%) | 0.946 |
| Mineralocorticoid receptor antagonists (n, %) | 69 (63.3%) | 52 (73.2%) | 0.165 |
| Amiodarone (n, %) | 66 (60.5%) | 36 (50.7%) | 0.193 |
| Inotropic agents (n, %) | 51 (46.7%) | 16 (22.5%) | 0.001 |
| High ALBI Group (n = 109) | Low ALBI Group (n = 71) | p Value | |
|---|---|---|---|
| Laboratory data | |||
| Hemoglobin (g/dL) | 12.4 ± 2.2 | 13.4 ± 1.7 | 0.002 |
| Platelet (×103/μL) | 172.0 (147.5–215.5) | 168.0 (150.0–211.0) | 0.701 |
| Albumin (g/dL) | 3.4 ± 0.4 | 4.2 ± 0.2 | <0.001 |
| Total bilirubin (mg/dL) | 1.0 ± 0.7 | 0.8 ± 0.3 | 0.036 |
| Aspartate aminotransferase (U/L) | 25.0 (19.5–32.0) | 26.0 (20.0–35.0) | 0.459 |
| Alanine aminotransferase (U/L) | 21.0 (13.5–32.5) | 22.0 (13.0–33.0) | 0.645 |
| Lactate dehydrogenase (U/L) | 199.0 (167.0–251.0) | 213.5 (185.5–240.0) | 0.129 |
| Blood urea nitrogen (mg/dL) | 20.5 (16.2–27.0) | 21.0 (17.0–29.0) | 0.519 |
| Creatinine (mg/dL) | 1.09 (0.83–1.41) | 1.09 (0.92–1.38) | 0.816 |
| eGFR (mL/min/1.73 m2) | 48.0 (37.0–68.0) | 52.0 (37.0–61.0) | 0.965 |
| Sodium (mEq/L) | 138.0 (136.0–140.0) | 139.0 (137.0–141.0) | 0.320 |
| C-reactive protein (mg/dL) | 0.24 (0.08–0.72) | 0.11 (0.05–0.21) | <0.001 |
| Brain natriuretic peptide (pg/mL) | 379.7 (184.8–615.3) | 190.5 (121.8–340.0) | <0.001 |
| ALBI score | −2.15 ± 0.32 | −2.89 ± 0.22 | <0.001 |
| Echocardiographic data | |||
| Left atrial diameter (mm) | 46.1 ± 8.7 | 45.9 ± 9.7 | 0.870 |
| LV end-diastolic diameter (mm) | 62.8 ± 9.4 | 61.7 ± 8.7 | 0.422 |
| LV end-systolic diameter (mm) | 54.5 ± 11.2 | 52.9 ± 10.0 | 0.335 |
| LV end-diastolic volume index (mL/m2) | 110.2 ± 47.1 | 109.8 ± 46.6 | 0.956 |
| LV end-systolic volume index (mL/m2) | 79.4 ± 41.5 | 80.0 ± 38.0 | 0.921 |
| LV ejection fraction (%) | 30.2 ± 11.0 | 30.9 ± 9.3 | 0.630 |
| TRPG (mmHg) | 28.3 ± 11.4 | 24.7 ± 8.4 | 0.034 |
| Inferior vena cava diameter (mm) | 15.8 ± 5.3 | 14.2 ± 4.4 | 0.041 |
| Heart Failure Deaths (41 Events/180 Patients) | Hazard Ratio | 95% Confidence Interval | p Value |
| Low/Low ALBI group | Reference | – | – |
| Low/High ALBI group | 2.765 | 0.842–9.086 | 0.094 |
| High/Low ALBI group | 1.852 | 0.669–5.129 | 0.235 |
| High/High ALBI group | 3.449 | 1.383–8.600 | 0.008 |
| High/High ALBI group * | 2.687 | 1.047–6.899 | 0.040 |
| Lethal Arrhythmic Events (79 Events/180 Patients) | Hazard Ratio | 95% Confidence Interval | pValue |
| Low/Low ALBI group | Reference | – | – |
| Low/High ALBI group | 1.189 | 0.562–2.515 | 0.651 |
| High/Low ALBI group | 0.922 | 0.493–1.724 | 0.799 |
| High/High ALBI group | 1.339 | 0.766–2.340 | 0.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Kaneshiro, T.; Yoshihisa, A.; Nodera, M.; Amami, K.; Nehashi, T.; Takeishi, Y. Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy. J. Clin. Med. 2021, 10, 5378. https://doi.org/10.3390/jcm10225378
Yamada S, Kaneshiro T, Yoshihisa A, Nodera M, Amami K, Nehashi T, Takeishi Y. Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy. Journal of Clinical Medicine. 2021; 10(22):5378. https://doi.org/10.3390/jcm10225378
Chicago/Turabian StyleYamada, Shinya, Takashi Kaneshiro, Akiomi Yoshihisa, Minoru Nodera, Kazuaki Amami, Takeshi Nehashi, and Yasuchika Takeishi. 2021. "Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy" Journal of Clinical Medicine 10, no. 22: 5378. https://doi.org/10.3390/jcm10225378
APA StyleYamada, S., Kaneshiro, T., Yoshihisa, A., Nodera, M., Amami, K., Nehashi, T., & Takeishi, Y. (2021). Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy. Journal of Clinical Medicine, 10(22), 5378. https://doi.org/10.3390/jcm10225378






