Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer
Abstract
:1. Introduction
2. Patients and Methods
Characterization of the Study Population
3. Cytotoxic Drugs and Methods
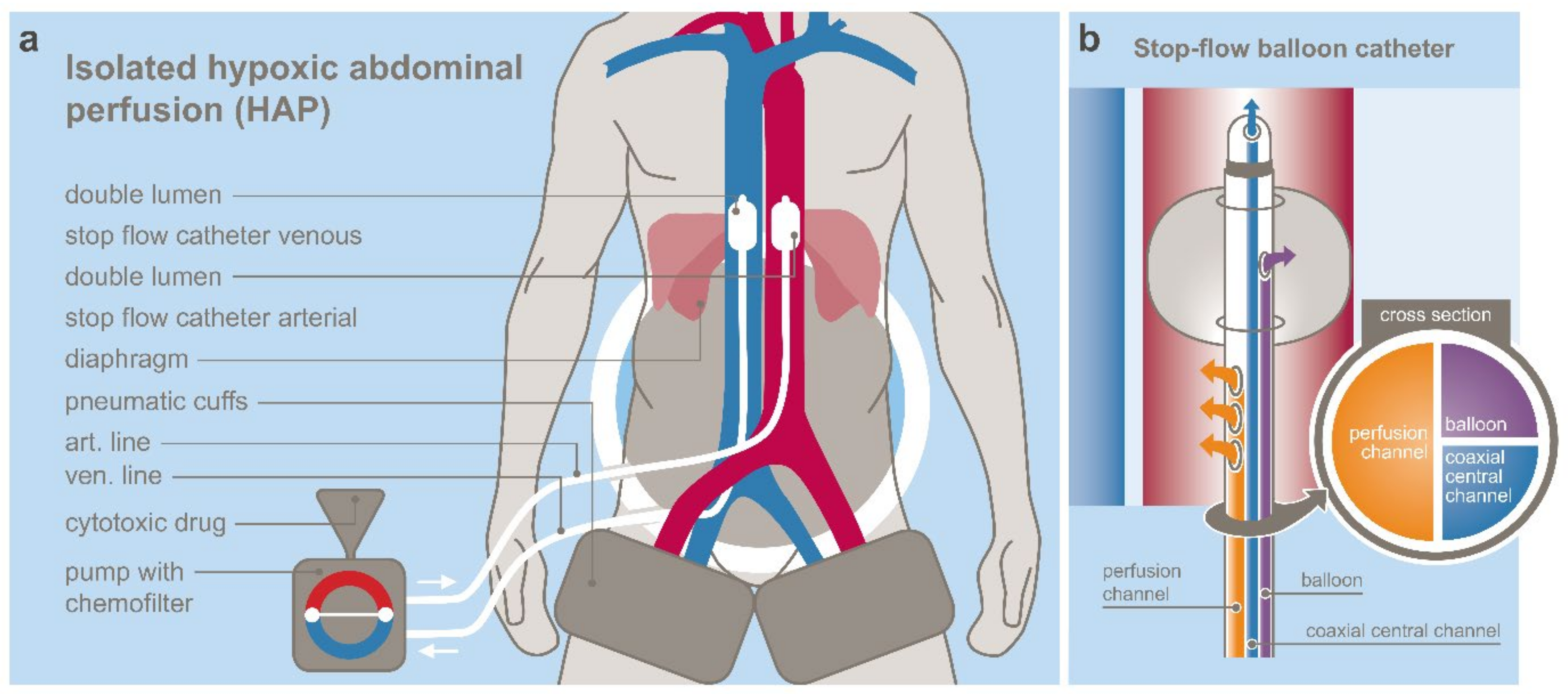
3.1. Regional Chemotherapy Techniques
3.2. Cytotoxic Drugs
3.3. Treatment Cycles
Statistical Analysis
4. Results
4.1. Adverse Events
4.2. Response
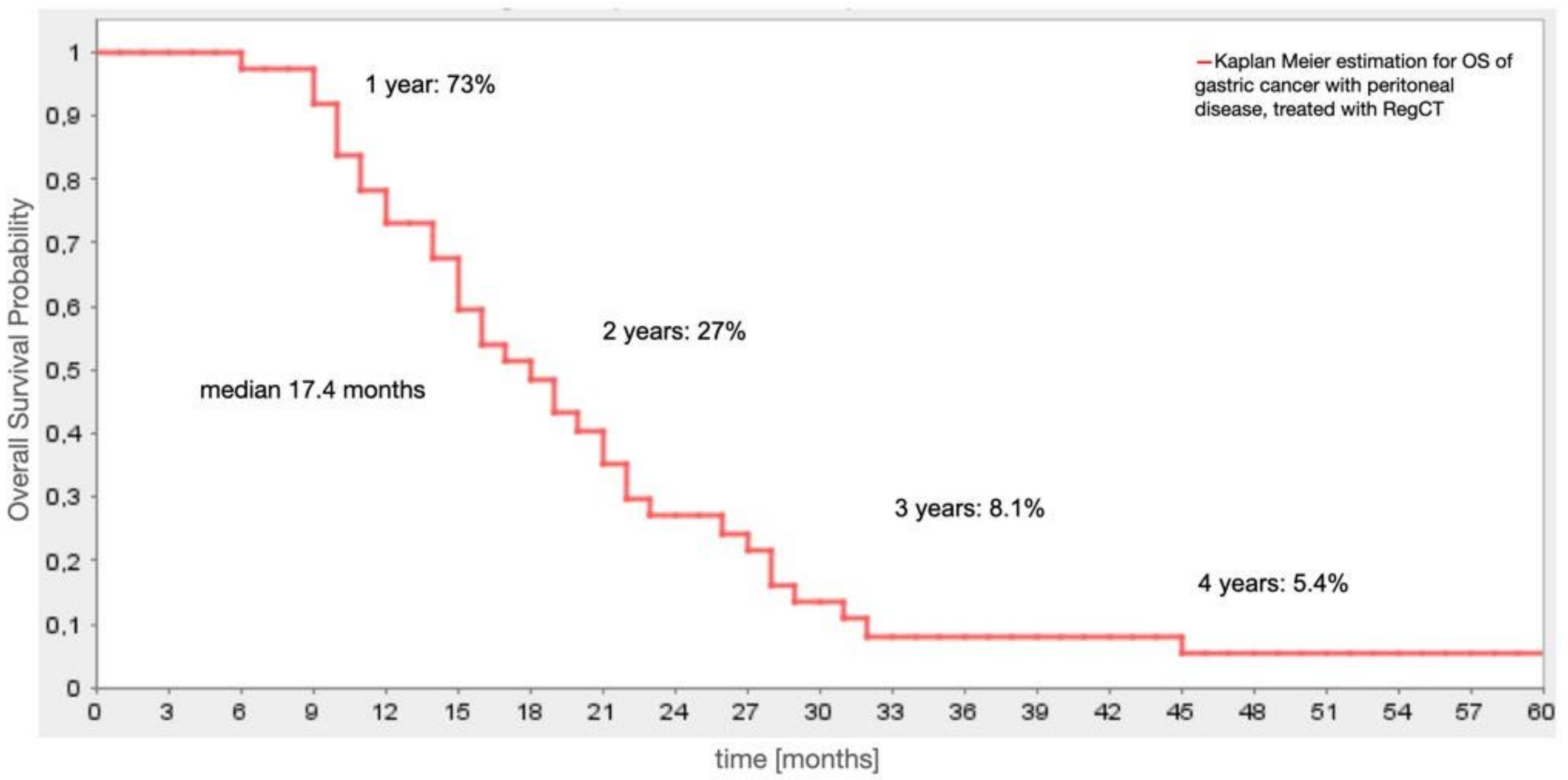
4.3. Survival
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moehler, M.; Al-Batran, S.E.; Andus, T.; Arends, J.; Arnold, D.; Baretton, G.; Bornschein, J.; Budach, W.; Daum, S.; Dietrich, C.; et al. S3-Leitlinie Magenkarzinom—Diagnostik und Therapie der Adenokarzinome des Magens und des ösophagogastralen Übergangs—Langversion 2.0—August 2019. AWMF-Registernummer: 032/009OL. Z. Gastroenterol. 2019, 57, 1517–1632. [Google Scholar] [CrossRef] [PubMed]
- Milano, A.F. 20-Year Comparative Survival and Mortality of Cancer of the Stomach by Age, Sex, Race, Stage, Grade, Cohort Entry Time-Period, Disease Duration & Selected ICD-O-3 Oncologic Phenotypes: A Systematic Review of 157,258 Cases for Diagnosis Years 1973–2014: (SEER*Stat 8.3.4). J. Insur. Med. 2019, 48, 5–23. [Google Scholar] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: A systematic review including evidence from Japan. Surg. Today 2021, 51, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Brandl, A.; Prabhu, A. Intraperitoneal chemotherapy in the treatment of gastric cancer peritoneal metastases: An overview of common therapeutic regimens. J. Gastrointest. Oncol. 2021, 12, S32–S44. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, in press. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef]
- Kitayama, J.; Ishigami, H.; Yamaguchi, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Lefor, A.K.; Sata, N. Treatment of patients with peritoneal metastases from gastric cancer. Ann. Gastroenterol. Surg. 2018, 2, 116–123. [Google Scholar] [CrossRef]
- Muro, K.; Van Cutsem, E.; Narita, Y.; Pentheroudakis, G.; Baba, E.; Li, J.; Ryu, M.H.; Zamaniah, W.I.W.; Yong, W.P.; Yeh, K.H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 19–33. [Google Scholar] [CrossRef]
- Aigner, K.R.; Gailhofer, S.; Kopp, S. Regional versus systemic chemotherapy for advanced pancreatic cancer: A randomized study. Hepatogastroenterology 1998, 45, 1125–1129. [Google Scholar]
- Aigner, K.R. Intra-arterial infusion: Overview and novel approaches. Semin. Surg. Oncol. 1998, 14, 248–253. [Google Scholar] [CrossRef]
- Aigner, K.R.; Gailhofer, S.; Selak, E.; Aigner, K. Intra-arterial infusion chemotherapy versus isolated upper abdominal perfusion for advanced pancreatic cancer: A retrospective cohort study on 454 patients. J. Cancer Res. Clin. Oncol. 2019, 145, 2855–2862. [Google Scholar] [CrossRef] [Green Version]
- Aigner, K.R.; Gailhofer, S.; Aigner, K. Hypoxic isolated abdominal perfusion breaks through chemoresistance in recurrent FIGO stage IIIC and IV ovarian cancer. Mol. Clin. Oncol. 2021, 14, 129. [Google Scholar] [CrossRef]
- Meyer, F.; Gebauer, T.; Grote, R.; Martens-Lobenhoffer, J.; Ridwelski, K.; Lippert, H. Results of regional chemotherapy using the aortic stop-flow technique in advanced pancreatic carcinoma. Surg. Today 2006, 36, 155–161. [Google Scholar] [CrossRef]
- Aigner, K.R.; Gailhofer, S. Celiac axis infusion and microembolization for advanced stage III/IV pancreatic cancer—A phase II study on 265 cases. Anticancer Res. 2005, 25, 4407–4412. [Google Scholar]
- Runnels, J.; Acosta, G.; Rose, A.; Haynes, M.; Nikolaidis, D.; Wong, A.; Fiani, B. The role for intra-arterial chemotherapy for refractory retinoblastoma: A systematic review. Clin. Transl. Oncol. 2021, 23, 2066–2077. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Furrer, M.; Friedel, G. Locoregional therapy. J. Thorac. Oncol. 2010, 5, S151–S154. [Google Scholar] [CrossRef] [Green Version]
- Van Schil, P.E.; Hendriks, J.M.; van Putte, B.P.; Stockman, B.A.; Lauwers, P.R.; Ten Broecke, P.W.; Grootenboers, M.J.; Schramel, F.M. Isolated lung perfusion and related techniques for the treatment of pulmonary metastases. Eur. J. Cardiothorac. Surg. 2008, 33, 487–496. [Google Scholar] [CrossRef]
- Link, K.H.; Aigner, K.R.; Kuehn, W.; Schwemmle, K.; Kern, D.H. Prospective correlative chemosensitivity testing in high-dose intraarterial chemotherapy for liver metastases. Cancer Res. 1986, 46, 4837–4840. [Google Scholar]
- Teicher, B.A.; Lazo, J.S.; Sartorelli, A.C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981, 41, 73–81. [Google Scholar] [PubMed]
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J. Clin. Oncol. 2018, 36, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Prabhu, A.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; Nishihara, K.; et al. Long Term Survival after Cytoreductive Surgery Combined with Perioperative Chemotherapy in Gastric Cancer Patients with Peritoneal Metastasis. Cancers 2020, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Koemans, W.J.; van der Kaaij, R.T.; Boot, H.; Buffart, T.; Veenhof, A.A.F.A.; Hartemink, K.J.; Grootscholten, C.; Snaebjornsson, P.; Retel, V.P.; van Tinteren, H.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 2019, 19, 420. [Google Scholar] [CrossRef]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Königsrainer, A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: A phase II study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846402. [Google Scholar] [CrossRef] [Green Version]
| Value | N (%) | |
|---|---|---|
| Total | 38 (100) | |
| Gender | male | 23 (60) |
| female | 15 (40) | |
| Age—median (range) | 54 (37–74) | |
| Karnofsky Index | 30–60% | 24 (63) |
| 70–100% | 14 (37) | |
| Ascites | 2 quadrants | 4 (11) |
| 4 quadrants | 18 (47) | |
| Metastases Spectrum | peritoneal | 38 |
| multiple sites (other than peritoneal) | 14 (37) | |
| single site (other than peritoneal) | 12 (32) | |
| peritoneal only | 12 (32) | |
| liver | 11 (29) | |
| lymphatic | 13 (34) | |
| lung | 1 (2) | |
| bone | 2 (5) | |
| other | 10 (26) | |
| Pre-treatment | systemic chemotherapy | 19 (50) |
| surgery | 18 (47) | |
| no pre-treatment | 12 (32) | |
| radiotherapy | 4 (10) | |
| Chemotherapy Spectrum | first line chemotherapy | 9 (24) |
| multiple lines chemotherapy | 10 (26) | |
| platin-based chemotherapy | 18 (47) | |
| others | 15 (39) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aigner, K.; Vashist, Y.K.; Selak, E.; Gailhofer, S.; Aigner, K.R. Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer. J. Clin. Med. 2021, 10, 5322. https://doi.org/10.3390/jcm10225322
Aigner K, Vashist YK, Selak E, Gailhofer S, Aigner KR. Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer. Journal of Clinical Medicine. 2021; 10(22):5322. https://doi.org/10.3390/jcm10225322
Chicago/Turabian StyleAigner, Kornelia, Yogesh Kumar Vashist, Emir Selak, Sabine Gailhofer, and Karl Reinhard Aigner. 2021. "Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer" Journal of Clinical Medicine 10, no. 22: 5322. https://doi.org/10.3390/jcm10225322
APA StyleAigner, K., Vashist, Y. K., Selak, E., Gailhofer, S., & Aigner, K. R. (2021). Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer. Journal of Clinical Medicine, 10(22), 5322. https://doi.org/10.3390/jcm10225322






