The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. DNA Isolation
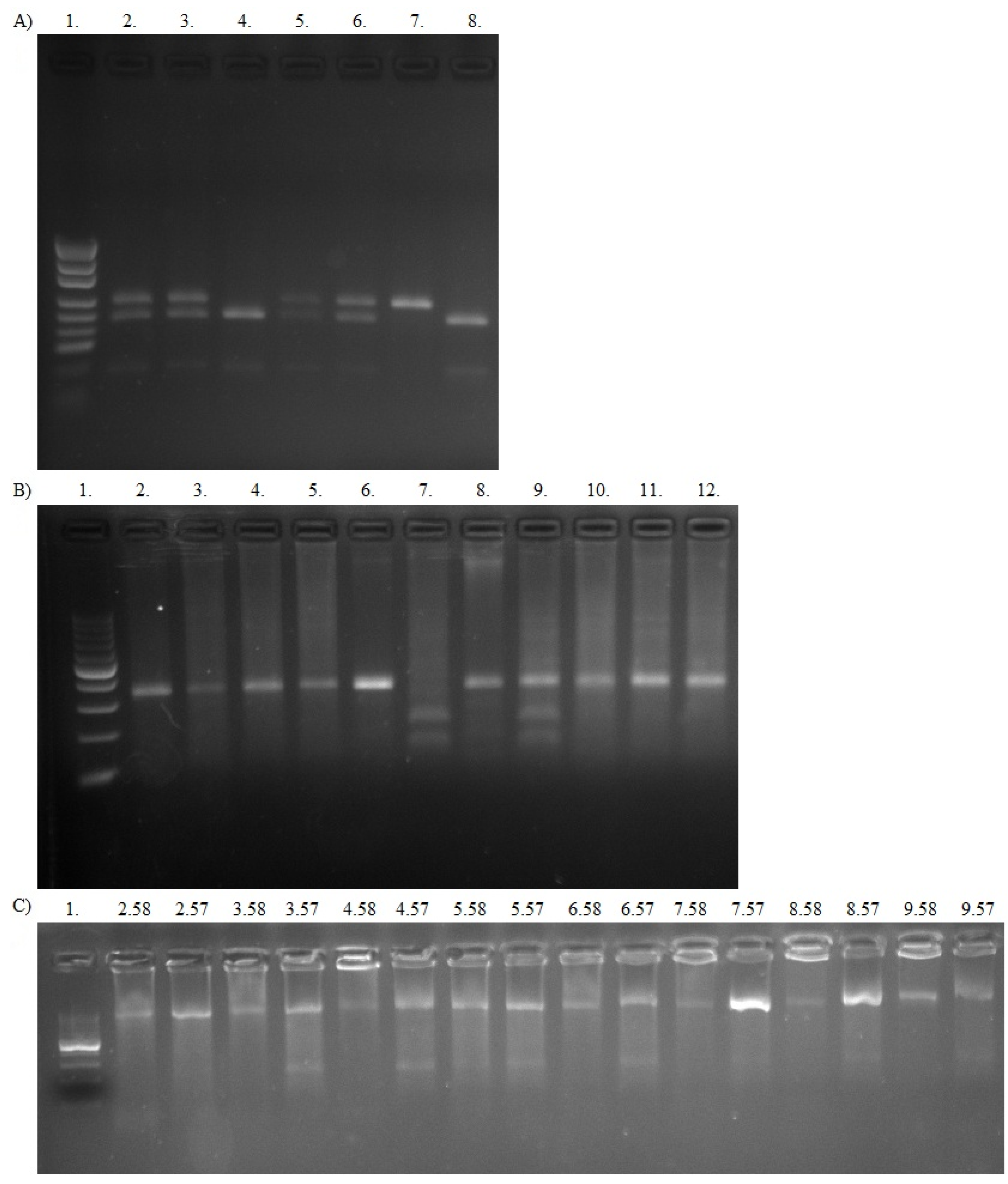
2.3. ABCB1 Genotyping
2.3.1. Automated DNA Sequencing
- forward 5′-TCA GTT ACC CAT CTC GAA AAG AA-3′
- reverse 5′-ACA TCA GAA AGA TGT GCA ATG TG-3′
- forward 5′-TAT GGT TGG CAA CTA ACA CT-3′
- reverse 5′-CAT GAA AAA GAT TGC TTT GA-3′
2.3.2. PCR-RFLP
- -
- forward 5′-TGC TGG TCC TGA AGT TGA TCT GTG AAC-3′
- -
- reverse 5′-ACA TTA GGC AGT GAC TCG ATG AAG GCA-3′
2.4. CYP1A1 Genotyping
- -
- forward 5′-AAG AGG TGT AGC CGC TGC ACT-3′
- -
- reverse 5′-TAG GAG TCT TGT CTC ATG CCT-3′
- -
- forward 5′-ATA GGG TTA GTG GGA GGG ACA CG-3′
- -
- reverse 5′-GCT CAA TGC AGG CTA GAA CTA GAA TAG AAG-3′
2.5. Cytogenetic Analyses
2.6. Bortezomib In Vitro Treatment
2.7. Statistical Analysis
3. Results
3.1. Frequencies of Alleles and Genotypes
3.2. Studied Variants and the Risk of MM Development
3.3. ABCB1 and CYP1A1 Variants as a Risk Factors of Death or MM Progression
3.4. Correlation between Analyzed Polymorphisms and MM Clinical Features
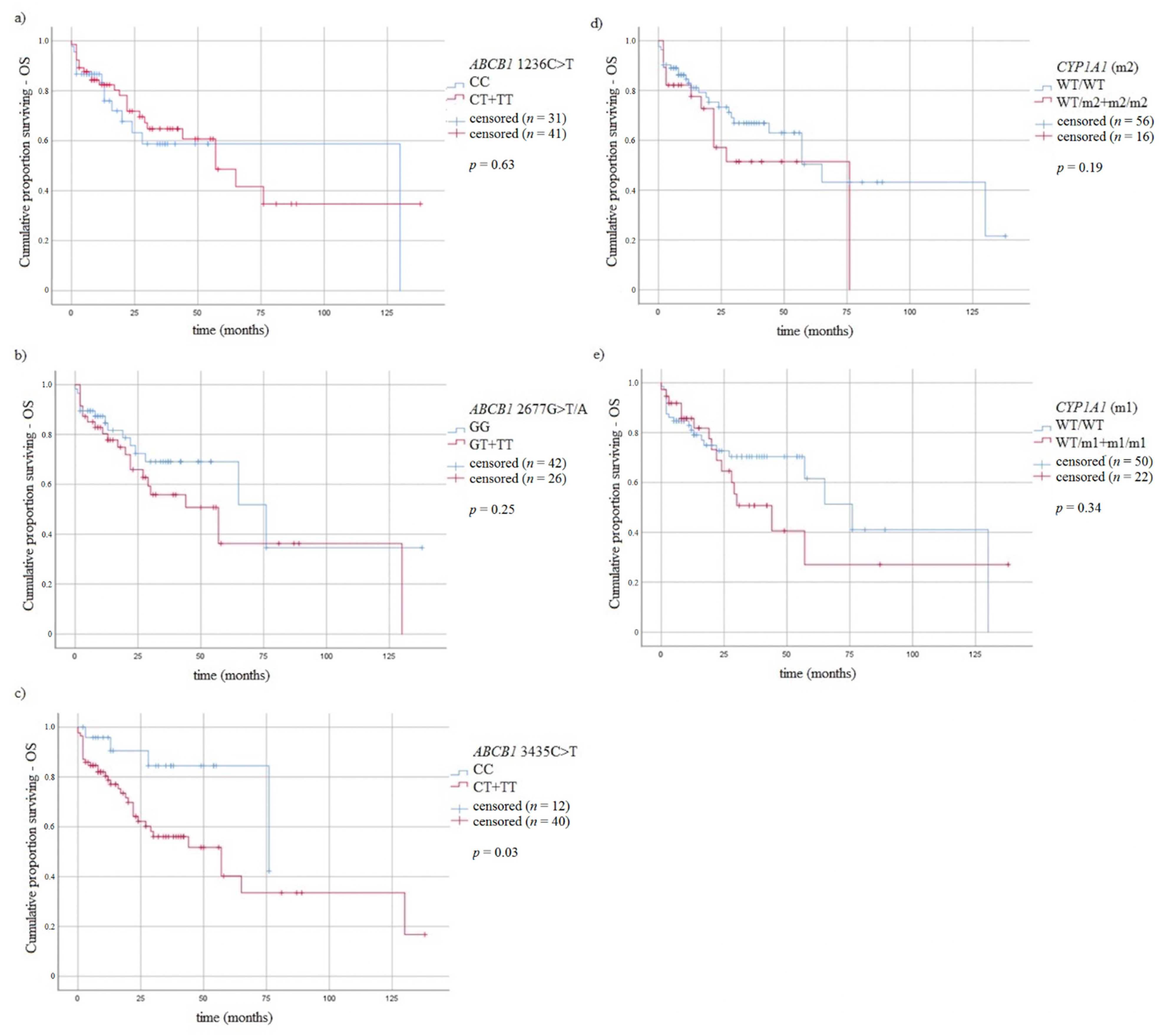
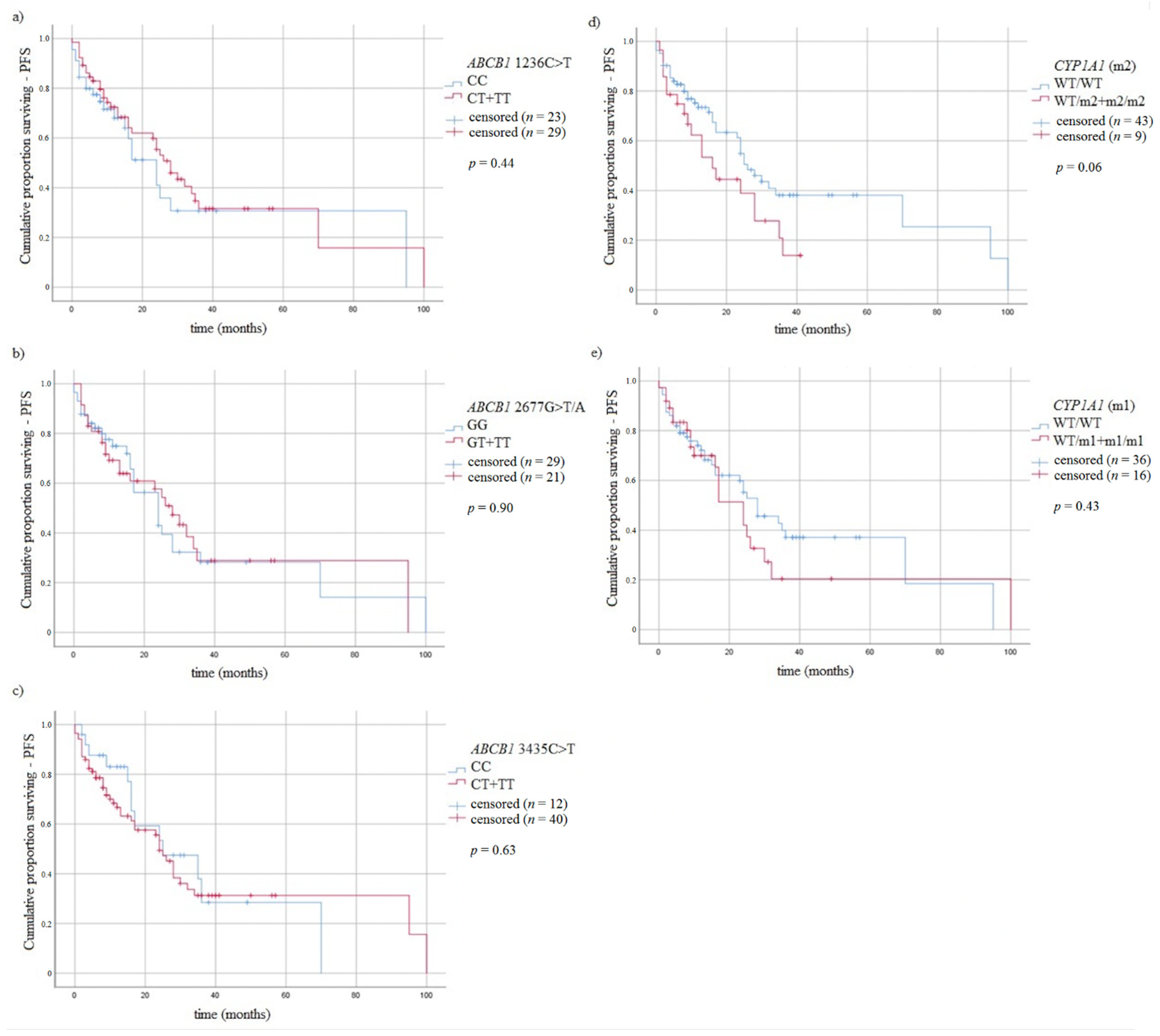
3.5. Survival of MM Patients and Studied Variants
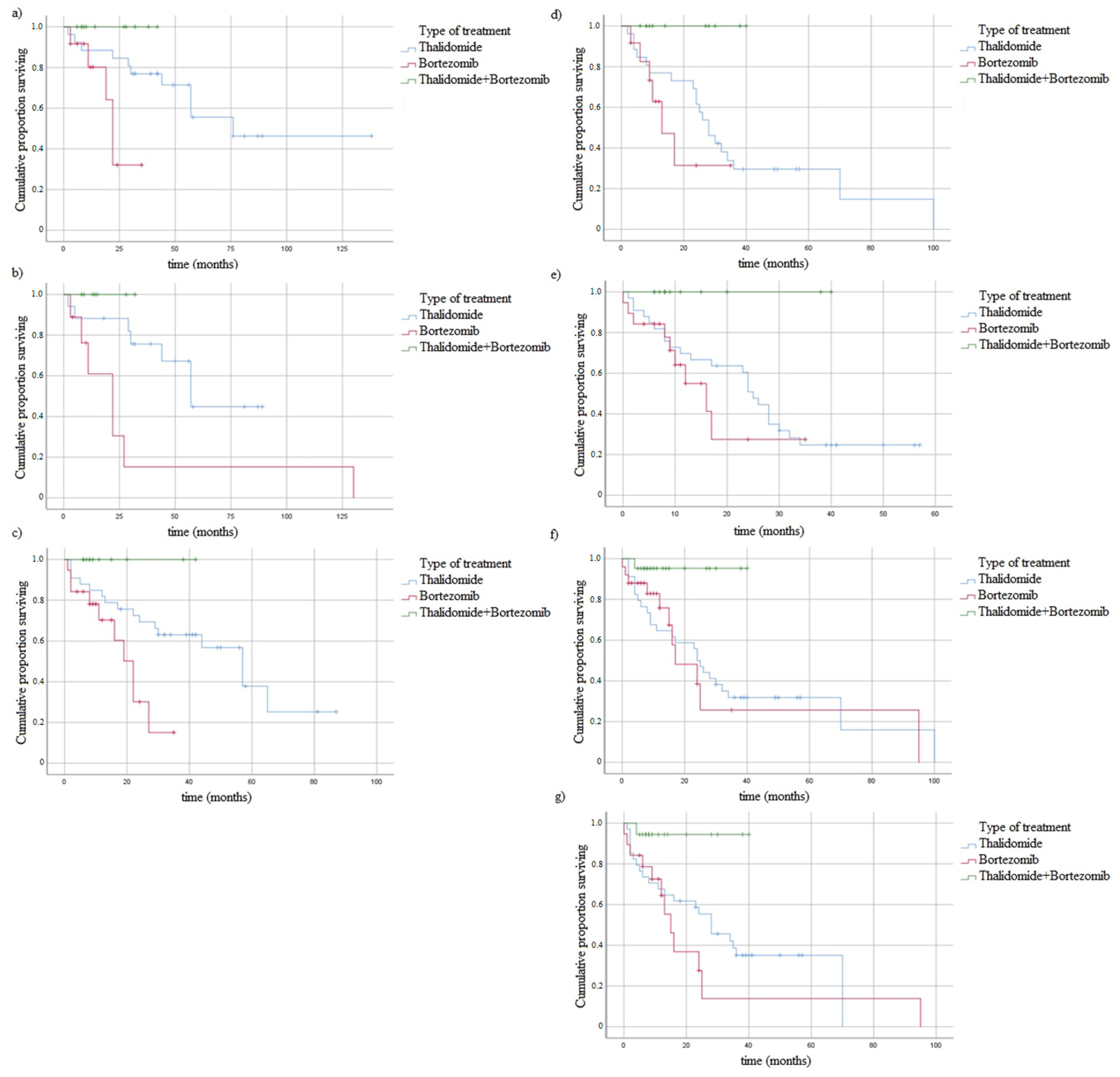
3.6. In Vitro Study with Bortezomib
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K. Management of Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2018, 16, 624–627. [Google Scholar] [CrossRef]
- Iida, S. Mechanisms of action and resistance for multiple myeloma novel drug treatments. Int. J. Hematol. 2016, 104, 271–272. [Google Scholar] [CrossRef][Green Version]
- Toru, U.; Ayada, C.; Genç, O.; Turgut, S.; Turgut, G.; Bulut, I. MDR-1 gene C/T polymorphism in COPD: Data from Aegean part of Turkey. Int. J. Clin. Exp. Med. 2014, 7, 3573–3577. [Google Scholar] [PubMed]
- Dogan, O.T.; Katrancıoglu, N.; Karahan, O.; Sanlı, G.C.; Zorlu, A.; Manduz, S. Frequency of the mdr-1 C > T gene polymorphism in patients with COPD. Clinics 2010, 65, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Sakaeda, T. MDR1 Genotype-related Pharmacokinetics: Fact or Fiction? Drug Metab. Pharmacokinet. 2005, 20, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Jamroziak, K.; Balcerczak, E.; Calka, K.; Piaskowski, S.; Urbanska-Rys, H.; Salagacka, A.; Mirowski, M.; Robak, T. Polymorphisms and haplotypes in the multidrug resistance 1 gene (MDR1/ABCB1) and risk of multiple myeloma. Leuk. Res. 2009, 33, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, S.; Burk, O.; von Richter, O.; Arnold, H.P.; Brockmöller, J.; Johne, A.; Cascorbi, I.; Gerloff, T.; Roots, I.; Eichelbaum, M.; et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 3473–3478. [Google Scholar] [CrossRef]
- Drain, S.; Flannely, L.; Drake, M.B.; Kettle, P.; Orr, N.; Bjourson, A.J.; Catherwood, M.A.; Alexander, H.D. Multidrug resistance gene expression and ABCB1 SNPs in plasma cell myeloma. Leuk. Res. 2011, 35, 1457–1463. [Google Scholar] [CrossRef]
- Nauck, M.; Stein, U.; Von Karger, S.; März, W.; Wieland, H. Rapid detection of the C3435T polymorphism of multidrug resistance gene 1 using fluorogenic hybridization probes. Clin. Chem. 2000, 46, 1995–1997. [Google Scholar] [CrossRef]
- Rychlik-Sych, M.; Baranska, M.; Dudarewicz, M.; Skrętkowicz, J.; Żebrowska, A.; Woźniacka, A.; Owczarek, J.; Orszulak-Michalak, D.; Waszczykowska, E. Haplotypes of ABCB1 1236C > T (rs1128503), 2677G > T/A (rs2032582), and 3435C > T (rs1045642) in patients with bullous pemphigoid. Arch. Dermatol. Res. 2018, 310, 515–522. [Google Scholar] [CrossRef]
- Dessilly, G.; Panin, N.; Elens, L.; Haufroid, V.; Demoulin, J.-B. Impact of ABCB1 1236C > T-2677G > T-3435C > T polymorphisms on the anti-proliferative activity of imatinib, nilotinib, dasatinib and ponatinib. Sci. Rep. 2016, 6, 29559. [Google Scholar] [CrossRef]
- Hawley, T.S.; Riz, I.; Yang, W.; Wakabayashi, Y.; DePalma, L.; Chang, Y.-T.; Peng, W.; Zhu, J.; Hawley, R. Identification of an ABCB1 (P-glycoprotein)-positive carfilzomib-resistant myeloma subpopulation by the pluripotent stem cell fluorescent dye CDy1. Am. J. Hematol. 2013, 88, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Kastritis, E.; Dimopoulos, M.; Lonial, S. Treatment options for relapsed and refractory multiple myeloma. Blood 2015, 125, 3085–3099. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Harousseau, J.-L.; Durie, B.; Anderson, K.C.; Dimopoulos, M.; Kyle, R.; Blade, J.; Richardson, P.; Orlowski, R.; Siegel, D.; et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011, 117, 4691–4695. [Google Scholar] [CrossRef]
- Pérez-Galán, P.; Roué, G.; Villamor, N.; Montserrat, E.; Campo, E.; Colomer, D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 2006, 107, 257–264. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Q.; Xu, Y.; Chen, Z.; Xia, W.; Yang, Y.; Liu, D. Plasma enhance drug sensitivity to bortezomib by inhibition of cyp1a1 in myeloma cells. Transl. Cancer Res. 2019, 8, 2841–2847. [Google Scholar] [CrossRef]
- Besse, A.; Besse, A.; Stolze, S.C.; Stolze, S.C.; Rasche, L.; Rasche, L.; Weinhold, N.; Weinhold, N.; Morgan, G.; Morgan, G.; et al. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia 2017, 32, 391–401. [Google Scholar] [CrossRef]
- Rumpold, H.; Salvador, C.; Wolf, A.M.; Tilg, H.; Gastl, G.; Wolf, D. Knockdown of PgP resensitizes leukemic cells to proteasome inhibitors. Biochem. Biophys. Res. Commun. 2007, 361, 549–554. [Google Scholar] [CrossRef]
- Mandery, K.; Glaeser, H.; Fromm, M.F. Interaction of innovative small molecule drugs used for cancer therapy with drug transporters. Br. J. Pharmacol. 2011, 165, 345–362. [Google Scholar] [CrossRef]
- Ludwig, H.; Beksac, M.; Bladé, J.; Boccadoro, M.; Cavenagh, J.; Cavo, M.; Dimopoulos, M.; Drach, J.; Einsele, H.; Facon, T.; et al. Current Multiple Myeloma Treatment Strategies with Novel Agents: A European Perspective. Oncology 2010, 15, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Rajkumar, S.V. Treatment of newly diagnosed myeloma. Leukemia 2008, 23, 449–456. [Google Scholar] [CrossRef][Green Version]
- Paraskevaidis, A.; Drakoulis, N.; Roots, I.; Orfanos, C.; Zouboulis, C.P.D. Polymorphisms in the Human Cytochrome P-450 1A1 Gene (CYP1A1) as a Factor for Developing Acne. Dermatology 1998, 196, 171–175. [Google Scholar] [CrossRef] [PubMed]
- PBoffetta, P. Biomarkers in cancer epidemiology: An integrative approach. Carcinogenesis 2009, 31, 121–126. [Google Scholar] [CrossRef][Green Version]
- Zhuo, W.; Zhang, L.; Wang, Y.; Zhu, B.; Chen, Z. CYP1A1 MspI polymorphism and acute myeloid leukemia risk: Meta-analyses based on 5018 subjects. J. Exp. Clin. Cancer Res. 2012, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Shimada, T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat. Res. Mol. Mech. Mutagen. 1998, 400, 201–213. [Google Scholar] [CrossRef]
- Xu, D.; Hu, J.; De Bruyne, E.; Menu, E.; Schots, R.; Vanderkerken, K.; Van Valckenborgh, E. Dll1/Notch activation contributes to bortezomib resistance by upregulating CYP1A1 in multiple myeloma. Biochem. Biophys. Res. Commun. 2012, 428, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Pekol, T.; Daniels, J.S.; LaButti, J.; Parsons, I.; Nix, D.; Baronas, E.; Hsieh, F.; Gan, L.-S.; Miwa, G. Human Metabolism of the Proteasome Inhibitor Bortezomib: Identification of Circulating Metabolites. Drug Metab. Dispos. 2005, 33, 771–777. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, C.S.; Singh, S.; Goel, S.; Ahmed, R.S.; Gupta, S.; Grover, R.K.; Banerjee, B.D. CYP 1A1 polymorphism and organochlorine pesticides levels in the etiology of prostate cancer. Chemosphere 2010, 81, 464–468. [Google Scholar] [CrossRef]
- Kang, S.-H.; Kim, T.Y.; Kim, H.Y.; Yoon, J.-H.; Cho, H.-I.; Yoon, S.S.; Kang, D.H.; Suh, C.W.; Lee, J.H.; Lee, N.S. Protective Role of CYP1A1*2A in the Development of Multiple Myeloma. Acta Haematol. 2008, 119, 60–64. [Google Scholar] [CrossRef]
- Sánchez-Siles, M.; Pelegrín-Hernández, J.; Hellin-Meseguer, D.; Guerrero-Sánchez, Y.; Corno-Caparrós, A.; Cabezas-Herrera, J.; Pastor-Quirante, F.; Fernández-Ruiz, J.; Aliaga-Sánchez, A.; Lucero-Berdugo, M.; et al. Genotype of Null Polymorphisms in Genes GSTM1, GSTT1, CYP1A1, and CYP1A1*2A (rs4646903 T > C)/CYP1A1*2C (rs1048943 A > G) in Patients with Larynx Cancer in Southeast Spain. Cancers 2020, 12, 2478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-P.; He, X.-F.; Ye, X.-H. Association between the combined effects of GSTM1 present/null and CYP1A1 MspI polymorphisms with lung cancer risk: An updated meta-analysis. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Jyala, N.S.; Bag, N. Cytochrome P450 1A1 genetic polymorphisms as cancer biomarkers. Indian J. Cancer 2015, 52, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Saurabh, K.; Pant, M.C.; Mathur, N.; Parmar, D. Evidence for increased cytochrome P450 1A1 expression in blood lymphocytes of lung cancer patients. Mutat. Res. Mol. Mech. Mutagen. 2009, 670, 74–78. [Google Scholar] [CrossRef] [PubMed]
- KKawajiri, K.; Nakachi, K.; Imai, K.; Watanabe, J.; Hayashi, S.-I. The CYP1A1 gene and cancer susceptibility. Crit. Rev. Oncol. 1993, 14, 77–87. [Google Scholar] [CrossRef]
- Zmorzyński, S.; Popek-Marciniec, S.; Styk, W.; Wojcierowska-Litwin, M.; Korszeń-Pilecka, I.; Szudy-Szczyrek, A.; Chocholska, S.; Hus, M.; Filip, A.A. The Impact of the NOD2/CARD15 Variant (3020insC) and PSMA6 Polymorphism (-8C > G) on the Development and Outcome of Multiple Myeloma. BioMed Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; Miguel, J.S.; Larocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group Consensus Statement for the Management, Treatment, and Supportive Care of Patients With Myeloma Not Eligible for Standard Autologous Stem-Cell Transplantation. J. Clin. Oncol. 2014, 32, 587–600. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Bladé, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef]
- Federal Drug Administration. Guidance for Industry: Clinical Trial Endpoints for the Approval of Non-Small Cell Lung Cancer Drugs and Biologics. 2018. Available online: https://www.fda.gov/media/71195/download (accessed on 14 February 2021).
- Turgut, S.; Turgut, G.; Atalay, E. Genotype and allele frequency of human multidrug resistance (MDR1) gene C3435T polymorphism in Denizli province of Turkey. Mol. Biol. Rep. 2006, 33, 295–300. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Yadav, C.S.; Ahmed, R.S.; Gupta, S.; Pasha, S.T.; Tripathi, A.K.; Banerjee, B.D. CYP1A1 and CYP3A4 polymorphic variations in Delhi population of Northern India. Environ. Toxicol. Pharmacol. 2010, 29, 126–130. [Google Scholar] [CrossRef]
- Drakoulis, N.; Cascorbi, I.; Gross, C.R.; Roots, I. Polymorphisms in the human CYP1A1 gene as susceptibility factors for lung cancer: Exon-7 mutation (4889 A to G), and a T to C mutation in the 3?-flanking region. J. Mol. Med. 1994, 72, 240–248. [Google Scholar] [CrossRef]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutiérrez, N.C.; Liebisch, P.; O’Connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef]
- Ahmann, G.J.; Jalal, S.M.; Juneau, A.L.; Christensen, E.R.; Hanson, C.A.; Dewald, G.W.; Greipp, P.R. A novel three-color, clone-specific fluorescence in situ hybridization procedure for monoclonal gammopathies. Cancer Genet. Cytogenet. 1998, 101, 7–11. [Google Scholar] [CrossRef]
- Dmoszyńska, A.; Chocholska, S. Molecular Biology Methods in the Diagnosis of Multiple Myeloma. In Molecular Aspects of Hematologic Malignancies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 443–449. [Google Scholar] [CrossRef]
- Zmorzynski, S.; Szudy-Szczyrek, A.; Popek-Marciniec, S.; Korszen-Pilecka, I.; Wojcierowska-Litwin, M.; Luterek, M.; Chocholska, S.; Styk, W.; Swiderska-Kołacz, G.; Januszewska, J.; et al. ACE Insertion/Deletion Polymorphism (rs4646994) Is Associated with the Increased Risk of Multiple Myeloma. Front. Oncol. 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Hocherman, I.; Linn, S.; Cohen, Y.; Tatarsky, I. Prognostic significance of plasma cell morphology in multiple myeloma. Cancer 1987, 60, 1060–1065. [Google Scholar] [CrossRef]
- Preacher, K.J. Calculation for the Chi-Square Test: An Interactive Calculation Tool for Chi-Square Tests of Goodness of Fit and Independence. 2019. Available online: http://quantpsy.org/ (accessed on 30 April 2021).
- Ashariati, A. Polymorphism C3435T of the MDR-1 gene predict response to preoperative chemotherapy in locally advanced breast cancer with Her2/neu expression. Acta Med. Indones. 2008, 40, 187–191. [Google Scholar]
- Razi, B.; Sarab, G.A.; Omidkhoda, A.; Alizadeh, S. Multidrug resistance 1 (MDR1/ABCB1) gene polymorphism (rs1045642 C > T) and susceptibility to multiple myeloma: A systematic review and meta-analysis. Hematology 2018, 23, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Uludag, A.; Silan, F.; Atik, S.Y.; Turgut, B.; Ozdemir, O. Possible roles of the xenobiotic transporter P-glycoproteins encoded by the MDR1 3435 C > T gene polymorphism in differentiated thyroid cancers. Asian Pac. J. Cancer Prev. 2013, 14, 3213–3217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drain, S.; Catherwood, M.A.; Orr, N.; Galligan, L.; Rea, I.M.; Hodkinson, C.; Drake, M.B.; Kettle, P.J.; Morris, T.C.M.; Alexander, H.D. ABCB1 (MDR1)rs1045642 is associated with increased overall survival in plasma cell myeloma. Leuk. Lymphoma 2009, 50, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.-W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “Silent” Polymorphism in the MDR 1 Gene Changes Substrate Specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Gonzalez-Roces, S.; Pollán, M.; Lucas, R.; Tejerina, A.; Martin, M.; Alba, A. Expression of LRP and MDR1 in locally advanced breast cancer predicts axillary node invasion at the time of rescue mastectomy after induction chemotherapy. Breast Cancer Res. 2001, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lincz, L.F.; Kerridge, I.; Scorgie, F.E.; Bailey, M.; Enno, A.; Spencer, A. Xenobiotic gene polymorphisms and susceptibility to multiple myeloma. Haematologica 2004, 89, 628–629. [Google Scholar] [PubMed]
- Zhuo, W.-L.; Wang, Y.; Zhuo, X.-L.; Zhu, B.; Zhu, Y.; Chen, Z.-T. Polymorphisms of CYP1A1 and GSTM1 and laryngeal cancer risk: Evidence-based meta-analyses. J. Cancer Res. Clin. Oncol. 2009, 135, 1081–1090. [Google Scholar] [CrossRef]
- Shaik, A.P.; Jamil, K.; Das, P. CYP1A1 Polymorphisms and Risk of Prostate Cancer: A Meta-analysis. Urol. J. 2009, 6, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Wang, Q.; Qian, Q.; Wei, S.-Z.; Yu, L.-K. CYP1A1 MspI and exon7 gene polymorphisms and lung cancer risk: An updated meta-analysis and review. J. Exp. Clin. Cancer Res. 2011, 30, 99. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and cervical cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 6647–6654. [Google Scholar] [CrossRef]
- Zhuo, W.-L.; Zhang, Y.-S.; Wang, Y.; Zhuo, X.-L.; Zhu, B.; Cai, L.; Chen, Z.-T. Association Studies of CYP1A1 and GSTM1 Polymorphisms with Esophageal Cancer Risk: Evidence-based Meta-analyses. Arch. Med. Res. 2009, 40, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Economopoulos, K.P. Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2009, 122, 459–469. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.-J.; Sun, L.; Li, H.-L. Association between CYP1A1 polymorphism and colorectal cancer risk: A meta-analysis. Mol. Biol. Rep. 2011, 39, 3533–3540. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X. Quantitative assessment of the associations between CYP1A1 polymorphisms and gastric cancer risk. Tumor Biol. 2012, 33, 1125–1132. [Google Scholar] [CrossRef]
- Krishnan, S.R.; Jaiswal, R.; Brown, R.D.; Luk, F.; Bebawy, M. Multiple myeloma and persistence of drug resistance in the age of novel drugs (Review). Int. J. Oncol. 2016, 49, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Nikesitch, N.; Ling, S.C.W. Molecular mechanisms in multiple myeloma drug resistance. J. Clin. Pathol. 2016, 69, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Boysen, J.; Nelson, M.; Secreto, C.; Warner, S.L.; Bearss, D.; Lesnick, C.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination with BTK Inhibitors. Clin. Cancer Res. 2015, 21, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.H.; Dobbins, S.E.; Cornish, A.J.; Chubb, D.; Law, P.J.; Kaiser, M. Whole-genome sequencing of multiple myeloma reveals oncogenic pathways are targeted somatically through multiple mechanisms. Leukemia 2018, 32, 2459–2470. [Google Scholar] [CrossRef]
- Mitchell, J.; Li, N.; Weinhold, N.; Försti, A.; Ali, M.; van Duin, M.; Thorleifsson, G.; Johnson, D.C.; Chen, B.; Halvarsson, B.-M.; et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma Title. Nat. Commun. 2016, 7, 12050. [Google Scholar] [CrossRef] [PubMed]
- Ziv, E.; Dean, E.; Hu, D.; Martino, A.; Serie, D.; Curtin, K.; Campa, D.; Aftab, B.; Bracci, P.; Buda, G.; et al. Genome-wide association study identifies variants at 16p13 associated with survival in multiple myeloma patients. Nat. Commun. 2015, 6, 7539. [Google Scholar] [CrossRef]
- Agrawal, A.; Chou, Y.L.; Carey, C.E.; Baranger, D.A.; Zhang, B.; Sherva, R.; Wetherill, L.; Kapoor, M.; Wang, J.C.; Bertelsen, S.; et al. Genome-Wide Association Study Identifies a Novel Locus for Bortezomib-Induced Peripheral Neuropathy in European Patients with Multiple Myeloma. Clin. Cancer Res. 2016, 22, 4350–4355. [Google Scholar]
- Johnson, D.C.; Weinhold, N.; Mitchell, J.S.; Chen, B.; Kaiser, M.; Hillengass, J.; Bertsch, U.; Gregory, W.A.; Cairns, D.; Jackson, G.H.; et al. Genome-wide association study identifies variation at 6q25.1 associated with survival in multiple myeloma. Nat. Commun. 2016, 7, 10290. [Google Scholar] [CrossRef]


| Variables | MM Patients, n = 110 |
|---|---|
| Age (years) * | |
| Median | 65.36 |
| Range | 42–83 |
| Sex | |
| Male | 53 (48.18%) |
| Female | 57 (51.82%) |
| Type of MM * | |
| IgG | 60 (54.54%) |
| IgA | 27 (24.54%) |
| Light chain | 23 (20.9%) |
| Serum M protein (g/dL) * | |
| Median | 4.96 |
| Range | 1.06–8.3 |
| Stage according to the International Staging System * | |
| I | 30 (27.27%) |
| II | 33 (30%) |
| III | 47 (42.72%) |
| Smoking | |
| Yes | 20 (18.18%) |
| No: Non-smokers | 77 (70%) |
| No: Ex-smokers | 13 (11.81%) |
| Serum β2-microglobulin * | |
| ≤3.5 mg/L | 34 (30.9%) |
| >3.5 mg/L | 33 (30%) |
| >5.5 mg mg/L | 43 (39.1%) |
| Hypercalcemia * | |
| No | 97 (88.18%) |
| Yes | 13 (11.81%) |
| Renal failure * | |
| No | 86 (78.18%) |
| Yes | 24 (21.81%) |
| The stage of chronic kidney disease (grade) * | |
| G1 | 31 (28.18%) |
| G2 | 30 (27.27%) |
| G3A | 17 (15.45%) |
| G3B | 15 (13.63%) |
| G4 | 8 (7.27%) |
| G5 | 9 (8.18%) |
| Anemia grade before treatment (WHO) * | |
| Absent | 30 (27.27%) |
| I—mild | 36 (32.73%) |
| II—moderate | 32 (29.09%) |
| III—severe | 12 (10.9%) |
| Cytogenetic changes * | |
| del(17p13.1) | 11 (10%) |
| del(17p13.1) and t(4;14) | 4 (3.63%) |
| del(17p13.1) and t(14;16) | 1 (0.9%) |
| t(4;14) | 12 (10.9%) |
| t(14;16) | 1 (0.9%) |
| Other IgH gene rearrangement | 13 (11.81%) |
| First line treatment | |
| CTD | 52 (47.27%) |
| VCD | 33 (30%) |
| VTD | 23 (20.9%) |
| Transplant | |
| ASCT | 35 (31.81%) |
| Second line treatment | |
| Rd | 16 (14.54%) |
| VCD | 18 (16.36%) |
| VD | 5 (4.54%) |
| Number of relapses | |
| 1 | 31 (28.18%) |
| 2 | 8 (7.27%) |
| Follow-up (months) | |
| Median | 18.5 |
| Range | 2–138 |
| Deaths | |
| Before chemotherapy | 2 (1.81%) |
| Total | 35 (31.81%) |
| Groups | Genotypes | HWE p Value and χ2 * | ||
|---|---|---|---|---|
| ABCB1 gene 1236C > T | ||||
| - | CC | CT | TT | - |
| CONTROL | ||||
| E | 32.49 | 49.02 | 18.49 | p = 0.46, χ2 = 0.54 |
| O | 30 | 54 | 16 | |
| CASE | ||||
| E | 43.91 | 51.17 | 14.91 | p = 0.72 χ2 = 0.12 |
| O | 45 | 49 | 16 | |
| ABCB1 gene 2677G > T/A | ||||
| - | GG | GT + GA | AA + TT + AT | - |
| CONTROL | ||||
| E | 29.16 | 49.68 | 21.16 | p = 0.16, χ2 = 1.93 |
| O | 34 | 40 | 26 | |
| CASE | ||||
| E | 51.82 | 47.36 | 10.82 | p = 0.08, χ2 = 3.01 |
| O | 57 | 37 | 16 | |
| ABCB1 gene 3435C > T | ||||
| - | CC | CT | TT | - |
| CONTROL | ||||
| E | 19.36 | 49.28 | 31.36 | p = 0.17, χ2 = 0.67 |
| O | 18 | 52 | 30 | |
| CASE | ||||
| E | 30 | 54.90 | 25.10 | p = 0.17, χ2 = 1.88 |
| O | 25 | 65 | 20 | |
| CYP1A1 gene 6235T > C (m1, CYP1A1*2A) | ||||
| - | WT | WT/m1 | m1/m1 | - |
| CONTROL | ||||
| E | 79.21 | 19.58 | 1.21 | p = 0.07, χ2 = 3.27 |
| O | 82 | 14 | 4 | |
| CASE | ||||
| E | 70.4 | 35.2 | 4.4 | p = 0.64, χ2 = 0.42 |
| O | 72 | 32 | 6 | |
| CYP1A1 gene 4889A > G (m2, CYP1A1*2C) | ||||
| - | WT | WT/m2 | m2/m2 | - |
| CONTROL | ||||
| E | 90.25 | 9.5 | 0.25 | p = 0.55, χ2 = 0.35 |
| O | 90 | 10 | 0 | |
| CASE | ||||
| E | 76.94 | 30.1 | 2.94 | p = 0.002, χ2 = 9.07 |
| O | 82 | 20 | 8 | |
| Gene Variants and Alleles | MM n (%) | Controls n (%) | Odds Ratio | 95% CI | p Values |
|---|---|---|---|---|---|
| ABCB1 gene 1236C > T | |||||
| CC | 45 (41%) | 30 (30%) | 1 | - | - |
| CT | 49 (44.5%) | 54 (54%) | 1.65 | 0.90–3.02 | 0.10 |
| TT | 16 (14.5%) | 16 (16%) | 1.50 | 0.65–3.44 | 0.33 |
| Total: | 110 (100%) | 100 (100%) | |||
| C | 139 (63%) | 114 (57%) | 1 | - | - |
| T | 81 (37%) | 86 (43%) | 1.29 | 0.87–1.91 | 0.19 |
| Total: | 220 (100%) | 200 (100%) | |||
| ABCB1 gene 2677G > T/A | |||||
| GG | 57 (51.7%) | 34 (34%) | 1 | - | - |
| GT | 33 (30%) | 36 (36%) | 1.82 | 0.97–3.45 | 0.06 |
| TT | 14 (12.8%) | 24 (24%) | 2.87 | 1.31–6.29 | 0.007 |
| GA | 4 (3.7%) | 4 (4%) | 1.67 | 0.39–7.14 | 0.74 |
| AT | 1 (0.9%) | 2 (2%) | 3.35 | 0.29–38.38 | 0.67 |
| AA | 1 (0.9%) | 0 | * | * | * |
| Total: | 110 (100) | 100 (100) | |||
| G | 152 (69%) | 108 (54%) | 1 | - | - |
| T | 62 (28%) | 86 (43%) | 1.95 | 1.29–2.93 | 0.001 |
| A | 6 (3%) | 6 (3%) | 1.40 | 0.44–4.48 | 0.55 |
| Total: | 220 (100) | 200 (100) | |||
| ABCB1 gene 3435C > T | |||||
| CC | 25 (22.7%) | 18 (18%) | 1 | - | - |
| CT | 65 (59.1%) | 52 (52%) | 1.11 | 0.54–2.25 | 0.76 |
| TT | 20 (18.2%) | 30 (30%) | 2.08 | 0.90–4.77 | 0.08 |
| Total: | 110 (100) | 100 (100) | |||
| C | 114 (52%) | 88 (44%) | 1 | - | - |
| T | 106 (48%) | 112 (56%) | 1.36 | 0.93–2.01 | 0.10 |
| Total: | 220 (100) | 200 (100) | |||
| CYP1A1 gene 6235T > C (m1, CYP1A1*2A) | |||||
| WT/WT | 72 (65.5%) | 82 (82%) | 1 | - | - |
| WT/m1 | 32 (29.1%) | 14 (14%) | 0.38 | 0.19–0.77 | 0.006 |
| m1/m1 | 6 (5.4%) | 4 (4%) | 0.68 | 0.15–2.15 | 0.62 |
| Total: | 110 (100) | 100 (100) | |||
| WT | 176 (80%) | 178 (89%) | 1 | - | - |
| m1 | 44 (20%) | 22 (11%) | 0.49 | 0.28–0.85 | 0.011 |
| Total: | 220 (100) | 200 (100) | |||
| CYP1A1 gene 4889A > G (m2, CYP1A1*2C) | |||||
| WT/WT | 82 (74.5%) | 90 (90%) | 1 | - | - |
| WT/m2 | 20 (18.2%) | 10 (10%) | 0.45 | 0.20–1.03 | 0.054 |
| m2/m2 | 8 (7.3%) | 0 | * | * | 0.011 |
| Total: | 110 (100) | 100 (100) | |||
| WT | 185 (84%) | 190 (95%) | 1 | - | - |
| m2 | 35 (16%) | 10 (5%) | 0.27 | 0.13–0.57 | 0.0003 |
| Total: | 220 (100) | 200 (100) | |||
| Variable | Univariate Cox Analysis for OS | Univariate Cox Analysis for PFS | ||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| ISS | ||||||
| I + II | - | R | - | - | R | - |
| III | 0.001 | 3.03 | 0.17–0.64 | 0.001 | 3.12 | 0.18–0.54 |
| Auto-HSCT | ||||||
| yes | - | R | - | - | R | - |
| no | <0.001 | 6.05 | 2.44–14.97 | 0.001 | 3.07 | 1.68–5.62 |
| ABCB1 gene 1236C > T | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.64 | 1.17 | 0.60–2.30 | 0.45 | 1.23 | 0.72–2.10 |
| ABCB1 gene 2677G > T/A * | ||||||
| GG | - | R | - | - | R | - |
| GT + TT | 0.26 | 0.68 | 0.35–1.33 | 0.91 | 1.03 | 0.60–1.76 |
| ABCB1 gene 3435C > T | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.04 | 0.34 | 0.12–0.96 | 0.64 | 0.86 | 0.46–1.60 |
| CYP1A1 gene 6235T > C (m1, CYP1A1*2A) | ||||||
| WT/WT | - | R | - | - | R | - |
| WT/m1 + m1/m1 | 0.35 | 0.73 | 0.38–1.41 | 0.44 | 0.80 | 0.46–1.39 |
| CYP1A1 gene 4889A > G (m2, CYP1A1*2C) | ||||||
| WT/WT | - | R | - | - | R | - |
| WT/m2 + m2/m2 | 0.20 | 0.64 | 0.32–1.27 | 0.07 | 0.60 | 0.34–1.05 |
| Variable | Multivariate Cox Analysis for OS | Multivariate Cox Analysis for PFS | ||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| ISS | ||||||
| I + II | - | reference | - | - | reference | - |
| III | 0.05 | 0.44 | 0.20–0.99 | 0.003 | 0.35 | 0.18–0.70 |
| Auto-HSCT | ||||||
| yes | - | reference | - | - | reference | - |
| no | 0.02 | 3.64 | 1.27–10.50 | 0.35 | 1.43 | 0.67–3.04 |
| ABCB1 gene 1236C > T | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.74 | 0.86 | 0.37–2.04 | 0.95 | 1.02 | 0.50–2.05 |
| ABCB1 gene 2677G > T/A * | ||||||
| GG | - | R | - | - | R | - |
| GT + TT | 0.56 | 1.26 | 0.56–2.83 | 0.33 | 1.36 | 0.73–2.53 |
| ABCB1 gene 3435C > T | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.04 | 0.29 | 0.09–0.93 | 0.31 | 0.69 | 0.34–1.40 |
| CYP1A1 gene 6235T > C (m1, CYP1A1*2A) | ||||||
| WT/WT | - | R | - | - | R | - |
| WT/m1 + m1/m1 | 0.17 | 0.60 | 0.29–1.23 | 0.11 | 0.61 | 0.33–1.13 |
| CYP1A1 gene 4889A > G (m2, CYP1A1*2C) | ||||||
| WT/WT | - | R | - | - | R | - |
| WT/m2 + m2/m2 | 0.51 | 0.76 | 0.33–1.73 | 0.29 | 0.69 | 0.35–1.37 |
| Variables | MM Patients | 1236C > T | 2677G > T/A | 3435C > T | 6235T > C (m1, CYP1A1*2A) | 4889A > G (m2, CYP1A1*2C) |
|---|---|---|---|---|---|---|
| CC vs. CT + TT p-Value | GG vs. GT + TT p-Value | CC vs. CT + TT p-Value | WT/WT vs. WT/m1 and m1/m1 p-Value | WT/WT vs. WT/m and m2/m2 p-Value | ||
| Mean age (years) * | 65.36 | 0.11 | 0.67 | 0.69 | 0.70 | 0.26 |
| Free light chain ratio * | 292.64 | 0.15 | 0.21 | 0.17 | 0.89 | <0.001 |
| % of plasma cells in bone marrow * | 30.85 | 0.16 | 0.10 | 0.78 | 0.24 | 0.37 |
| Albumins (g/dL) * | 3.58 | 0.88 | 0.57 | 0.76 | 0.71 | 0.31 |
| β2-microglobulin * (mg/L) | 6.18 | 0.53 | 0.18 | 0.71 | 0.63 | 0.03 |
| Calcium * (mM/L) | 2.43 | 0.22 | 0.83 | 0.37 | 0.19 | 0.64 |
| Hemoglobin * (g/dL) | 10.58 | 0.89 | 0.62 | 0.45 | 0.94 | 0.31 |
| Creatinine * (mg/dL) | 1.66 | 0.44 | 0.61 | 0.77 | 0.44 | <0.001 |
| Platelets (K/μL) | 210.64 | 0.54 | 0.83 | 0.32 | 0.01 | 0.26 |
| C-reactive protein * (mg/L) | 15.16 | 0.96 | 0.60 | 0.63 | 0.88 | 0.08 |
| Estimated glomerular filtration rate * mL/min/1.73 m2 | 60.31 | 0.50 | 0.56 | 0.09 | 0.20 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zmorzynski, S.; Wojcierowska-Litwin, M.; Popek-Marciniec, S.; Szudy-Szczyrek, A.; Styk, W.; Chocholska, S.; Filip, A.A. The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma. J. Clin. Med. 2021, 10, 5276. https://doi.org/10.3390/jcm10225276
Zmorzynski S, Wojcierowska-Litwin M, Popek-Marciniec S, Szudy-Szczyrek A, Styk W, Chocholska S, Filip AA. The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma. Journal of Clinical Medicine. 2021; 10(22):5276. https://doi.org/10.3390/jcm10225276
Chicago/Turabian StyleZmorzynski, Szymon, Magdalena Wojcierowska-Litwin, Sylwia Popek-Marciniec, Aneta Szudy-Szczyrek, Wojciech Styk, Sylwia Chocholska, and Agata Anna Filip. 2021. "The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma" Journal of Clinical Medicine 10, no. 22: 5276. https://doi.org/10.3390/jcm10225276
APA StyleZmorzynski, S., Wojcierowska-Litwin, M., Popek-Marciniec, S., Szudy-Szczyrek, A., Styk, W., Chocholska, S., & Filip, A. A. (2021). The Relationship of ABCB1/MDR1 and CYP1A1 Variants with the Risk of Disease Development and Shortening of Overall Survival in Patients with Multiple Myeloma. Journal of Clinical Medicine, 10(22), 5276. https://doi.org/10.3390/jcm10225276






