Low eGFR Is a Strong Predictor of Worse Outcome in Hospitalized COVID-19 Patients
Abstract
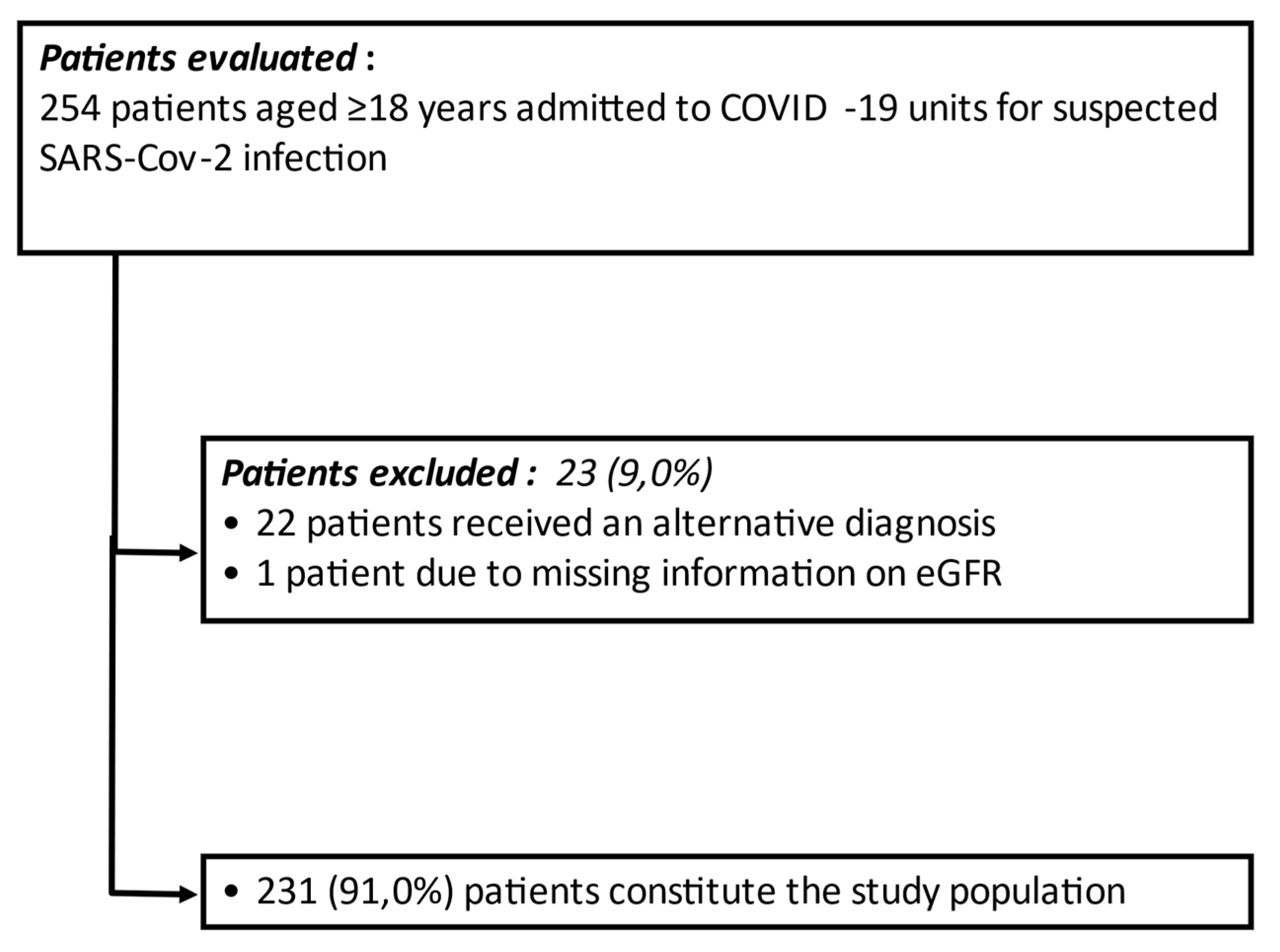
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinelli, M.; Quattrociocchi, W.; Galeazzi, A.; Valensise, C.M.; Brugnoli, E.; Schmidt, A.L.; Zola, P.; Zollo, F.; Scala, A. The COVID-19 social media infodemic. Sci. Rep. 2020, 10, 16598. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Ventura, P.; Ageno, W.; Cogliati, C.B.; Muiesan, M.L.; Girelli, D.; Pirisi, M.; Gasbarrini, A.; Angeli, P.; Querini, P.R.; et al. Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: Results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI). Intern. Emerg. Med. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Miyashita, H.; Yamada, T.; Harrington, M.; Steinberg, D.; Dunn, A.; Siau, E. Risk Factors for Mortality in Patients with COVID-19 in New York City. J. Gen. Intern. Med. 2021, 36, 17–26. [Google Scholar] [CrossRef]
- De Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef]
- Grams, M.E.; Chow, E.K.; Segev, D.L.; Coresh, J. Lifetime incidence of CKD stages 3–5 in the United States. Am. J. Kidney Dis. 2013, 62, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.T.; De Cosmo, S.; Viazzi, F.; Mirijello, A.; Ceriello, A.; Guida, P.; Giorda, C.; Cucinotta, D.; Pontremoli, R.; Fioretto, P.; et al. Diabetic kidney disease in the elderly: Prevalence and clinical correlates. BMC Geriatr. 2018, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Carmena, R.; Ascaso, J.F.; Redon, J. Chronic kidney disease as a cardiovascular risk factor. J. Hypertens. 2020, 38, 2110–2121. [Google Scholar] [CrossRef]
- Lamacchia, O.; Viazzi, F.; Fioretto, P.; Mirijello, A.; Giorda, C.; Ceriello, A.; Russo, G.; Guida, P.; Pontremoli, R.; De Cosmo, S. Normoalbuminuric kidney impairment in patients with T1DM: Insights from annals initiative. Diabetol. Metab. Syndr. 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Matsushita, K.; Abate, K.H.; Al-Aly, Z.; Ärnlöv, J.; Asayama, K.; Atkins, R.; Badawi, A.; Ballew, S.H.; Banerjee, A.; et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J. Am. Soc. Nephrol. 2017, 28, 2167–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Hong, X.W.; Chi, Z.P.; Liu, G.Y.; Huang, H.; Guo, S.Q.; Fan, J.R.; Lin, X.W.; Qu, L.Z.; Chen, R.L.; Wu, L.J.; et al. Characteristics of Renal Function in Patients Diagnosed With COVID-19: An Observational Study. Front. Med. 2020, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Uribarri, A.; Núñez-Gil, I.J.; Aparisi, A.; Becerra-Muñoz, V.M.; Feltes, G.; Trabattoni, D.; Fernández-Rozas, I.; Viana-Llamas, M.C.; Pepe, M.; Cerrato, E.; et al. Impact of renal function on admission in COVID-19 patients: An analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J. Nephrol. 2020, 33, 737–745. [Google Scholar] [CrossRef]
- Li, W.X.; Xu, W.; Huang, C.L.; Fei, L.; Xie, X.D.; Li, Q.; Chen, L. Acute cardiac injury and acute kidney injury associated with severity and mortality in patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2114–2122. [Google Scholar] [CrossRef]
- Wang, A.Z.; Ehrman, R.; Bucca, A.; Croft, A.; Glober, N.; Holt, D.; Lardaro, T.; Musey, P.; Peterson, K.; Trigonis, R.; et al. Can we predict which COVID-19 patients will need transfer to intensive care within 24 hours of floor admission? Acad. Emerg. Med. 2021, 6, 511–518. [Google Scholar] [CrossRef]
- Gok, M.; Cetinkaya, H.; Kandemir, T.; Karahan, E.; Tuncer, İ.B.; Bukrek, C.; Sahin, G. Chronic kidney disease predicts poor outcomes of COVID-19 patients. Int. Urol. Nephrol. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals with Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Flacco, M.E.; Carradori, T.; Volta, C.A.; Cosenza, G.; De Togni, A.; Acuti Martellucci, C.; Parruti, G.; Mantovani, L.; Manzoli, L. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS ONE 2020, 15, e0235248. [Google Scholar] [CrossRef] [PubMed]
- Mirijello, A.; Zarrelli, M.; Miscio, G.; de Matthaeis, A.; Piscitelli, P.; Carbonelli, C.M.; Di Giorgio, A.; Inglese, M.; Ciliberti, G.L.; Marciano, C.; et al. Diagnosis of COVID-19 in Patients with Negative Nasopharyngeal Swabs: Reliability of Radiological and Clinical Diagnosis and Accuracy Versus Serology. Diagnostics 2021, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. Available online: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 (accessed on 27 April 2021).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. R Development Core Team, Version 3.3.2. Available online: https://www.r-project.org/ (accessed on 2 February 2021).
- Gansevoort, R.T.; Hilbrands, L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020, 16, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B. Mortality in COVID-19: Further Evidence for a Sex-Based Difference in the OpenSAFELY Study. J. Womens Health 2021, 30, 61–63. [Google Scholar] [CrossRef]
- Cho, S.I.; Yoon, S.; Lee, H.J. Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Sci. Rep. 2021, 11, 6375. [Google Scholar] [CrossRef] [PubMed]


| Main Patients’ Clinical Features | Whole Population (n = 231) | eGFR ≥ 60 mL/min/1.73 m2 (n = 168) | eGFR < 60 mL/min/1.73 m2 (n = 63) | p Value |
|---|---|---|---|---|
| Age (years old) | 68.6 ± 15.0 | 65.3 ± 15.1 | 77.3 ± 10.4 | <0.001 |
| Gender (M/F) | 125/106 (54.1%/45.9%) | 97/71 (57.7%/42.3%) | 28/35 (44.4%/55.6%) | 0.071 |
| BMI kg/m2 | 26.9 ± 4.6 | 26.7 ± 4.5 | 27.5 ± 5.0 | 0.328 |
| GCS (points) | 14.4 ± 2.1 | 14.6 ± 1.6 | 13.7 ± 3.1 | 0.003 |
| CCI (points) | 3.9 ± 2.5 | 3.2 ± 2.3 | 5.7 ± 2.0 | <0.001 |
| Diabetes, n (%) | 44 (19.0%) | 22 (13.1%) | 22 (34.9%) | <0.001 |
| Hypertension, n (%) | 109 (47.2%) | 67 (39.9%) | 42 (66.7%) | <0.001 |
| Dyslipidemia, n (%) | 24 (10.4%) | 16 (9.5%) | 8 (12.7%) | 0.938 |
| Atrial Fibrillation, n (%) | 33 (14.3%) | 20 (11.9%) | 13 (20.6%) | 0.091 |
| COPD, n (%) | 25 (10.8%) | 17 (10.1%) | 8 (12.7%) | 0.574 |
| Dementia, n (%) | 41 (17.7%) | 20 (11.9%) | 21 (33.3%) | <0.001 |
| Antiplatelet treatment, n (%) | 50 (21.6%) | 27 (16.1%) | 23 (36.5%) | <0.001 |
| Anti-hypertensive treatment, n (%) | 105 (45.4%) | 62 (36.9%) | 42 (66.7%) | <0.001 |
| ACEi/ARB, n (%) | 73 (31.6%) | 48 (28.6%) | 25 (39.7%) | 0.106 |
| Serum creatinine (mg/dL) | 1.2 ± 1.2 | 0.7 ± 0.2 | 2.3 ± 1.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | 88.6 ± 45.3 | 108.4 ± 36.0 | 35.9 ± 15.5 | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 63 (27.2%) | - | - | - |
| CKD, n (%) | 39 (16.9%) | - | 39 (61.9%) | - |
| AKI, n (%) | 24 (10.4%) | - | 24 (38.1%) | - |
| Glycemia (mg/dL) | 121.8 ± 56.2 | 110.8 ± 46.2 | 150.0 ± 71.0 | <0.001 |
| Triglycerides (mg/dL) | 124.5 ± 52.8 | 123.1 ± 54.4 | 128.6 ± 48.4 | 0.354 |
| Cholesterol (mg/dL) | 138.7 ± 40.4 | 143.1 ± 40.3 | 126.3 ± 38.3 | 0.011 |
| LDH (UI/L) | 284.4 ± 160.9 | 275.5 ± 142.8 | 307.2 ± 199.9 | 0.997 |
| SpO2 (%) | 92.6 ± 5.7 | 92.9 ± 5.3 | 91.6 ± 7.0 | 0.598 |
| Troponin (ng/mL) | 238.7 ± 1580.1 | 63.2 ± 209.0 | 698.5 ± 2975.6 | <0.001 |
| D-dimer (ng/mL) | 3325.0 ± 8053.6 | 2585.2 ± 6115.9 | 5409.9 ± 11821.7 | 0.013 |
| CRP (mg/dL) | 7.4 ± 7.2 | 6.7 ± 6.7 | 9.3 ± 8.2 | 0.035 |
| PCT (ng/mL) | 1.2 ± 4.0 | 0.9 ± 4.0 | 2.2 ± 4.0 | <0.001 |
| IL-6 (pg/mL) | 73.0 ± 196.0 | 68.2 ± 196.2 | 87.9 ± 201.0 | 0.226 |
| Occurrence of primary outcome, n (%) | 79 (34.2%) | 45 (26.8%) | 34 (54.0%) | <0.001 |
| Time to Combined Endpoint | ||
|---|---|---|
| Variable | HR (95% CI) | p Value |
| Age (per 5 years) | 1.26 (1.15–1.39) | <0.001 |
| Gender (M/F) | 1.04 (0.66–1.62) | 0.871 |
| BMI kg/m2 | 1.02 (0.97–1.07) | 0.457 |
| CCI (points) | 1.26 (1.15–1.38) | <0.001 |
| Diabetes, n (%) | 1.58 (0.95–2.64) | 0.079 |
| Antiplatelet treatment, n (%) | 1.96 (1.21–3.19) | 0.006 |
| Anti-dyslipidemia treatment, n (%) | 0.94 (0.48–1.84) | 0.854 |
| Anti-hypertensive treatment, n (%) | 1.20 (0.77–1.87) | 0.427 |
| Serum creatinine (mg/dL) | 1.28 (1.15–1.44) | <0.001 |
| eGFR (per 10 mL/min/1.73 m2) | 0.93 (0.88–0.97) | 0.003 |
| eGFR < 60 mL/min/1.73 m2 | 2.40 (1.53–3.76) | <0.001 |
| Blood glucose (mg/dL) | 1 (1–1.01) | <0.001 |
| Triglycerides (mg/dL) | 1 (1–1.01) | 0.853 |
| Cholesterol (mg/dL) | 0.99 (0.99–1) | 0.115 |
| LDH (UI/L) | 1 (1–1) | 0.003 |
| SpO2 (%) | 0.93 (0.89–0.96) | <0.001 |
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Age (per 5 years) | 1.22 (1.10–1.35) | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 1.64 (1.02–2.63) | 0.040 |
| Group | HR | p Value |
|---|---|---|
| Baseline eGFR ≥ 60 mL/min/1.73 m2 | 1 | n.a. |
| AKI | 1.89 (0.97–3.68) | 0.059 |
| CKD | 2.59 (1.64–4.54) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirijello, A.; Piscitelli, P.; de Matthaeis, A.; Inglese, M.; D’Errico, M.M.; Massa, V.; Greco, A.; Fontana, A.; Copetti, M.; Florio, L.; et al. Low eGFR Is a Strong Predictor of Worse Outcome in Hospitalized COVID-19 Patients. J. Clin. Med. 2021, 10, 5224. https://doi.org/10.3390/jcm10225224
Mirijello A, Piscitelli P, de Matthaeis A, Inglese M, D’Errico MM, Massa V, Greco A, Fontana A, Copetti M, Florio L, et al. Low eGFR Is a Strong Predictor of Worse Outcome in Hospitalized COVID-19 Patients. Journal of Clinical Medicine. 2021; 10(22):5224. https://doi.org/10.3390/jcm10225224
Chicago/Turabian StyleMirijello, Antonio, Pamela Piscitelli, Angela de Matthaeis, Michele Inglese, Maria Maddalena D’Errico, Valentina Massa, Antonio Greco, Andrea Fontana, Massimiliano Copetti, Lucia Florio, and et al. 2021. "Low eGFR Is a Strong Predictor of Worse Outcome in Hospitalized COVID-19 Patients" Journal of Clinical Medicine 10, no. 22: 5224. https://doi.org/10.3390/jcm10225224
APA StyleMirijello, A., Piscitelli, P., de Matthaeis, A., Inglese, M., D’Errico, M. M., Massa, V., Greco, A., Fontana, A., Copetti, M., Florio, L., Leone, M. A., Prencipe, M. A., Aucella, F., & De Cosmo, S., on behalf of the CSS-COVID-19 Group. (2021). Low eGFR Is a Strong Predictor of Worse Outcome in Hospitalized COVID-19 Patients. Journal of Clinical Medicine, 10(22), 5224. https://doi.org/10.3390/jcm10225224








