Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives
Abstract
1. Introduction
2. ROD and Bone Markers
3. ROD and PTH
4. ROD and Osteoporosis
5. ROD and Non-Invasive Diagnostic Approach
6. ROD and Bone Biopsy
7. Indications of Bone Biopsy in CKD
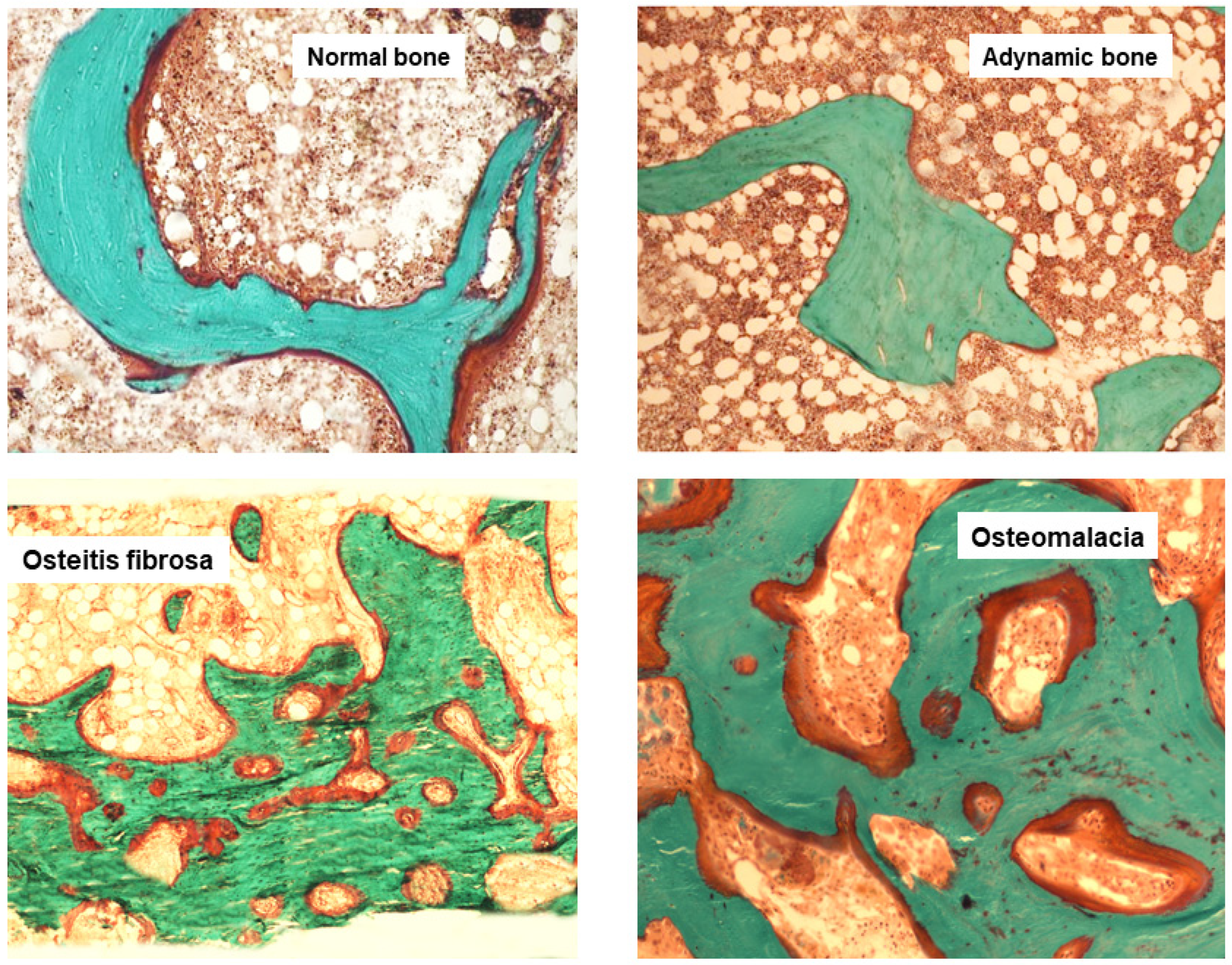
8. Classification of ROD by Histomorphometry
9. Limitations of Bone Biopsy
10. New Perspectives
11. Conclusions
12. Highlights
- Bone and mineral metabolism alterations are common in chronic kidney disease (CKD);
- In borderline cases, the accuracy of markers are low and inadequate for the management of CKD patients;
- PTH has a poor predictive power in low turnover disease in CKD;
- In CKD patients, the predictive powers of Bone Densitometryand non-invasive approaches are low because they provide a two-dimensional evaluation of a three-dimensional structure, and thus offer a very poor spatial resolution;
- The use of bone biopsy is the gold standard for the diagnosis and specific classification of Renal Osteodystrophy (ROD);
- The Italian biopsy protocol, as well as the built network based on the hub/spoke model, can ameliorate the appeal of this procedure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| ROD | renal osteodystrophy |
| KDIGO | Kidney Disease Improving Global Outcomes |
| CKD-MBD | Chronic Kidney Disease Mineral and Bone Disorder |
| PTH | Parathyroid Hormone |
| ERA-EDTA | European Renal Association—European Dialysis and Transplant Association |
| DXA | Dual X-rays absorptiometry |
| TBS | Trabecular Bone Score |
| HR-pQCT | High Resolution-peripheral Quantitative Computed Tomography |
| MSCs | Mesenchymal stem cells |
References
- Carbonare, L.D.; Valenti, M.; Bertoldo, F.; Zanatta, M.; Zenari, S.; Realdi, G.; Cascio, V.L.; Giannini, S. Bone microarchitecture evaluated by histomorphometry. Micron 2005, 36, 609–616. [Google Scholar] [CrossRef]
- Carbonare, L.D.; Giannini, S. Histologic diagnosis of metabolic bone diseases: Bone histomorphometry. Reumatismo 2004, 56, 15–23. [Google Scholar] [CrossRef][Green Version]
- Carbonare, L.D.; Ballanti, P.; Bertoldo, F.; Valenti, M.T.; Giovanazzi, B.; Giannini, S.; Realdi, G.; Cascio, V.L. Trabecular bone microarchitecture in mild primary hyperparathyroidism. J. Endocrinol. Investig. 2008, 31, 525–530. [Google Scholar] [CrossRef]
- Salam, S.; Gallagher, O.; Hughes, D.; Khwaja, A.; Eastell, R. The role of static bone histomorphometry in diagnosing renal osteodystrophy. Bone 2021, 142, 115689. [Google Scholar] [CrossRef]
- Ramalho, J.; Marques, I.; Hans, D.; Dempster, D.; Zhou, H.; Patel, P.; Pereira, R.; Jorgetti, V.; Moyses, R.; Nickolas, T.L. The trabecular bone score: Relationships with trabecular and cortical microarchitecture measured by HR-pQCT and histomorphometry in patients with chronic kidney disease. Bone 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Tregnago, P.; Valenti, M.T.; Bertoldo, F.; Ferronato, G.; et al. Osteomalacia: The Missing Link in the Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaws? Oncologist 2012, 17, 1114–1119. [Google Scholar] [CrossRef]
- Recker, R.; Dempster, D.; Langdahl, B.; Giezek, H.; Clark, S.; Ellis, G.; De Villiers, T.; Valter, I.; Zerbini, C.A.; Cohn, D.; et al. Effects of Odanacatib on Bone Structure and Quality in Postmenopausal Women with Osteoporosis: 5-Year Data from the Phase 3 Long-Term Odanacatib Fracture Trial (LOFT) and its Extension. J. Bone Miner. Res. 2020, 35, 1289–1299. [Google Scholar] [CrossRef]
- Evenepoel, P.; Behets, G.; Laurent, M.; D’Haese, P. Update on the role of bone biopsy in the management of patients with CKD–MBD. J. Nephrol. 2017, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Burton, I.S.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Bone Histomorphometry and 18F-Sodium Fluoride Positron Emission Tomography Imaging: Comparison Between only Bone Turnover-based and Unified TMV-based Classification of Renal Osteodystrophy. Calcif. Tissue Int. 2021, 1–10. [Google Scholar] [CrossRef]
- Lavigne, F.; Desbiens, L.-C.; Garneau, G.; Côté, F.; Mac-Way, F. Iliac crest bone biopsy by interventional radiologists to improve access to bone biopsy in chronic kidney disease populations: Technical note and a case series. J. Nephrol. 2021, 34, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, C.E.M.; Dos Reis, L.M.; Quadros, K.R.D.S.; Roza, N.A.V.; Sano, R.; Carvalho, A.B.; Jorgetti, V.; De Oliveira, R.B. Renal osteodystrophy and clinical outcomes: Data from the Brazilian Registry of Bone Biopsies—REBRABO. Braz. J. Nephrol. 2020, 42, 138–146. [Google Scholar] [CrossRef]
- Novel-Catin, E.; Pelletier, S.; Fouque, D.; Roux, J.-P.; Chapurlat, R.; D’Haese, P.; Behets, G.; Evenepoel, P.; Nickolas, T.L.; Lafage-Proust, M.-H. Quantitative histomorphometric analysis of halved iliac crest bone biopsies yield comparable ROD diagnosis as full 7.5mm wide samples. Bone 2020, 138, 115460. [Google Scholar] [CrossRef]
- Liangos, O.; Kirchhoff, S.; Buchholz, J.; Ketteler, M. Bone Biopsy Results in Chronic Kidney Disease: A Single-Center Experience. Kidney Blood Press. Res. 2018, 43, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Toussaint, N.D.; Masterson, R.; Holt, S.G.; Rajapakse, C.S.; Ebeling, P.R.; Mohanty, S.T.; Baldock, P.; Elder, G.J. Deterioration of Cortical Bone Microarchitecture: Critical Component of Renal Osteodystrophy Evaluation. Am. J. Nephrol. 2018, 47, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Behets, G.J.; Viaene, L.; D’Haese, P.C. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int. 2017, 91, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drueke, T.B.; Du, H.; Manley, T.; et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients With CKD Treated by Dialysis. Am. J. Kidney Dis. 2016, 67, 559–566. [Google Scholar] [CrossRef]
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.-C. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2011, 26, 1368–1376. [Google Scholar] [CrossRef]
- Lehmann, G.; Ott, U.; Kämmerer, D.; Schütze, J.; Wolf, G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3–5. Clin. Nephrol. 2008, 70, 296–305. [Google Scholar] [CrossRef]
- Miller, P.D. The Role of Bone Biopsy in Patients with Chronic Renal Failure. Clin. J. Am. Soc. Nephrol. 2008, 3, S140–S150. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Bonucci, E.; Calabria, S.; Costantini, S.; Ferrannini, M.; Giustini, M.; Giordano, R.; Nicolai, G.; Manni, M.; et al. Renal Osteodystrophy in Predialysis and Hemodialysis Patients: Comparison of Histologic Patterns and Diagnostic Predictivity of Intact PTH. Nephron 2002, 91, 103–111. [Google Scholar] [CrossRef]
- Gerakis, A.; Hadjidakis, D.; Kokkinakis, E.; Apostolou, T.; Raptis, S.; Billis, A. Correlation of bone mineral density with the histological findings of renal osteodystrophy in patients on hemodialysis. J. Nephrol. 2000, 13, 437–443. [Google Scholar]
- Jørgensen, H.S.; Behets, G.; Viaene, L.; Bammens, B.; Claes, K.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D.; D’Haese, P.C.; et al. Static HISTOMORPHOMETRY allows for a diagnosis of bone turnover in renal OSTEODYSTROPHY in the absence of tetracycline labels. Bone 2021, 152, 116066. [Google Scholar] [CrossRef]
- Gerakis, A.; Hutchison, A.; Apostolou, T.; Freemont, A.; Billis, A. Biochemical markers for non-invasive diagnosis of hyperparathyroid bone disease and adynamic bone in patients on haemodialysis. Nephrol. Dial. Transplant. 1996, 11, 2430–2438. [Google Scholar] [CrossRef]
- Bakkaloglu, S.A.; Bacchetta, J.; Lalayiannis, A.D.; Leifheit-Nestler, M.; Stabouli, S.; Haarhaus, M.; Reusz, G.; Groothoff, J.; Schmitt, C.P.; Evenepoel, P.; et al. Bone evaluation in paediatric chronic kidney disease: Clinical practice points from the European Society for Paediatric Nephrology CKD-MBD and Dialysis working groups and CKD-MBD working group of the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 413–425. [Google Scholar] [CrossRef]
- Khairallah, P.; Nickolas, T.L. Management of Osteoporosis in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.; Barreto, D.; Moysés, R.; Neves, K.; Canziani, M.; Draibe, S.; Jorgetti, V.; Carvalho, A. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008, 73, 771–777. [Google Scholar] [CrossRef]
- Moore, C.; Yee, J.; Malluche, H.; Rao, D.S.; Monier-Faugere, M.-C.; Adams, E.; Daramola-Ogunwuyi, O.; Fehmi, H.; Bhat, S.; Osman-Malik, Y. Relationship between Bone Histology and Markers of Bone and Mineral Metabolism in African-American Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Jannot, M.; Mac-Way, F.; Lapierre, V.; Lafage-Proust, M.-H. The use of bone mineral density measured by dual energy X-ray absorptiometry (DXA) and peripheral quantitative computed microtomography in chronic kidney disease. J. Nephrol. 2017, 30, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Akizawa, T.; Bavanandan, S.; Hamano, T.; Liew, A.; Lu, K.-C.; Lumlertgul, D.; Oh, K.-H.; Zhao, M.-H.; Fung, S.; et al. 2017 Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update Implementation: Asia Summit Conference Report. Kidney Int. Rep. 2019, 4, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L.; Baim, S.; Bishop, N.J.; Gordon, C.M.; Hans, D.B.; Langman, C.B.; Leonard, M.B.; Kalkwarf, H.J. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr. Nephrol. 2010, 25, 37–47. [Google Scholar] [CrossRef]
- Jamal, S.A.; Chase, C.; Goh, Y.; Richardson, R.; Hawker, G.A. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am. J. Kidney Dis. 2002, 39, 843–849. [Google Scholar] [CrossRef]
- Christoforidis, A.; Printza, N.; Gkogka, C.; Siomou, E.; Challa, A.; Kazantzidou, E.; Kollios, K.; Papachristou, F. Comparative study of quantitative ultrasonography and dual-energy X-ray absorptiometry for evaluating renal osteodystrophy in children with chronic kidney disease. J. Bone Miner. Metab. 2011, 29, 321–327. [Google Scholar] [CrossRef]
- Moe, S.M. Renal Osteodystrophy or Kidney-Induced Osteoporosis? Curr. Osteoporos. Rep. 2017, 15, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Ureña-Torres, P.; Cozzolino, M.; Rodríguez-García, M.; Gómez-Alonso, C. The Non-invasive Diagnosis of Bone Disorders in CKD. Calcif. Tissue Int. 2021, 108, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Shevroja, E.; Lamy, O.; Hans, D. Review on the Utility of Trabecular Bone Score, a Surrogate of Bone Micro-architecture, in the Chronic Kidney Disease Spectrum and in Kidney Transplant Recipients. Front. Endocrinol. 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Pocock, N. Use of dual energy X-ray absorptiometry, the trabecular bone score and quantitative computed tomography in the evaluation of chronic kidney disease-mineral and bone disorders. Nephrology 2017, 22, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Geusens, P.; Chapurlat, R.; Schett, G.; Ghasem-Zadeh, A.; Seeman, E.; De Jong, J.; Bergh, J.V.D. High-resolution in vivo imaging of bone and joints: A window to microarchitecture. Nat. Rev. Rheumatol. 2014, 10, 304–313. [Google Scholar] [CrossRef]
- Carvalho, C.; Alves, C.M.; Frazão, J.M. The role of bone biopsy for the diagnosis of renal osteodystrophy: A short overview and future perspectives. J. Nephrol. 2016, 29, 617–626. [Google Scholar] [CrossRef]
- Evenepoel, P.; D’Haese, P.; Bacchetta, J.; Cannata-Andia, J.; Ferreira, A.; Haarhaus, M.; Mazzaferro, S.; Lafage-Proust, M.-H.; Salam, S.; Spasovski, G.; et al. Bone biopsy practice patterns across Europe: The European renal osteodystrophy initiative—A position paper. Nephrol. Dial. Transplant. 2017, 32, 1608–1613. [Google Scholar] [CrossRef][Green Version]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.-C. Bone Mineral Density and Serum Biochemical Predictors of Bone Loss in Patients with CKD on Dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Durbridge, T.C.; Woods, A.E.; Vernon-Roberts, B. Comparison of two bone trephine instruments used for quantitative histomorphometry. J. Clin. Pathol. 1989, 42, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Carbonare, L.D.; Innamorati, G.; Valenti, M.T. Transcription Factor Runx2 and its Application to Bone Tissue Engineering. Stem Cell Rev. Rep. 2011, 8, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Carbonare, L.D.; Valenti, M.T.; Zanatta, M.; Donatelli, L.; Cascio, V.L. Circulating mesenchymal stem cells with abnormal osteogenic differentiation in patients with osteoporosis. Arthritis Rheum. 2009, 60, 3356. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Carbonare, L.D.; Donatelli, L.; Bertoldo, F.; Zanatta, M.; Cascio, V.L. Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone 2008, 43, 1084–1092. [Google Scholar] [CrossRef]
- Chong, P.-P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J. Orthop. Res. 2012, 30, 634–642. [Google Scholar] [CrossRef]
- Fei, D.; Wang, Y.; Zhai, Q.; Zhang, X.; Zhang, Y.; Wang, Y.; Li, B.; Wang, Q. KAT6A regulates stemness of aging bone marrow-derived mesenchymal stem cells through Nrf2/ARE signaling pathway. Stem Cell Res. Ther. 2021, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Carbonare, L.D.; Mottes, M. Role of microRNAs in progenitor cell commitment and osteogenic differentiation in health and disease (Review). Int. J. Mol. Med. 2018, 41, 2441–2449. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y. The potential role of lncRNAs in osteoporosis. J. Bone Miner. Metab. 2021, 39, 341–352. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.; Ren, Y.; Han, X.; Wang, J.; Hong, W. Kcnq1ot1 promotes osteogenic differentiation and suppresses osteoclast differentiation. J. South. Med. Univ. 2021, 41, 31–38. [Google Scholar]
- Zhou, Z.; Hossain, M.S.; Liu, D. Involvement of the long noncoding RNA H19 in osteogenic differentiation and bone regeneration. Stem Cell Res. Ther. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Gu, P.-C.; Xu, S.-Z.; Lin, X.-J. Long non-coding RNA-DANCR in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. Biosci. Biotechnol. Biochem. 2015, 79, 732–737. [Google Scholar] [CrossRef]
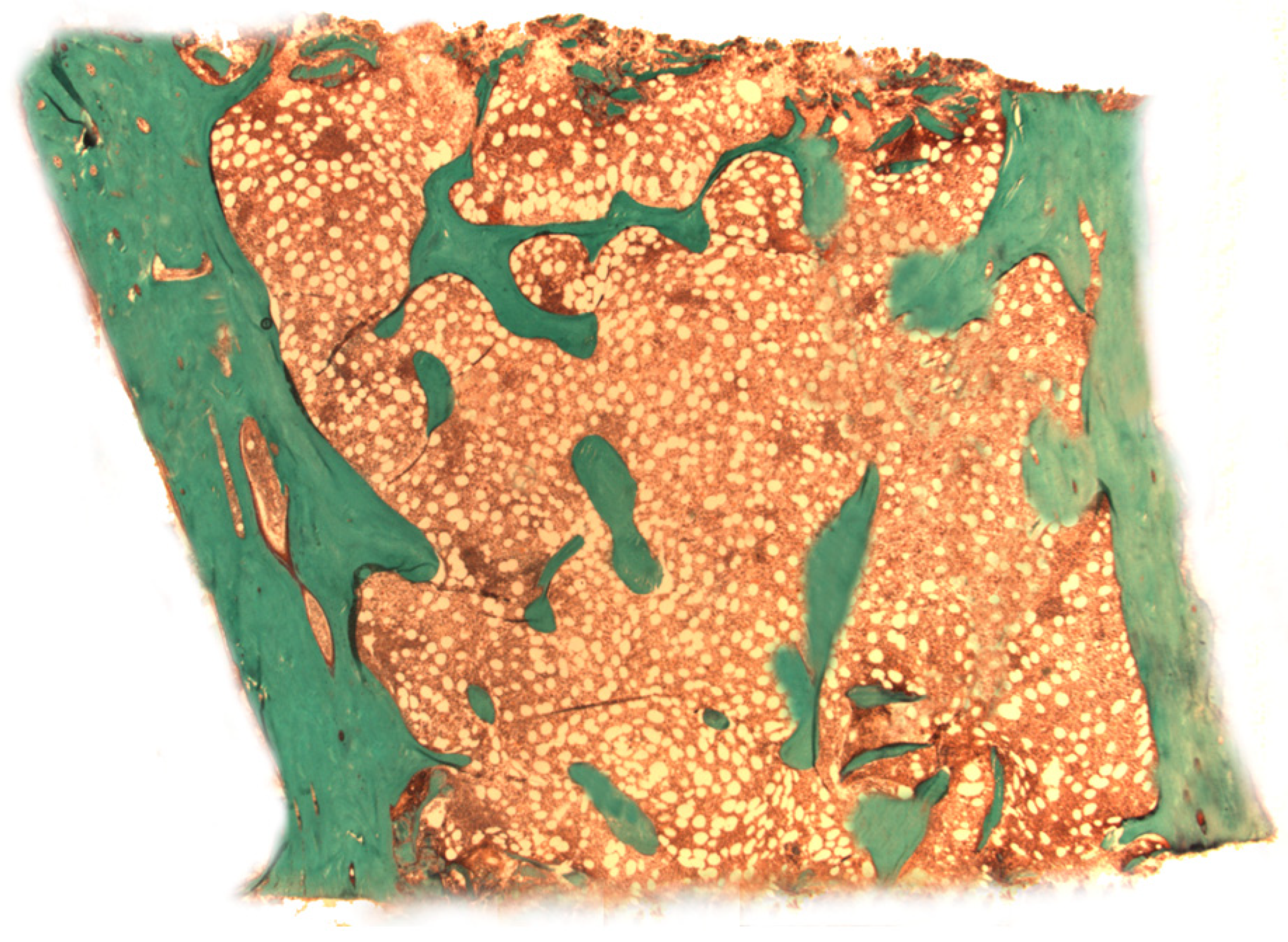
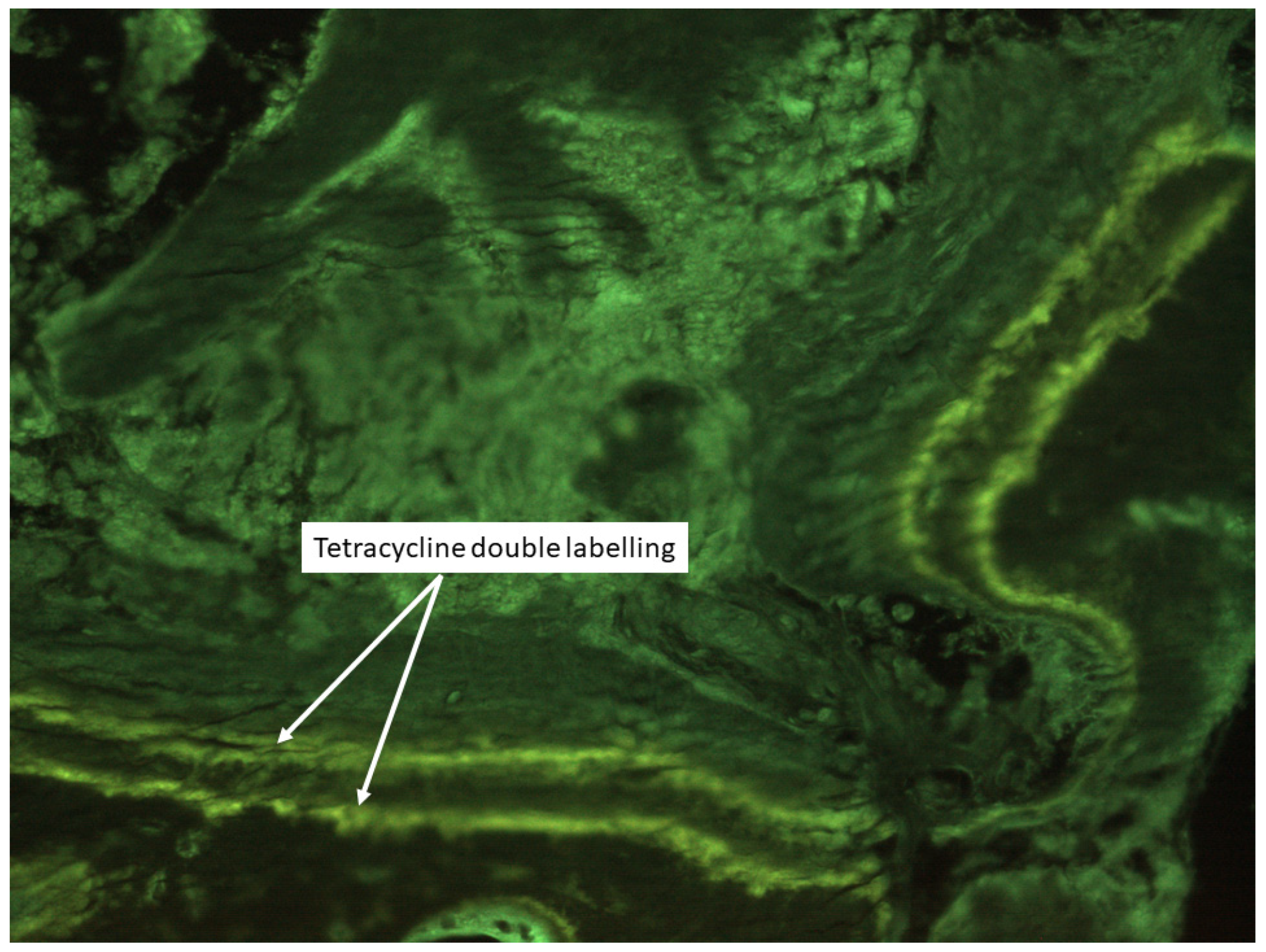
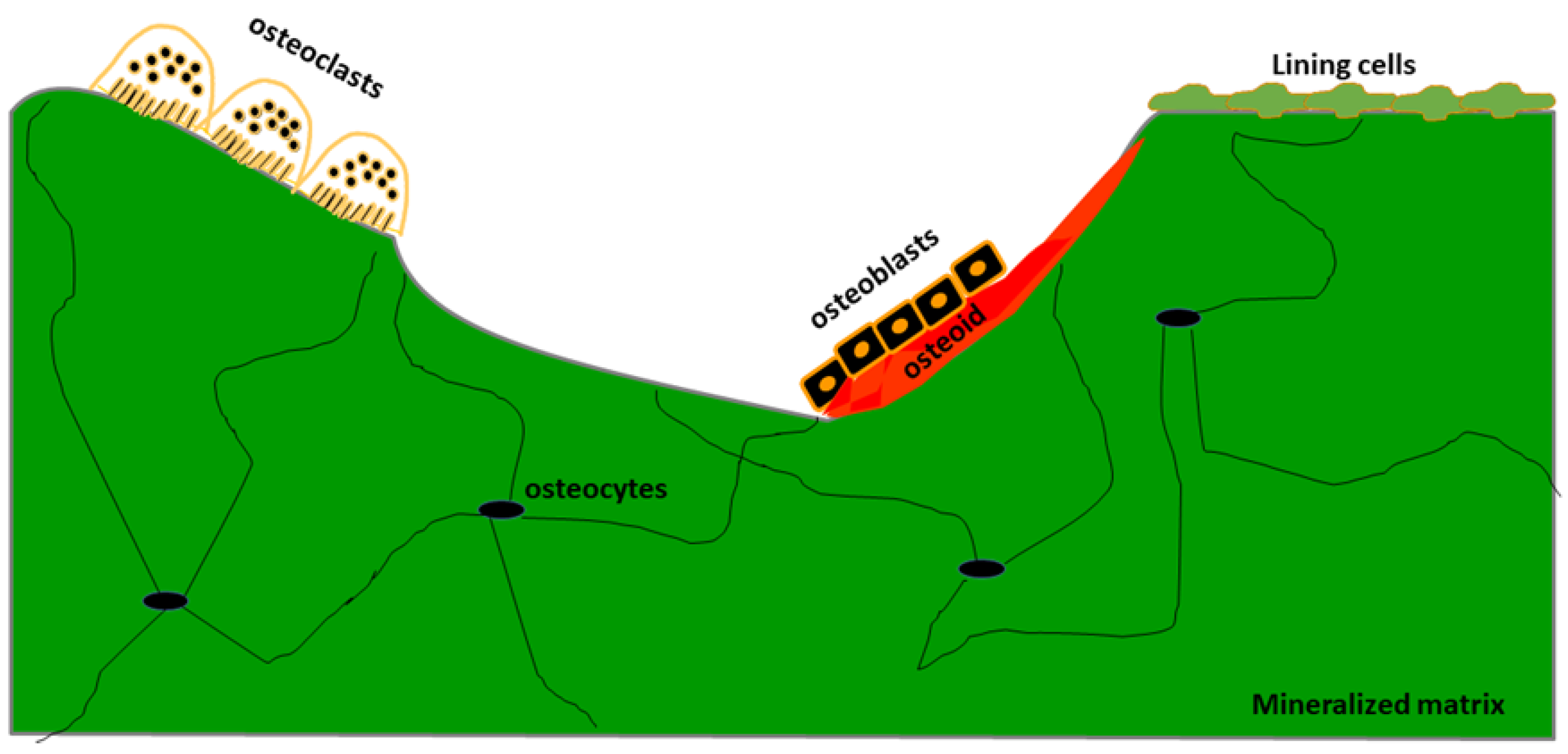
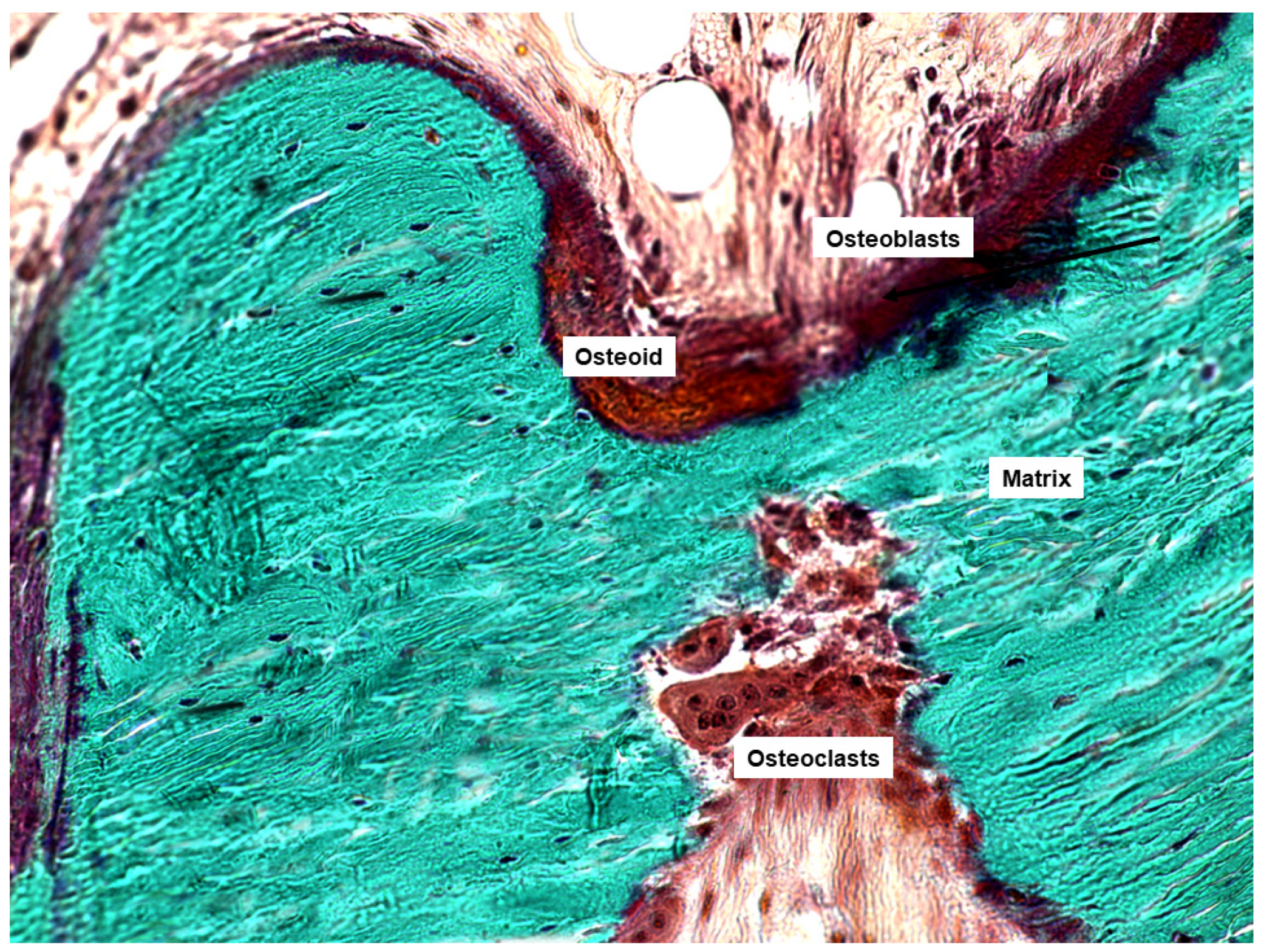
| Authors | Design of the Study | Number of Patients | Type of Bone Disease | Journal, Year |
|---|---|---|---|---|
| Aaltonen et al. | Observational | n = 26 ESRD | 61% low, 27% normal, 12% high bone turnover | Calcif Tissue Int. 2021 |
| Lavigne et al. | Observational | n = 11 CKD | 45.4% adynamic bone disease 18.1% high 18.1% mixed renal osteodystrophy 9.1% osteomalacia 9.1% not defined | J Nephrol. 2021 |
| Salam et al. | Cross-sectional | n = 43 CKD stages IV-V | 40% high, 34% normal, 26% low bone turnover | Bone, 2021 |
| Carbonara et al. | Observational | n = 260 CKD stages III-V | High bone turnover | J. Bras. Nefrol. 2020 |
| Novel-Catin et al. | Observational | n = 68, ESRD | 45% Osteitis fibrosa 21% Mixed uremic osteodystrophy 12% Adynamic bone disease 10% Osteomalacia | Bone, 2020 |
| Liangos et al. | Observational | 33, CKD stage III-V 53, on hemodialysis | High-turnover mixed uremic osteodystrophy | Kidney Blood Press Res. 2018 |
| Sharma et al. | Observational | 14, CKD stage V | 50% high, 29% normal, 21% low bone turnover | Am J Nephrol, 2018 |
| Evenepoel et al. | Observational | 36, kidney transplant | 44.4% low, 52.8% normal, 2.8% high bone turnover | Kidney Int., 2017 |
| Sprague et al. | Cross-sectional | 492, on hemodialysis | 59% low, 24% normal, 17% high bone turnover | Am J Kidney Dis., 2016 |
| Malluche et al. | Observational | 630, CKD stage V | 58% low, 24% high, 18% normal bone turnover | JBMR 2011 |
| Lehmann et al. | Observational | 36, CKD stages III-IV 92, CKD stage V | 47.2% Osteitis fibrosa 61.4% Osteitis fibrosa | Clin Nephrol, 2008 |
| Miller et al. | Observational | 6, CKD stages IV-V | 33% low, 33% high bone turnover, 33% osteomalacia | CJASN 2008 |
| Coen et al. | Observational | 79, CKD 107, on hemodialysis | 69% mixed osteodystrophy 11% adynamic bone disease 2.5% hyperparathyroidism 1% osteomalacia 57% hyperparathyroidism 28% mixed osteodystrophy 11% adynamic bone disease 2.8% osteomalacia | Nephron, 2002 |
| Gerakis et al. | Observational | 62, on hemodialysis | 64.5% hyperparathyroidism 22.6% adynamic bone disease 9.7% mixed bone disease 3.2% osteomalacia | J Nephrol., 2000 |
| Jørgensen et al. | Observational | 205, kidney transplan | 24% low, 60% normal, 16% high turnover | Bone, 2021 |
| (1) In patients with CKD G3a–G5D, it is reasonable to perform a bone biopsy if knowledge of the type of renal osteodystrophy will impact treatment decisions (evidence not graded) |
| (2) In patients with CKD G3a–G5D with biochemical abnormalities of CKD-MBD and low BMD and/or fragility fractures, we suggest that treatment choices take into account the magnitude and reversibility of the biochemical abnormalities and the progression of CKD, with consideration of a bone biopsy (evidence 2D). |
| (3) During the first 12 months after kidney transplant, in patients in with an estimated glomerular filtration rate greater than approximately 30 mL/min/1.73 m2 and a low BMD, it is reasonable to consider a bone biopsy to guide treatment (evidence not graded). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalle Carbonare, L.; Valenti, M.T.; Giannini, S.; Gallieni, M.; Stefani, F.; Ciresa, R.; Politi, C.; Fusaro, M. Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives. J. Clin. Med. 2021, 10, 4617. https://doi.org/10.3390/jcm10194617
Dalle Carbonare L, Valenti MT, Giannini S, Gallieni M, Stefani F, Ciresa R, Politi C, Fusaro M. Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives. Journal of Clinical Medicine. 2021; 10(19):4617. https://doi.org/10.3390/jcm10194617
Chicago/Turabian StyleDalle Carbonare, Luca, Maria Teresa Valenti, Sandro Giannini, Maurizio Gallieni, Francesca Stefani, Roberto Ciresa, Cristina Politi, and Maria Fusaro. 2021. "Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives" Journal of Clinical Medicine 10, no. 19: 4617. https://doi.org/10.3390/jcm10194617
APA StyleDalle Carbonare, L., Valenti, M. T., Giannini, S., Gallieni, M., Stefani, F., Ciresa, R., Politi, C., & Fusaro, M. (2021). Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives. Journal of Clinical Medicine, 10(19), 4617. https://doi.org/10.3390/jcm10194617









