B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. EGPA Characteristics
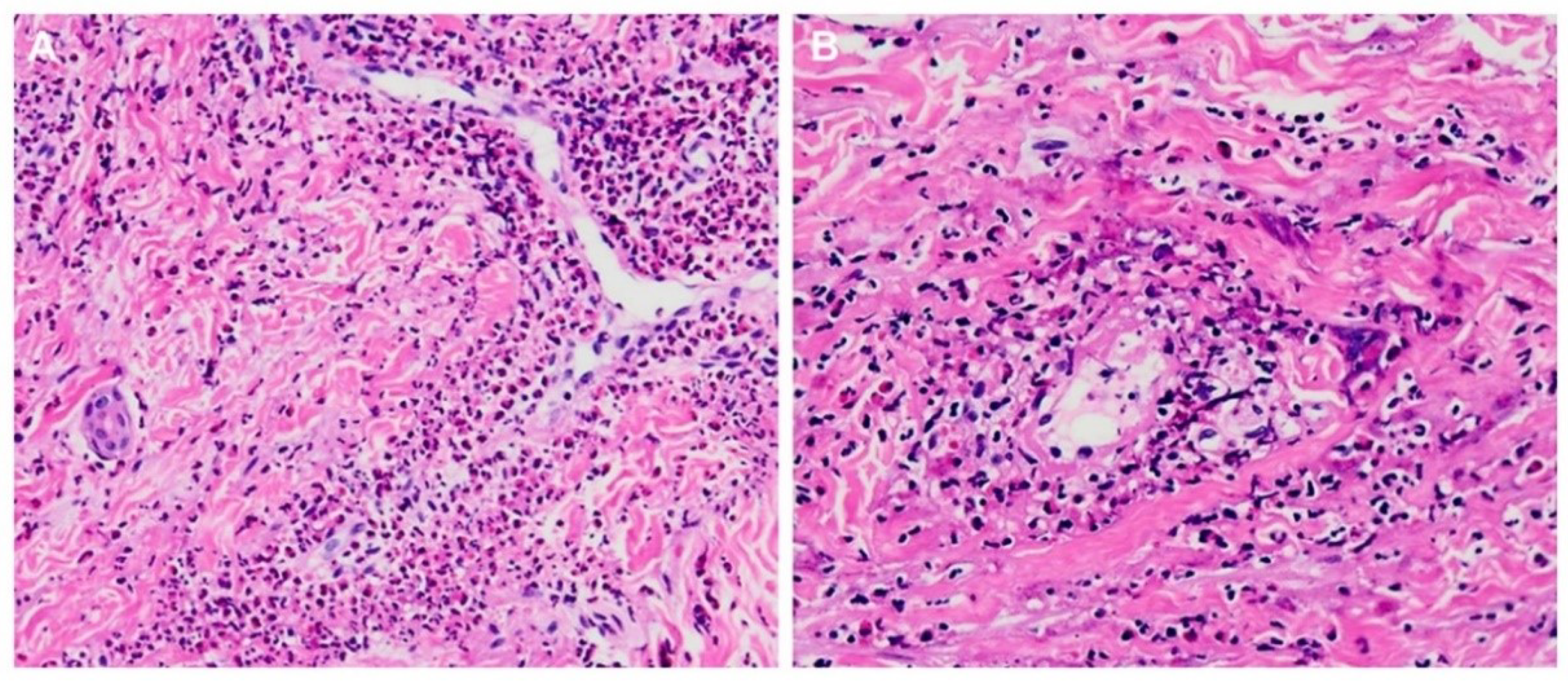
3.2. Myocarditis Characteristics
3.3. RTX Treatment
3.4. Therapeutic Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almaani, S.; Fussner, L.A.; Brodsky, S.; Meara, A.S.; Jayne, D. ANCA-associated vasculitis: An update. J. Clin. Med. 2021, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W. Vasculitis: From target molecules to novel therapeutic approaches. Biomedicines 2021, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, D.C. Cardiac involvement in systemic inflammatory diseases. Eur. Heart J. 2007, 2, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological impact of myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L. French Vasculitis Study Group (FVSG): The Five-factor score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef]
- Wu, E.Y.; Hernandez, M.L.; Jennette, J.C.; Falk, R.J. Eosinophilic granulomatosis with polyangiitis: Clinical pathology conference and review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1496–1504. [Google Scholar] [CrossRef]
- Gioffredi, A.; Maritati, F.; Oliva, E.; Buzio, C. Eosinophilic granulomatosis with polyangiitis: An overview. Front. Immunol. 2014, 5, 549. [Google Scholar] [CrossRef] [Green Version]
- Trivioli, G.; Terrier, B.; Vaglio, A. Eosinophilic granulomatosis with polyangiitis: Understanding the disease and its management. Rheumatology 2020, 59 (Suppl. 3), iii84–iii94. [Google Scholar] [CrossRef]
- Khoury, P.; Grayson, P.C.; Klion, A.D. Eosinophils in vasculitis: Characteristics and roles in pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Cusack, R.P.; Whetstone, C.E.; Xie, Y.; Ranjbar, M.; Gauvreau, G.M. Regulation of eosinophilia in asthma-new therapeutic approaches for asthma treatment. Cells 2021, 10, 817. [Google Scholar] [CrossRef]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021, 73, 1366–1383. [Google Scholar] [CrossRef]
- Canzian, A.; Venhoff, N.; Urban, M.L.; Sartorelli, S.; Ruppert, A.M.; Groh, M.; Girszyn, N.; Taillé, C.; Maurier, F.; Cottin, V.; et al. Use of biologics to treat relapsing and/or refractory eosinophilic granulomatosis with polyangiitis: Data from a european collaborative study. Arthritis Rheumatol. 2021, 73, 498–503. [Google Scholar] [CrossRef]
- Groh, M.; Pagnoux, C.; Baldini, C.; Bel, E.; Bottero, P.; Cottin, V.; Dalhoff, K.; Dunogué, B.; Gross, W.; Holle, J.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur. J. Intern. Med. 2015, 26, 545–553. [Google Scholar] [CrossRef]
- Isozaki, T.; Homma, T.; Sagara, H.; Kasama, T. Role of cytokines in EGPA and the possibility of treatment with an anti-IL-5 antibody. J. Clin. Med. 2020, 9, 3890. [Google Scholar] [CrossRef]
- Taimeh, Z.; Tang, W.H.W. New Advances and ongoing challenges in the use of biologic agents in cardiac sarcoidosis and other Inflammatory cardiomyopathies. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 39. [Google Scholar] [CrossRef]
- Wang, C.R.; Tsai, Y.S.; Tsai, H.W. Acute myocarditis in anti-neutrophil cytoplasmic antibody-positive microscopic polyangiitis patients receiving the rituximab therapy. J. Rheumatol. 2019, 46, 1645–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.C.; Constantine, M.; Ahmadi, A.; Shiau, C.; Chen, L.Y.C. Eosinophilic myocarditis. Am. J. Med. Sci. 2017, 354, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.T.; Hunder, G.G.; Lie, J.T.; Michel, B.A.; Bloch, D.A.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990, 33, 1094–1100. [Google Scholar] [CrossRef]
- Wang, C.R.; Tsai, Y.S.; Li, W.T. Lupus myocarditis receiving the rituximab therapy-a monocentric retrospective study. Clin. Rheumatol. 2018, 37, 1701–1707. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, A.J.; Hot, A.; Arndt, F.; Moosig, F.; Guerry, M.J.; Amudala, N.; Smith, R.; Sivasothy, P.; Guillevin, L.; Merkel, P.A.; et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 2016, 75, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, H.; Chatterjee, S.; Langford, C.A. Eosinophilia in rheumatologic/vascular disorders. Immunol. Allergy Clin. N. Am. 2015, 35, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Neumann, T.; Manger, B.; Schmid, M.; Kroegel, C.; Hansch, A.; Kaiser, W.A.; Reinhardt, D.; Wolf, G.; Hein, G.; Mall, G.; et al. Cardiac involvement in Churg-Strauss syndrome: Impact of endomyocarditis. Medicine 2009, 88, 236–243. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronbichler, A.; Jayne, D.R.; Mayer, G. Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis. Eur. J. Clin. Investig. 2015, 45, 346–368. [Google Scholar] [CrossRef]
- Moosig, F.; Bremer, J.P.; Hellmich, B.; Holle, J.U.; Holl-Ulrich, K.; Laudien, M.; Matthis, C.; Metzler, C.; Nölle, B.; Richardt, G.; et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): Monocentric experiences in 150 patients. Ann. Rheum. Dis. 2013, 72, 1011–1017. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zhou, J.; Li, J.; Wu, Q.; Yang, H.; Zhang, S.; Fei, Y.; Zhang, W.; Zhao, Y.; et al. Cardiac involvement in eosinophilic granulomatosis with polyangiitis: A retrospective study in the Chinese population. Front. Med. 2020, 7, 583944. [Google Scholar] [CrossRef] [PubMed]
- Thiel, J.; Troilo, A.; Salzer, U.; Schleyer, T.; Halmschlag, K.; Rizzi, M.; Frede, N.; Venhoff, A.; Voll, R.E.; Venhoff, N. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: A 36-month follow-up analysis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1556–1563. [Google Scholar] [CrossRef]
- Theis, D.; Langford, C.A.; Hoffman, G.S.; Villa-Forte, A. Long-term use of rituximab for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. 2015, 67 (Suppl. 10), 891. [Google Scholar]
- Menditto, V.G.; Rossetti, G.; Olivari, D.; Angeletti, A.; Rocchi, M.; Gabrielli, A.; Pomponio, G. Rituximab for eosinophilic granulomatosis with polyangiitis: A systematic review of observational studies. Rheumatology 2021, 60, 1640–1650. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detoraki, A.; Tremante, E.; Poto, R.; Morelli, E.; Quaremba, G.; Granata, F.; Romano, A.; Mormile, I.; Rossi, F.W.; de Paulis, A.; et al. Real-life evidence of low-dose mepolizumab efficacy in EGPA: A case series. Respir. Res. 2021, 22, 185. [Google Scholar] [CrossRef]
- Faverio, P.; Bonaiti, G.; Bini, F.; Vaghi, A.; Pesci, A. Mepolizumab as the first targeted treatment for eosinophilic granulomatosis with polyangiitis: A review of current evidence and potential place in therapy. Ther. Clin. Risk. Manag. 2018, 14, 2385–2396. [Google Scholar] [CrossRef] [Green Version]
- Vultaggio, A.; Nencini, F.; Bormioli, S.; Vivarelli, E.; Dies, L.; Rossi, O.; Parronchi, P.; Maggi, E.; Matucci, A. Low-Dose mepolizumab effectiveness in patients suffering from eosinophilic granulomatosis with polyangiitis. Allergy Asthma Immunol. Res. 2020, 12, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Shenoy, S.N. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol. Int. 2017, 37, 151–167. [Google Scholar] [CrossRef]
- Jones, R.B.; Ferraro, A.J.; Chaudhry, A.N.; Brogan, P.; Salama, A.D.; Smith, K.G.; Savage, C.O.; Jayne, D.R. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009, 60, 2156–2168. [Google Scholar] [CrossRef]
- Vaglio, A.; Strehl, J.D.; Manger, B.; Maritati, F.; Alberici, F.; Beyer, C.; Rech, J.; Sinico, R.A.; Bonatti, F.; Battistelli, L.; et al. IgG4 immune response in Churg-Strauss syndrome. Ann. Rheum. Dis. 2012, 71, 390–393. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Tsurikisawa, N.; Tsuburai, T.; Oshikata, C.; Akiyama, K. Cytokine production profile of CD4+ T cells from patients with active Churg-Strauss syndrome tends toward Th17. Int. Arch. Allergy Immunol. 2009, 149 (Suppl. 1), 61–65. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Lauwerys, B.; Marijnissen, R.J.; Timmermans, K.; Di Padova, F.; Koenders, M.I.; Gutierrez-Roelens, I.; Durez, P.; Netea, M.G.; van der Meer, J.W.; et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011, 63, 1507–1516. [Google Scholar] [CrossRef]
- Khosroshahi, A.; Bloch, D.B.; Deshpande, V.; Stone, J.H. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010, 62, 1755–1762. [Google Scholar] [CrossRef]
- Miloslavsky, E.; Unizony, S. The heart in vasculitis. Rheum. Dis. Clin. N. Am. 2014, 40, 11–26. [Google Scholar] [CrossRef]
- Ogbogu, P.U.; Rosing, D.R.; Horne, M.K., 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol. Allergy Clin. N. Am. 2007, 27, 457–475. [Google Scholar] [CrossRef] [Green Version]
- Colantuono, S.; Pellicano, C.; Leodori, G.; Cilia, F.; Francone, M.; Visentini, M. Early benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Allergol. Int. 2020, 69, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Jones, D.M.; Homsi, Y. Therapeutic effect of anti-IL-5 on eosinophilic myocarditis with large pericardial effusion. BMJ Case Rep. 2017, 2017, bcr-2016-218992. [Google Scholar] [CrossRef]
- Pepper, R.J.; Fabre, M.A.; Pavesio, C.; Gaskin, G.; Jones, R.B.; Jayne, D.; Pusey, C.D.; Salama, A.D. Rituximab is effective in the treatment of refractory Churg-Strauss syndrome and is associated with diminished T-cell interleukin-5 production. Rheumatology 2008, 47, 1104–1105. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.; Hösli, S.; Kostylina, G.; Yawalkar, N.; Simon, H.U. Anti-CD20 (rituximab) treatment improves atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sedivá, A.; Kayserová, J.; Vernerová, E.; Poloucková, A.; Capková, S.; Spísek, R.; Bartůnková, J. Anti-CD20 (rituximab) treatment for atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 1515–1516. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.; Veraitch, O.; Szydlo, R.; Charles, P.; Kelleher, P.; Gazzard, B.; Nelson, M.; Stebbing, J. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood 2009, 113, 4521–4524. [Google Scholar] [CrossRef] [Green Version]


| No. | Age @ Sex | Fever @ | Skin | Sinus | Joint | Mus | Lung | Heart | GI | Renal | PNS | CNS | FFS @ | BVAS @ | Pathology Findings | CRP mg/L @ | Eosin /μL @ | ANCA Status @ | ACR Item @ | Medication Profile | Final Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS/CYC/AZA/MTX/RTX | |||||||||||||||||||||
| 1 | 45 M | Yes | Yes | Yes | Nil | Nil | Yes | Yes | Yes | Nil | Yes | Nil | 1 | 31 | Eosinophilia Vasculitis | 51.1 | 6090 | Negative | 6 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 2 | 30 M | Nil | Yes | Yes | Nil | Yes | Yes | Yes | Yes | Yes | Yes | Nil | 2 | 36 | Eosinophilia Vasculitis | 92.6 | 11,567 | Negative | 6 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 3 | 45 F | Nil | Yes | Nil | Yes | Yes | Yes | Yes | Nil | Yes | Yes | Nil | 2 | 29 | Eosinophilia Vasculitis | 46.5 | 16,947 | Negative | 5 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 4 | 36 M | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Nil | Yes | Nil | 2 | 37 | Eosinophilia Vasculitis | 171.9 | 17,424 | Positive, anti-MPO | 6 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 5 | 47 F | Yes | Nil | Yes | Nil | Nil | Yes | Yes | Nil | Nil | Yes | Nil | 1 | 30 | Eosinophilia | 183.3 | 26,781 | Negative | 6 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 6 | 55 F | Yes | Yes | Nil | Yes | Nil | Yes | Yes | Nil | Nil | Yes | Nil | 1 | 28 | Eosinophilia Vasculitis | 165.1 | 5806 | Negative | 5 | Yes/Yes/Yes/Nil/Yes | Survival, remission |
| 7 | 39 F | Nil | Yes | Nil | Nil | Nil | Yes | Yes | Nil | Nil | Yes | Nil | 1 | 20 | Eosinophilia | 39.8 | 10,140 | Positive, anti-MPO | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 8 | 40 M | Yes | Yes | Yes | Nil | Nil | Yes | Yes | Nil | Nil | Yes | Nil | 1 | 24 | Eosinophilia Vasculitis | 47.5 | 23,392 | Negative | 6 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 9 | 37 F | Nil | Yes | Yes | Yes | Nil | Yes | Yes | Nil | Nil | Nil | Nil | 1 | 19 | Eosinophilia | 42.5 | 11,139 | Positive, anti-MPO | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 10 | 20 F | Yes | Yes | Nil | Nil | Yes | Yes | Yes | Nil | Nil | Yes | Yes | 2 | 26 | Eosinophilia Vasculitis | 98.8 | 8019 | Negative | 5 | Yes/Yes/Yes/Nil/Nil | Death due to activity |
| 11 | 47 M | Yes | Nil | Nil | Nil | Nil | Yes | Nil | Nil | Yes | Yes | Nil | 1 | 30 | Eosinophilia | 54.8 | 9750 | Positive, anti-MPO | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 12 | 29 F | Yes | Yes | Nil | Nil | Nil | Yes | Nil | Nil | Nil | Yes | Nil | 1 | 21 | Eosinophilia Vasculitis | 80.7 | 12,816 | Negative | 5 | Yes/Nil/Yes/Yes/Nil | Survival, remission |
| 13 | 66 M | Nil | Nil | Yes | Yes | Nil | Yes | Nil | Nil | Nil | Yes | Nil | 1 | 20 | Eosinophilia | 76.3 | 2541 | Negative | 6 | Yes/Nil/Yes/Nil/Nil | Survival, remission |
| 14 | 70 M | Nil | Yes | Yes | Nil | Nil | Nil | Nil | Nil | Nil | Yes | Nil | 1 | 21 | Eosinophilia | 44.8 | 3744 | Negative | 5 | Yes/Nil/Yes/Nil/Nil | Survival, remission |
| 15 | 32 M | Yes | Yes | Nil | Yes | Yes | Yes | Nil | Nil | Yes | Yes | Nil | 2 | 39 | Eosinophilia Vasculitis | 80.1 | 2314 | Positive, anti-MPO | 5 | Yes/Yes/Yes/Nil/Nil | Death due to activity |
| 16 | 56 F | Nil | Nil | Yes | Yes | Nil | Yes | Nil | Yes | Nil | Yes | Nil | 1 | 25 | Eosinophilia | 70.0 | 13,029 | Negative | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 17 | 49 F | Yes | Yes | Yes | Nil | Nil | Yes | Nil | Yes | Nil | Nil | Nil | 1 | 21 | Eosinophilia | 32.1 | 4429 | Negative | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 18 | 54 M | Yes | Nil | Yes | Nil | Nil | Yes | Nil | Nil | Yes | Yes | Yes | 1 | 32 | Eosinophilia | 61.9 | 8775 | Positive, anti-MPO | 6 | Yes/Yes/Yes/Nil/Nil | Death due to infection |
| 19 | 38 M | Nil | Nil | Yes | Yes | Nil | Yes | Nil | Yes | Nil | Yes | Nil | 1 | 27 | Eosinophilia | 62.1 | 7696 | Negative | 6 | Yes/Yes/Yes/Yes/Nil | Survival, remission |
| 20 | 41 M | Nil | Yes | Nil | Yes | Yes | Yes | Nil | Nil | Nil | Yes | Nil | 1 | 23 | Eosinophilia | 29.4 | 3952 | Positive, anti-MPO | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| 21 | 55 M | Nil | Yes | Nil | Yes | Nil | Yes | Nil | Nil | Nil | Yes | Nil | 1 | 18 | Eosinophilia | 32.1 | 4356 | Negative | 5 | Yes/Yes/Yes/Nil/Nil | Survival, remission |
| Stat # | F 43% 44 ± 12 | 52% | 71% | 57% | 48% | 29% | 95% | 48% | 29% | 24% | 95% | 10% | 1.3 ± 0.4 | 27 ± 6 | Vasculitis 43% | 74.9 ± 45.5 | 10,034 ± 6641 | Negative 67% | 5.4 ± 0.5 | 100%/86%/100%/10%/29% | Survival 86% |
| No. | Age @ Sex | FFS @/ BVAS @ | ANCA Status before/after | Myocarditis Type/Onset | NYHAFC, Rhythm before/after | Biomarker before/after | Impaired LVEF before/after | Cardiac Therapeutics before/after | Disease Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 M | 2/23 | Negative/ Negative | Myocarditis/ Disease relapse | II/I SB, SP/NSR | Elevated/ Normalized | Mildly/ Normalized | Combined CSA/ Nil | Complete remission |
| 2 | 31 M | 3/29 | Negative/ Negative | Myocarditis/ Disease relapse | III/I ST, PVC/NSR | Elevated/ Normalized | Moderately/ Normalized | Combined CSA, ART/ low-dose ARB | Complete remission |
| 3 | 46 F | 2/20 | Negative/ Negative | Endomyocarditis Myopericarditis/ Disease onset | III/I ST, PVC/NSR | Elevated/ Normalized | Moderately/ Normalized | Combined CSA, ART/ low-dose ACEI | Complete remission |
| 4 | 36 M | 2/15 | Positive/ Negative | Endomyocarditis Myopericarditis/ Disease onset | III/I ST/NSR | Elevated/ Normalized | Moderately/ Normalized | Combined CSA/ low-dose ACEI | Partial remission |
| 5 | 48 F | 1/21 | Negative/ Negative | Myocarditis/ Disease onset | II/I ST/NSR | Elevated/ Normalized | Mildly/ Normalized | Combined CSA/ Nil | Partial remission |
| 6 | 56 F | 2/20 | Negative/ Negative | Endomyocarditis/ Disease relapse | III/II ST/NSR | Elevated/ Normalized # | Moderately/ Normalized | Combined CSA /ACEI | Partial remission |
| 7 | 39 F | 2/13 | Positive/ Negative | Endomyocarditis/ Disease onset | II/I ST, PAC, PAT/NSR | Elevated/ Normalized | Mildly/ Normalized | Combined CSA, ART /ACEI | Complete remission |
| 8 | 40 M | 1/24 | Negative/ Negative | Myopericarditis/ Disease onset | II/I ST/NSR | Elevated/ Normalized # | Mildly/ Normalized | Combined CSA/ Combined CSA | Partial remission |
| 9 | 37 F | 1/19 | Positive/ Negative | Myocarditis/ Disease onset | II/I ST/NSR | Elevated/ Normalized # | Mildly/ Normalized | Combined CSA/ ARB | Partial remission |
| 10 | 20 F | 2/26 | Negative/ Negative | Myopericarditis/ Disease onset | III/IV ST, PAC, PVC/ST, PVC | Elevated/ Elevated | Moderately/ Severely | Combined CSA, ART/ Combined CSA, ART | Death due to heart failure |
| No. | Age Sex /FFS | ANCA before/ after RTX | Biologics Indication | Biologics Regimen (Course) | @ B Cell (/μL) Diff | @ BVAS Diff | @ Eosinophil (/μL) Diff (Inh %) | @ CRP (mg/L) Diff | Side Effects | # Follow Up Time | Biologics Therapeutic Response | CS and IS before/after Biologics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 M 2 | Negative/ negative | Induction for disease relapse /maintenance | 375 mg/m2 weekly × 4 RTX (4) | 35 to 0 | 23 to 0 | 1170 to 149 (87.3%) | 24.0 to 1.8 | Low IgM | 67 m | Complete remission | AZ, CS, CYC /AZ, + low-dose CS |
| 2 | 31 M 3 | Negative/ negative | Induction for disease relapse /maintenance | 375 mg/m2 weekly × 4 RTX (3) | 103 to 0 | 29 to 0 | 826 to 95 (88.5%) | 8.0 to 2.2 | Low IgG/M | 56 m | Complete remission | AZ, CS, CYC /+ low-dose CS |
| 3 | 46 F 2 | Negative/ negative | Induction for refractory /maintenance | 375 mg/m2 weekly × 4 RTX (3) | 71 to 0 | 20 to 0 | 882 to 159 (82.0%) | 23.0 to 1.1 | Nil | 53 m | Complete remission | CS, CYC /AZ, + low-dose CS |
| 4 | 36 M 2 | Positive/ negative Negative/ ND | Induction for refractory /maintenance Induction for disease relapse | 375 mg/m2 weekly × 4 RTX (3), 100 mg quadri-weekly × 4 MEP | ND to 0 104 to ND | 15 to 4 9 to 3 | 1206 to 322 (73.3%) 1,493 to 164 (89.0%) | 17.9 to 3.2 15.6 to 1.9 | Nil Nil | 42 m 4 m | Partial remission after RTX, relapse at 43th m Partial remission after MEP | CS, CYC /AZ, + low-dose CS CS /+ low-dose CS |
| 5 | 48 F 1 | Negative/ negative | Induction for refractory /maintenance | 375 mg/m2 weekly × 4 RTX (2) | 159 to 0 | 21 to 3 | 755 to 172 (77.2%) | 51.6 to 1.5 | Low IgM | 44 m | Partial remission | CS, CYC /+ low-dose CS |
| 6 | 56 F 2 | Negative/ Negative Negative/ negative | Induction for disease relapse Induction for disease relapse | 375 mg/m2 weekly × 4 RTX (1), 100 mg quadri-weekly × 6 MEP | 316 to 0 340 to 329 | 20 to 6 11 to 4 | 1006 to 437 (56.6%) 953 to 92 (90.3%) | 35.4 to 6.4 11.5 to 1.6 | Infusion reaction 1st dose Nil | 24 m 6 m | Partial remission after RTX, relapse at 25th m Partial remission after MEP | AZ, CS, CYC /AZ, + low-dose CS CS /+ low-dose CS |
| No. | Involved Cardiac Area | Symptoms, NYHAFC before/after | Rhythm before/ after | Biomarkers before/after | Image Findings of cMRI and TTE before (@ During) RTX Therapy | Image Findings of cMRI and ECG after RTX Therapy |
|---|---|---|---|---|---|---|
| 1 | Myocardium | Dyspnea /Nil II/I | SB with SP /NSR | Elevated /Normalized | Dilated LV Mildly impaired LVEF Myocardial edema Multifocal mid-wall DGE at basal LV, LV mid-cavity IVS | Normalized LV size Normalized LVEF Resolved myocardial edema Reduced mid-wall DGE |
| 2 | Myocardium | Dyspnea, orthopnea, palpitation /Nil III/I | ST with PVC /NSR | Elevated /Normalized | Dilated LV with global hypokinesia Moderately impaired LVEF Myocardial edema Patchy mid-wall DGE at LV mid-cavity IVS and inferolateral wall, apical LV anterolateral wall | Normalized LV size and motion Normalized LVEF Resolved myocardial edema Reduced mid-wall DGE |
| 3 | Endocardium, myocardium, pericardium | Dyspnea, orthopnea, chest pain /Nil III/I | ST with PVC /NSR | Elevated /Normalized | LV global hypokinesia Moderately impaired LVEF Pericardial effusion Myocardial edema Curvilinear mid-wall DGE at global LV Diffuse endocardial DGE at global LV | Normalized LV motion Normalized LVEF Resolved pericardial effusion Resolved myocardial edema Reduced mid-wall DGE Reduced endocardial DGE |
| 4 | Endocardium, myocardium, pericardium | Dyspnea, orthopnea, chest pain /Nil III/I | ST /NSR | Elevated /Normalized | LV global hypokinesia Moderately impaired LVEF Pericardial effusion @ Resolved myocardial edema @ Spotty mid-wall DGE at basal LV inferolateral wall @ Endocardial DGE at basal LV, LV mid-cavity | Normalized LV motion Normalized LVEF Resolved pericardial effusion Resolved myocardial edema Reduced mid-wall DGE Reduced endocardial DGE |
| 5 | Myocardium | Dyspnea /Nil II/I | ST /NSR | Elevated /Normalized | Dilated LV Mildly impaired LVEF Myocardial edema Curvilinear mid-wall DGE at basal LV anteroseptal wall, spotty mid-wall DGE at LV mid-cavity antero-lateral wall | Normalized LV size Normalized LVEF Resolved myocardial edema Reduced mid-wall DGE |
| 6 | Endocardium, myocardium | Dyspnea, orthopnea, palpitation /Dyspnea III/II | ST /NSR | Elevated /# Normalized | LV global hypokinesia Moderately impaired LVEF Myocardial edema Curvilinear mid-wall DGE at basal LV, LV mid-cavity Endocardial DGE at LV mid-cavity | Normalized LV motion Normalized LVEF Resolved myocardial edema Reduced mid-wall DGE Unreduced endocardial DGE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-R.; Tsai, Y.-S.; Tsai, H.-W.; Lee, C.-H. B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis. J. Clin. Med. 2021, 10, 4577. https://doi.org/10.3390/jcm10194577
Wang C-R, Tsai Y-S, Tsai H-W, Lee C-H. B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis. Journal of Clinical Medicine. 2021; 10(19):4577. https://doi.org/10.3390/jcm10194577
Chicago/Turabian StyleWang, Chrong-Reen, Yi-Shan Tsai, Hung-Wen Tsai, and Cheng-Han Lee. 2021. "B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis" Journal of Clinical Medicine 10, no. 19: 4577. https://doi.org/10.3390/jcm10194577
APA StyleWang, C.-R., Tsai, Y.-S., Tsai, H.-W., & Lee, C.-H. (2021). B-Cell-Depleting Therapy Improves Myocarditis in Seronegative Eosinophilic Granulomatosis with Polyangiitis. Journal of Clinical Medicine, 10(19), 4577. https://doi.org/10.3390/jcm10194577







