Biomarker Value in the Diagnosis of Community-Acquired Pneumonia with Concomitant Chronic Heart Failure
Abstract
1. Introduction
2. Materials and Methods
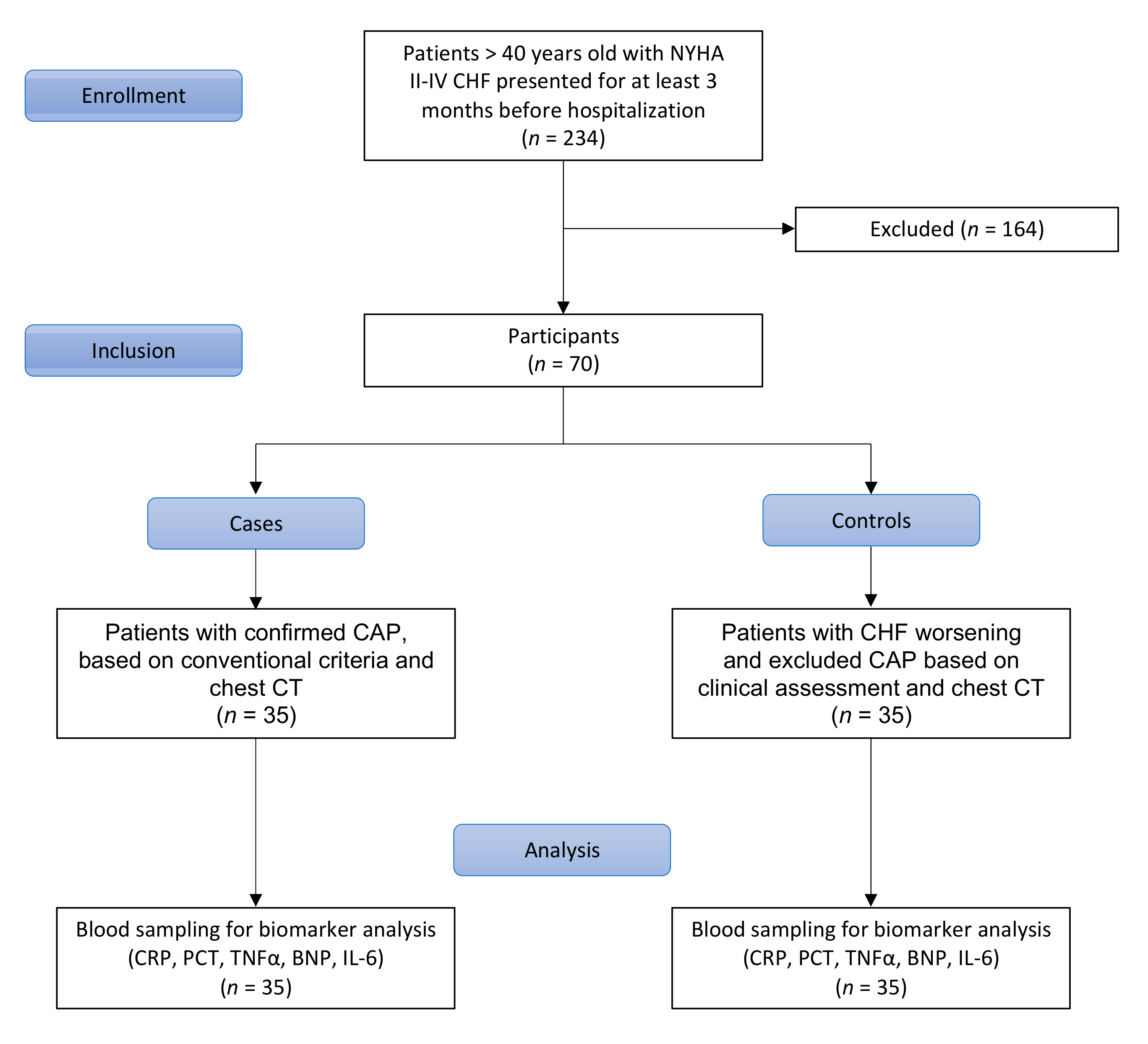
2.1. Study Design
2.2. Classification
2.3. Blood Sampling
2.4. Biomarker Analysis
2.5. Biomarker Reference
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
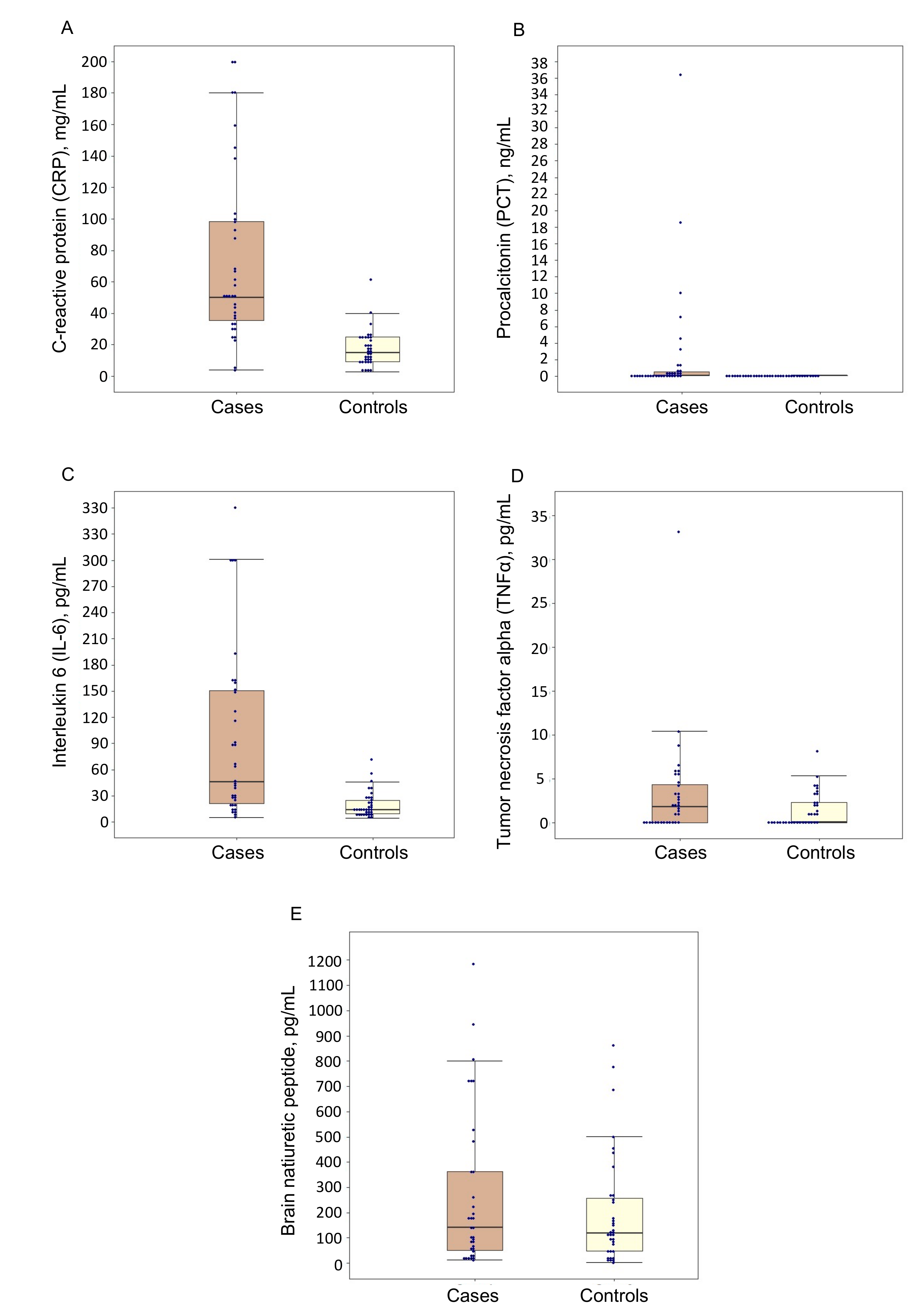
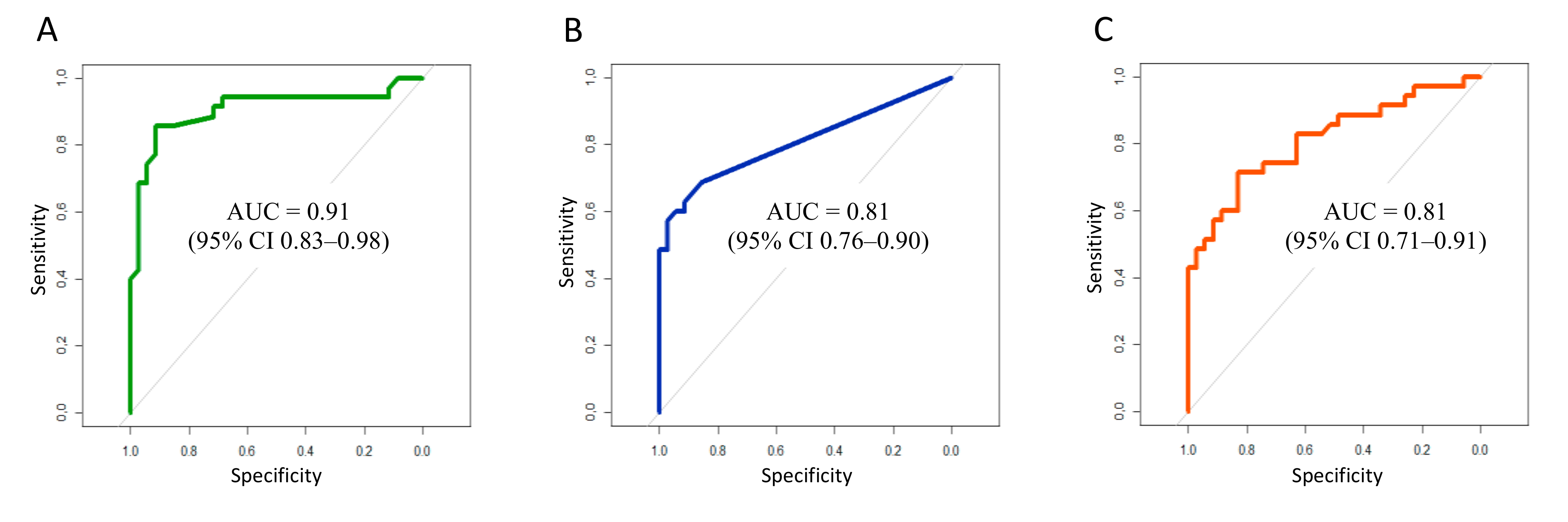
3.2. Biomarker Levels at Study Time Point
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broulette, J.; Yu, H.; Pyenson, B.; Iwasaki, K.; Sato, R. The Incidence Rate and Economic Burden of Community-Acquired Pneumonia in a Working-Age Population. Am. Health Drug Benefits 2013, 6, 494–503. [Google Scholar]
- Park, H.; Adeyemi, A.O.; Rascati, K.L. Direct Medical Costs and Utilization of Health Care Services to Treat Pneumonia in the United States: An Analysis of the 2007–2011 Medical Expenditure Panel Survey. Clin. Ther. 2015, 37, 1466–1476.e1. [Google Scholar] [CrossRef]
- Adamantia, L.; Sotiria, M.; Myrsini, M.; Michael, T. Managing CAP in the ICU. Curr. Infect. Dis. Rep. 2015, 17, 48. [Google Scholar] [CrossRef]
- Bartlett, J.G. Diagnostic Tests for Agents of Community-Acquired Pneumonia. Clin. Infect. Dis. 2011, 52, S296–S304. [Google Scholar] [CrossRef]
- Gadsby, N.J.; Russell, C.; McHugh, M.P.; Mark, H.; Morris, A.C.; Laurenson, I.F.; Hill, A.T.; Templeton, K. Comprehensive Molecular Testing for Respiratory Pathogens in Community-Acquired Pneumonia. Clin. Infect. Dis. 2016, 62, 817–823. [Google Scholar] [CrossRef]
- Cha, Y.S.; Lee, K.H.; Lee, J.W.; Kwon, W.; Lee, S.J.; Kang, K.S.; Kim, H.I.; Kim, O.H.; Cha, K.C.; Kim, H.; et al. The Usefulness of the Delta Neutrophil Index for Predicting Superimposed Pneumonia in Patients with Acute Decompensated Heart Failure in the Emergency Department. PLoS ONE 2016, 11, e0163461. [Google Scholar] [CrossRef]
- Maisel, A.; Neath, S.-X.; Landsberg, J.; Mueller, C.; Nowak, R.M.; Peacock, W.F.; Ponikowski, P.; Möckel, M.; Hogan, C.; Wu, A.H.; et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: Results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 278–286. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Garin, N.; Marti, C.; Scheffler, M.; Stirnemann, J.; Prendki, V. Computed tomography scan contribution to the diagnosis of community-acquired pneumonia. Curr. Opin. Pulm. Med. 2019, 25, 242–248. [Google Scholar] [CrossRef]
- Esayag, Y.; Nikitin, I.; Bar-Ziv, J.; Cytter, R.; Hadas-Halpern, I.; Zalut, T.; Yinnon, A.M. Diagnostic Value of Chest Radiographs in Bedridden Patients Suspected of Having Pneumonia. Am. J. Med. 2010, 123, 88.e1–88.e5. [Google Scholar] [CrossRef]
- Metlay, J.P.; Kapoor, W.N.; Fine, M.J. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997, 278, 1440–1445. [Google Scholar] [CrossRef]
- Kanwar, M.; Brar, N.; Khatib, R.; Fakih, M.G. Misdiagnosis of Community-Acquired Pneumonia and Inappropriate Utilization of Antibiotics. Chest 2007, 131, 1865–1869. [Google Scholar] [CrossRef]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64, iii1–iii55. [Google Scholar] [CrossRef]
- Brown, J.S. Community-acquired pneumonia. Clin. Med. 2012, 12, 538–543. [Google Scholar] [CrossRef]
- Siljan, W.W.; Holter, J.C.; Michelsen, A.E.; Nymo, S.H.; Lauritzen, T.; Oppen, K.; Husebye, E.; Ueland, T.; Mollnes, T.E.; Aukrust, P.; et al. Inflammatory biomarkers are associated with aetiology and predict outcomes in community-acquired pneumonia: Results of a 5-year follow-up cohort study. ERJ Open Res. 2019, 5, 00014-2019. [Google Scholar] [CrossRef]
- Joffe, E.; Justo, D.; Mashav, N.; Swartzon, M.; Gur, H.; Berliner, S.; Paran, Y. C-reactive protein to distinguish pneumonia from acute decompensated heart failure. Clin. Biochem. 2009, 42, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Leitner, I.; Egger, M.; Haltmayer, M.; Dieplinger, B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin. Chim. Acta 2015, 445, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Ge, N.; Liu, J.; Yuan, H.; Zhang, P.; Liu, W.; Wen, D. Procalcitonin testing for diagnosis and short-term prognosis in bacterial infection complicated by congestive heart failure: A multicenter analysis of 4698 cases. Crit. Care 2014, 18, R4. [Google Scholar] [CrossRef] [PubMed]
- Sinopal’nikov, A.I. Community-acquired pneumonia in adults: Approaches to antibacterial therapy in the context of cur-rent clinical guidelines. Ter. Arkh. 2010, 82, 5–10. [Google Scholar]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef]
- Komiya, K.; Akaba, T.; Kozaki, Y.; Kadota, J.-I.; Rubin, B.K. A systematic review of diagnostic methods to differentiate acute lung injury/acute respiratory distress syndrome from cardiogenic pulmonary edema. Crit. Care 2017, 21, 228. [Google Scholar] [CrossRef]
- Bobylev, A.A.; Rachina, S.; Avdeev, S.N.; Kozlov, R.S.; Mladov, V.V. C-reactive protein evaluation in community-acquired pneumonia with comorbid chronic heart failure as criterion of antibiotic prescription. Kardiologiia 2019, 59, 40–46. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.; Park, Y.S.; Lee, S.-M.; Yim, J.J.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Lee, C.H. Predictors of Cardiogenic and Non-Cardiogenic Causes in Cases with Bilateral Chest Infiltrates. Tuberc. Respir. Dis. 2013, 74, 15–22. [Google Scholar] [CrossRef][Green Version]
- Zalacain, R.; Torres, A.; Celis, R.; Blanquer, J.; Aspa, J.; Esteban, L.; Menéndez, R.; Blanquer, R.; Borderías, L. Community-acquired pneumonia in the elderly: Spanish multicentre study. Eur. Respir. J. 2003, 21, 294–302. [Google Scholar] [CrossRef]
- Flanders, S.A.; Stein, J.; Shochat, G.; Sellers, K.; Holland, M.; Maselli, J.; Drew, W.; Reingold, A.L.; Gonzales, R. Performance of a bedside c-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am. J. Med. 2004, 116, 529–535. [Google Scholar] [CrossRef]
- Bafadhel, M.; Clark, T.W.; Reid, C.; Medina, M.J.; Batham, S.; Barer, M.; Nicholson, K.G.; Brightling, C.E. Procalcitonin and C-Reactive Protein in Hospitalized Adult Patients with Community-Acquired Pneumonia or Exacerbation of Asthma or COPD. Chest 2011, 139, 1410–1418. [Google Scholar] [CrossRef]
- van Vugt, S.F.; Broekhuizen, B.D.L.; Lammens, C.; Zuithoff, N.P.A.; de Jong, P.A.; Coenen, S.; Ieven, M.; Butler, C.C.; Goossens, H.; Little, P.; et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: Diagnostic study. BMJ 2013, 346, f2450. [Google Scholar] [CrossRef]
- Stolz, D.; Christ-Crain, M.; Morgenthaler, N.G.; Leuppi, J.; Miedinger, D.; Bingisser, R.; Müller, C.; Struck, J.; Müller, B.; Tamm, M. Copeptin, C-Reactive Protein, and Procalcitonin as Prognostic Biomarkers in Acute Exacerbation of COPD. Chest 2007, 131, 1058–1067. [Google Scholar] [CrossRef]
- Moon, J.; Kang, S.M.; Cho, I.J.; Oh, J.; Shim, J.; Lee, S.H.; Jang, Y.; Chung, N. Clinical and Echocardiographic Findings of Newly Diagnosed Acute Decompensated Heart Failure in Elderly Patients. Yonsei Med. J. 2011, 52, 33–38. [Google Scholar] [CrossRef][Green Version]
- Foushee, J.A.; Hope, N.H.; Grace, E.E. Applying biomarkers to clinical practice: A guide for utilizing procalcitonin assays. J. Antimicrob. Chemother. 2012, 67, 2560–2569. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, J.W.; Mok, J.H.; Kim, M.H.; Cho, W.H.; Lee, K.; Kim, K.U.; Jeon, D.; Park, H.K.; Kim, Y.S.; et al. Usefulness of Plasma Procalcitonin to Predict Severity in Elderly Patients with Community-Acquired Pneumonia. Tuberc. Respir. Dis. 2013, 74, 207–214. [Google Scholar] [CrossRef]
- Horie, M.; Suzuki, M.; Noguchi, S.; Tanaka, W.; Yoshihara, H.; Kawakami, M.; Kichikawa, Y.; Sakamoto, Y.; Ugajin, M. Diagnostic and Prognostic Value of Procalcitonin in Community-Acquired Pneumonia. Am. J. Med Sci. 2012, 343, 30–35. [Google Scholar] [CrossRef]
- Buratti, T.; Ricevuti, G.; Pechlaner, C.; Joannidis, M.; Wiedermann, F.J.; Gritti, D.; Herold, M.; Wiedermann, C.J. Plasma levels of procalcitonin and interleukin-6 in acute myocardial infarction. Inflammation 2001, 25, 97–100. [Google Scholar] [CrossRef]
- Calbo, E.; Alsina, M.; Carballeira, M.R.; Lite, J.; Garau, J. The impact of time on the systemic inflammatory response in pneumococcal pneumonia. Eur. Respir. J. 2009, 35, 614–618. [Google Scholar] [CrossRef]
- Blum, A.; Miller, H. Role of cytokines in heart failure. Am. Heart J. 1998, 135, 181–186. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Vatasescu, R.G.; Stanciu, M.M.; Iorgulescu, C.; Vasile, A.I.; Dorobantu, M. Cardiac resynchronization therapy in patients with chronic heart failure is associated with anti-inflammatory and anti-remodeling effects. Clin. Biochem. 2013, 46, 230–234. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsujino, T.; Lee-Kawabata, M.; Naito, Y.; Sakoda, T.; Ohyanagi, M.; Masuyama, T. Serum interleukin-6 and C-reactive protein are markedly elevated in acute decompensated heart failure patients with left ventricular systolic dysfunction. Cytokine 2010, 49, 264–268. [Google Scholar] [CrossRef]
- Fink, A.M.; Gonzalez, R.C.; Lisowski, T.; Pini, M.; Fantuzzi, G.; Levy, W.C.; Piano, M.R. Fatigue, Inflammation, and Projected Mortality in Heart Failure. J. Card. Fail. 2012, 18, 711–716. [Google Scholar] [CrossRef]
- Niethammer, M.; Sieber, M.; von Haehling, S.; Anker, S.D.; Munzel, T.; Horstick, G.; Genth-Zotz, S. Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int. J. Cardiol. 2008, 129, 111–117. [Google Scholar] [CrossRef]
- Peschel, T.; Schönauer, M.; Thiele, H.; Anker, S.D.; Schuler, G.; Niebauer, J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Fail. 2003, 5, 609–614. [Google Scholar] [CrossRef]
- Komiya, K.; Ishii, H.; Teramoto, S.; Takahashi, O.; Eshima, N.; Yamaguchi, O.; Ebi, N.; Murakami, J.; Yamamoto, H.; Kadota, J.I. Diagnostic utility of C-reactive Protein combined with brain natriuretic peptide in acute pulmonary edema: A cross sectional study. Respir. Res. 2011, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Meng, L.; Lu, X.; Ou, N.; Li, X. Inflammatory Mediators in Chinese Patients With Congestive Heart Failure. J. Clin. Pharmacol. 2009, 49, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Parab, R.; Vasudevan, A.; Brensilver, J.; Gitler, B. Utility of Brain Natriuritic Peptide as a Diagnostic Tool for Congestive Heart Failure in the Elderly. Crit. Pathways Cardiol. A J. Evid.-Based Med. 2005, 4, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Boffa, G.M.; Zaninotto, M.; Sartor, R.; Mion, M.; Berton, A.; Pasqualetto, C.; Razzolini, R.; Plebani, M. Interleukin-6 and tumor necrosis factor-α as biochemical markers of heart failure: A head-to-head clinical comparison with B-type natriuretic peptide. J. Cardiovasc. Med. 2009, 10, 758–764. [Google Scholar] [CrossRef] [PubMed]
- De Denus, S.; Lavoie, J.; Ducharme, A.; O’Meara, E.; Racine, N.; Sirois, M.G.; Neagoe, P.-E.; Zhu, L.; Rouleau, J.-L.; White, M. Differences in Biomarkers in Patients With Heart Failure With a Reduced vs a Preserved Left Ventricular Ejection Fraction. Can. J. Cardiol. 2011, 28, 62–68. [Google Scholar] [CrossRef] [PubMed]
| Cases (n = 35) | Controls (n = 35) | p | |
|---|---|---|---|
| Female sex, n (%) | 24 (68.6) | 22 (62.9) | >0.05 |
| Median age, years | 78 (64–82) | 77 (71–82) | >0.05 |
| Time since CHF diagnosis, months | 65 (48–138) | 64 (49–125) | >0.05 |
| Number of CHF worsening episodes during past year | 2 (1–2) | 2 (1–2) | >0.05 |
| CHF functional class, n (%) | |||
| II III IV | 21 (60.0) | 17 (48.6) | |
| 12 (34.3) | 17 (48.6) | >0.05 | |
| 2 (5.7) | 1 (2.8) | ||
| Cough, n (%) | 33 (94.3) | 31 (88.6) | >0.05 |
| Sputum production, n (%) | 14 (40) | 12 (34.3) | >0.05 |
| Dyspnea, n (%) | 35 (100) | 35 (100) | >0.05 |
| Body temperature, °C | 36.8 (36.6–37.2) | 36.4 (36.3–36.8) | 0.0005 |
| Edema, n (%) | 29 (82.9) | 28 (80) | >0.05 |
| Edema location, n (%) Feet only Feet and shins Anasarca | |||
| 4 (13.8) | 8 (28.6) | >0.05 | |
| 25 (86.2) | 19 (67.9) | ||
| 0 (0) | 1 (3.5) | ||
| Fine crackles/rales on auscultation, n (%) | 33 (94.3) | 30 (85.7) | >0.05 |
| Dry rales on auscultation, n (%) | 7 (20) | 5 (14.3) | >0.05 |
| Infiltration on chest X-ray, n (%) | 28 (80) | 32 (91) | >0.05 |
| Pleural effusion on chest X-ray, n (%) | 23 (65.7) | 15 (42.9) | >0.05 |
| Peripheral WBC count (×109 L) | 8.4 (7.0–11.2) | 8.3 (7.0–9.5) | >0.05 |
| Band neutrophils, n (%) | 7 (5.0–9.8) | 6 (1.0–10.0) | >0.05 |
| In-hospital mortality, % | 2.9 | 2.9 | >0.05 |
| CRP | PCT | IL-6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sn (%) | Sp (%) | Cut-off (mg/L) | Sn (%) | Sp (%) | Cut-off (ng/mL) | Sn (%) | Sp (%) | Cut-off (pg/mL) |
| 100.0 | 8.6 | 3.5 | 68.6 | 85.7 | 0.050 | 88.6 | 48.6 | 13.3 |
| 97.1 | 11.4 | 5.0 | 62.9 | 91.4 | 0.055 | 85.6 | 48.6 | 13.5 |
| 94.3 | 11.4 | 7.0 | 60.0 | 91.4 | 0.065 | 85.7 | 51.4 | 13.9 |
| 94.3 | 25.7 | 9.5 | 60.0 | 94.3 | 0.075 | 82.9 | 54.3 | 14.4 |
| 94.3 | 34.3 | 11.5 | 57.1 | 97.1 | 0.085 | 82.9 | 57.1 | 15.1 |
| 94.3 | 48.6 | 14.5 | 54.3 | 97.1 | 0.095 | 82.9 | 60.0 | 15.9 |
| 94.3 | 57.1 | 17.5 | 48.6 | 97.1 | 0.115 | 80.0 | 62.9 | 18.7 |
| 94.3 | 68.6 | 21.0 | 48.6 | 100.0 | 0.145 | 74.3 | 65.7 | 20.2 |
| 91.4 | 71.4 | 23.5 | 45.7 | 100.0 | 0.185 | 74.3 | 68.6 | 21.5 |
| 88.6 | 71.4 | 24.5 | 42.9 | 100.0 | 0.220 | 74.3 | 74.3 | 23.9 |
| 85.7 | 85.7 | 25.5 | 40.0 | 100.0 | 0.235 | 71.4 | 77.1 | 26.3 |
| 85.7 | 88.6 | 26.5 | 37.1 | 100.0 | 0.265 | 71.4 | 82.9 | 27.6 |
| 85.7 | 91.4 | 28.5 | 34.3 | 100.0 | 0.335 | 65.7 | 82.9 | 28.4 |
| 80.0 | 91.4 | 31.5 | 31.4 | 100.0 | 0.395 | 60.0 | 82.9 | 31.9 |
| 77.1 | 91.4 | 33.5 | 28.6 | 100.0 | 0.460 | 60.0 | 85.7 | 35.7 |
| 74.3 | 94.3 | 35.5 | 25.7 | 100.0 | 0.545 | 57.1 | 88.6 | 39.3 |
| 71.4 | 94.3 | 38.0 | 22.6 | 100.0 | 0.945 | 54.3 | 91.4 | 42.5 |
| 68.6 | 97.1 | 40.5 | 20.0 | 100.0 | 1.355 | 51.4 | 91.4 | 44.3 |
| 62.9 | 97.1 | 45.0 | 17.1 | 100.0 | 2.255 | 48.6 | 94.3 | 49.9 |
| 60.0 | 97.1 | 48.0 | 14.3 | 100.0 | 3.845 | 48.6 | 97.1 | 58.5 |
| 45.7 | 97.1 | 54.0 | 11.4 | 100.0 | 5.855 | 42.9 | 98.1 | 67.7 |
| 42.9 | 97.1 | 60.0 | 8.6 | 100.0 | 8.665 | 42.9 | 100.0 | 78.8 |
| 40.0 | 100.0 | 64.0 | 5.7 | 100.0 | 14.44 | 40.0 | 100.0 | 87.3 |
| 37.1 | 100.0 | 67.0 | 2.9 | 100.0 | 27.56 | 37.1 | 100.0 | 88.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachina, S.; Bobylev, A.; Lazarev, P.; Mladov, V.; Carrouel, F.; Avdeev, S.; Kozlov, R.; Bourgeois, D. Biomarker Value in the Diagnosis of Community-Acquired Pneumonia with Concomitant Chronic Heart Failure. J. Clin. Med. 2021, 10, 4570. https://doi.org/10.3390/jcm10194570
Rachina S, Bobylev A, Lazarev P, Mladov V, Carrouel F, Avdeev S, Kozlov R, Bourgeois D. Biomarker Value in the Diagnosis of Community-Acquired Pneumonia with Concomitant Chronic Heart Failure. Journal of Clinical Medicine. 2021; 10(19):4570. https://doi.org/10.3390/jcm10194570
Chicago/Turabian StyleRachina, Svetlana, Andrey Bobylev, Pavel Lazarev, Vladimir Mladov, Florence Carrouel, Sergey Avdeev, Roman Kozlov, and Denis Bourgeois. 2021. "Biomarker Value in the Diagnosis of Community-Acquired Pneumonia with Concomitant Chronic Heart Failure" Journal of Clinical Medicine 10, no. 19: 4570. https://doi.org/10.3390/jcm10194570
APA StyleRachina, S., Bobylev, A., Lazarev, P., Mladov, V., Carrouel, F., Avdeev, S., Kozlov, R., & Bourgeois, D. (2021). Biomarker Value in the Diagnosis of Community-Acquired Pneumonia with Concomitant Chronic Heart Failure. Journal of Clinical Medicine, 10(19), 4570. https://doi.org/10.3390/jcm10194570










