Real-World Management of Patients with Primary Biliary Cholangitis—A Retrospective Study from a Tertiary Medical Center in Israel
Abstract
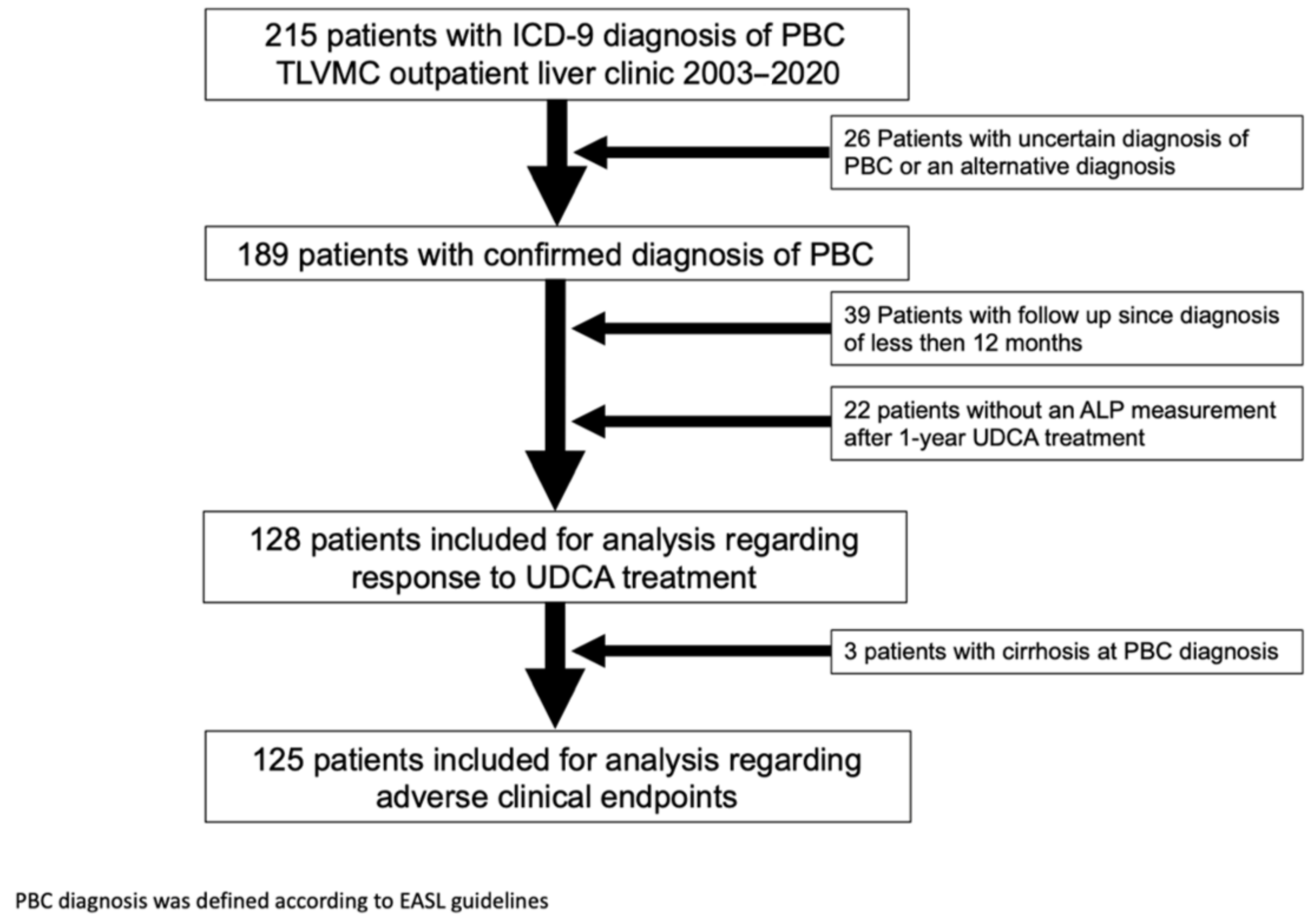
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patients and Disease Characteristics
3.2. Response to UDCA Treatment
3.3. Risk Factors Associated with Disease Progression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBC | Primary Biliary Cholangitis |
| AIH | Autoimmune hepatitis |
| ALP | Alkaline phosphates |
| GGT | Gamma glutamyl transferase |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| AMA | Anti-mitochondrial antibodies |
| ANA | Anti-nuclear antibodies |
| ASMA | Anti-smooth muscle antibodies |
| UDCA | Ursodeoxycholic acid |
| ULN | Upper limit of normal |
| OR | Odds ratio |
| HR | Hazard ratio |
| EASL | European Association for the Study of the Liver |
References
- Kaplan, M.M.; Gershwin, M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005, 353, 1261–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Wang, T.; Shen, Y.; Xi, X.; Yang, L. Underestimated Male Prevalence of Primary Biliary Cholangitis in China: Results of a 16-year cohort study involving 769 patients. Sci. Rep. 2017, 7, 6560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef] [PubMed]
- Sebode, M.; Weiler-Normann, C.; Liwinski, T.; Schramm, C. Autoantibodies in Autoimmune Liver Disease—Clinical and Diagnostic Relevance. Front. Immunol. 2018, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.H.; van Buuren, H.R.; Corpechot, C.; Thorburn, D.; Janssen, H.L.; Lindor, K.D.; Hirschfield, G.M.; Parés, A.; Floreani, A.; Mayo, M.J.; et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J. Hepatol. 2019, 71, 357–365. [Google Scholar] [CrossRef]
- Poupon, R.E.; Lindor, K.D.; Cauch-Dudek, K.; Dickson, E.R.; Heathcote, E.J. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997, 113, 884–890. [Google Scholar] [CrossRef]
- Lammers, W.; Hirschfield, G.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812. [Google Scholar] [CrossRef]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef]
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Sex and Age Are Determinants of the Clinical Phenotype of Primary Biliary Cirrhosis and Response to Ursodeoxycholic Acid. Gastroenterology 2013, 144, 560–569. [Google Scholar] [CrossRef]
- Kumagi, T.; Guindi, M.; Fischer, S.E.; Arenovich, T.; Abdalian, R.; Coltescu, C.; E Heathcote, J.; Hirschfield, G. Baseline Ductopenia and Treatment Response Predict Long-Term Histological Progression in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2010, 105, 2186–2194. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Galoosian, A.; Hanlon, C.; Zhang, J.; Holt, E.W.; Yimam, K.K. Clinical Updates in Primary Biliary Cholangitis: Trends, Epidemiology, Diagnostics, and New Therapeutic Approaches. J. Clin. Transl. Hepatol. 2020, 8, 49–60. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9151525. [Google Scholar] [CrossRef]
- Delgado, J.-S.; Vodonos, A.; Delgado, B.; Jotkowitz, A.; Rosenthal, A.; Fich, A.; Novack, V. Primary biliary cirrhosis in Southern Israel: A 20 year follow up study. Eur. J. Intern. Med. 2012, 23, e193–e198. [Google Scholar] [CrossRef]
- Lleo, A.; Colapietro, F. Changes in the Epidemiology of Primary Biliary Cholangitis. Clin. Liver Dis. 2018, 22, 429–441. [Google Scholar] [CrossRef]
- Zhang, H.; Carbone, M.; Lleo, A.; Invernizzi, P. Geoepidemiology, Genetic and Environmental Risk Factors for PBC. Dig. Dis. 2015, 33, 94–101. [Google Scholar] [CrossRef]
- Vespasiani-Gentilucci, U.; Rosina, F.; Pace-Palitti, V.; Sacco, R.; Pellicelli, A.; Chessa, L.; De Vincentis, A.; Barlattani, M.; Barlattani, A.; Feletti, V.; et al. Rate of non-response to ursodeoxycholic acid in a large real-world cohort of primary biliary cholangitis patients in Italy. Scand. J. Gastroenterol. 2019, 54, 1274–1282. [Google Scholar] [CrossRef]
- Örnolfsson, K.T.; Lund, S.H.; Olafsson, S.; Bergmann, O.M.; Björnsson, E.S. Biochemical response to ursodeoxycholic acid among PBC patients: A nationwide population-based study. Scand. J. Gastroenterol. 2019, 54, 609–616. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bruns, T.; Cheung, A.; Li, K.-K.; Kittler, C.; Kumagi, T.; Shah, H.; Corbett, C.; Al-Harthy, N.; Acarsu, U.; et al. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J. Hepatol. 2014, 60, 1249–1258. [Google Scholar] [CrossRef]
- Yang, F.; Yang, Y.; Wang, Q.; Wang, Z.; Miao, Q.; Xiao, X.; Wei, Y.; Bian, Z.; Sheng, L.; Chen, X.; et al. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: The additional effect of anti-gp210. Aliment. Pharmacol. Ther. 2017, 45, 733–743. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Liberal, R.; Lopes, S.; Machado, M.V.; Carvalho, J.; Dias, T.; Santos, A.; Agostinho, C.; Figueiredo, P.; Loureiro, R.; et al. Predictors for incomplete response to ursodeoxycholic acid in primary biliary cholangitis. Data from a national registry of liver disease. United Eur. Gastroenterol. J. 2021, 9, 699–706. [Google Scholar] [CrossRef]
- Wilde, A.-C.; Lieb, C.; Leicht, E.; Greverath, L.; Steinhagen, L.; de Chamorro, N.W.; Petersen, J.; Hofmann, W.; Hinrichsen, H.; Heyne, R.; et al. Real-World Clinical Management of Patients with Primary Biliary Cholangitis—A Retrospective Multicenter Study from Germany. J. Clin. Med. 2021, 10, 1061. [Google Scholar] [CrossRef]
- Wen, M.; Men, R.; Fan, X.; Shen, Y.; Ni, P.; Hu, Z.; Yang, L. Worse Response to Ursodeoxycholic Acid in Primary Biliary Cholangitis Patients with Autoimmune Hepatitis Features. Dig. Dis. 2021, 39, 366–374. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, B.-H.; Rodgers, B.; Pyrsopoulos, N. Characteristics and Inpatient Outcomes of Primary Biliary Cholangitis and Autoimmune Hepatitis Overlap Syndrome. J. Clin. Transl. Hepatol. 2021, 9, 392–398. [Google Scholar] [CrossRef]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: Development and validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.C.; Lammers, W.; Perez, C.F.M.; van Buuren, H.R.; Gulamhusein, A.; Trivedi, P.J.; Lazaridis, K.N.; Ponsioen, C.Y.; Floreani, A.; Hirschfield, G.M.; et al. Effects of Age and Sex of Response to Ursodeoxycholic Acid and Transplant-free Survival in Patients With Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2076–2084. [Google Scholar] [CrossRef]
- Floreani, A.; Mangini, C.; Reig, A.; Franceschet, I.; Cazzagon, N.; Perini, L.; Caballeria, L.; Cocchio, S.; Baldo, V.; Pares, A. Thyroid Dysfunction in Primary Biliary Cholangitis: A Comparative Study at Two European Centers. Am. J. Gastroenterol. 2017, 112, 114–119. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; Grgurevic, I.; Kanizaj, T.F.; Koller, T.; Bodorovska, B.; Bozin, T.; Mijic, M.; Rob, Z.; et al. Prognostic Factors in Primary Biliary Cholangitis: A Retrospective Study of Joint Slovak and Croatian Cohort of 249 Patients. J. Pers. Med. 2021, 11, 495. [Google Scholar] [CrossRef]
- Corpechot, C.; Abenavoli, L.; Rabahi, N.; Chrétien, Y.; Andréani, T.; Johanet, C.; Chazouillères, O.; Poupon, R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008, 48, 871–877. [Google Scholar] [CrossRef]
- Gerussi, A.; Bernasconi, D.P.; O’Donnell, S.E.; Lammers, W.J.; Van Buuren, H.; Hirschfield, G.; Janssen, H.; Corpechot, C.; Reig, A.; Pares, A.; et al. Measurement of Gamma Glutamyl Transferase to Determine Risk of Liver Transplantation or Death in Patients With Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1688–1697.e1614. [Google Scholar] [CrossRef]

| Number of Patients | 189 | |
|---|---|---|
| Demographics | Female gender, n (%) | 175 (92.6) |
| Age at diagnosis, years, mean (std) | 54.7 (13.0) | |
| Ethnicity, n (%) | ||
| Jewish Ashkenazi | 125 (72.3) | |
| Jewish non-Ashkenazi | 43 (24.9) | |
| Other | 5 (2.9) | |
| Smoking, n (%) | 31 (23.1) | |
| Symptoms | Fatigue, n (%) Severe fatigue, n (%) | 80 (47.9) 28 (14.8) |
| Pruritus, n (%) Severe pruritus, n (%) | 56 (29.6) 21 (11.1) | |
| Other liver diseases | NASH, n (%) | 37 (20.0) |
| Cholecystitis/Cholecystectomy, n (%) | 28 (15.1) | |
| PBC-AIH variant, n (%) | 11 (5.9) | |
| Chronic HBV, n (%) | 2 (1.1) | |
| Other autoimmune diseases | Any extrahepatic autoimmune disease, n (%) | 73 (39.2) |
| Hypothyroidism, n (%) | 37 (19.9) | |
| Celiac, n (%) | 3 (1.6) | |
| Scleroderma, n (%) | 4 (2.2) | |
| Sjogren, n (%) | 11 (5.9) | |
| Rheumatoid arthritis, n (%) | 9 (4.8) | |
| Psoriasis, n (%) | 4 (2.2) | |
| Systemic lupus erythematosus, n (%) | 2 (1.1) | |
| Ulcerative colitis, n (%) | 3 (1.6) | |
| Immune thrombocytopenic purpura, n (%) | 4 (2.2) | |
| Raynaud’s syndrome, n (%) | 3 (1.6) | |
| Other autoimmune disease, n (%) | 8 (4.3) | |
| Baseline Laboratory results (before UDCA) mean (std) | Hemoglobin (g/L) | 13.0 (1.2) |
| Platelets (×109/L) | 237 (80) | |
| Creatinine (mg/dL) | 0.78 (0.22) | |
| Total bilirubin (mg/dL) | 0.74 (0.56) | |
| AST (IU/L) | 59.2 (43.1) | |
| ALT (IU/L) | 72.7 (66.8) | |
| ALP (IU/L) | 280 (200) | |
| GGT (IU/L) | 342 (339) | |
| Albumin (g/L) | 40.3 (5.5) | |
| IgM (mg/dL) | 418 (382) | |
| Immune Serology | AMA, n (%) | 147 (86.5) |
| ANA, n (%) | 108 (75.5) | |
| ASMA, n (%) | 17 (13.5) | |
| Anti-gp210, n (%) | 9 (32.1) | |
| Anti-sp100, n (%) | 8 (29.6) | |
| UDCA Biochemical Response Rates | |||
|---|---|---|---|
| Criteria | Definition of Biochemical Response | n | Non-responder Rate |
| Toronto | ALP ≤ 1.67 × ULN | 128 | 28.1% |
| Barcelona | ALP normalization or decrease >40% | 69 | 40.6% |
| Paris-I | ALP ≤ 3 × ULN and AST ≤ 2 × ULN and normal total bilirubin | 86 | 17.4% |
| Paris-II | ALP ≤ 1.5 × ULN and AST ≤ 1.5 × ULN and normal total bilirubin | 86 | 38.4% |
| Rotterdam | Albumin and total bilirubin normalization | 96 | 16.7% |
| GLOBE score | Below age-specific threshold | 81 | 17.3% |
| UK PBC score | Risk of event in 5 years < 5% | 79 | 6.3% |
| Responder | Non-Responder | Univariable Analysis | |||
|---|---|---|---|---|---|
| Number of Patients, n (%) | 92 (71.9) | 36 (28.1) | OR (95% CI) | p-Value | |
| Demographics | Female gender, n (%) | 87 (94.6) | 32 (88.9) | 0.46 (0.12–1.82) | 0.268 |
| Age at diagnosis, years, mean (std) | 55.6 (12.6) | 48.8 (12.6) | 0.95 (0.92–0.99) | 0.009 | |
| Ethnicity, n (%) | |||||
| Jewish Ashkenazi | 61 (71.8) | 26 (78.8) | 1.3 (0.5–3.38) | 0.588 | |
| Jewish non-Ashkenazi | 21 (24.7) | 7 (21.2) | |||
| Other | 3 (3.5) | 0 (0) | |||
| Smoking, n (%) | 13 (19.1) | 7 (28.0) | 1.49 (0.68–3.23) | 0.316 | |
| Symptoms | Fatigue, n (%) | 38 (43.7) | 22 (62.9) | 0.50 (0.22–1.10) | 0.083 |
| Severe fatigue, n (%) | 13 (14.1) | 8 (22.2) | 1.37 (0.55–3.42) | 0.499 | |
| Pruritus, n (%) | 29 (32.6) | 15 (42.9) | 0.67 (0.30–1.46) | 0.311 | |
| Severe pruritus, n (%) | 9 (9.8) | 8 (22.2) | 2.22 (0.84–5.85) | 0.106 | |
| Other liver diseases | NASH, n (%) | 15 (16.7) | 10 (27.8) | 1.97 (0.79–4.93) | 0.145 |
| Cholecystitis/Cholecystectomy, n (%) | 9 (10.0) | 8 (22.2) | 2.63 (0.93–7.49) | 0.069 | |
| PBC-AIH variant, n (%) | 3 (3.3) | 5 (13.9) | 4.78 (1.08–21.2) | 0.039 | |
| Chronic HBV, n (%) | 0 (0) | 1 (2.8) | |||
| Other AI diseases | Extrahepatic autoimmune disease, n (%) | 41 (45.1) | 13 (36.1) | 0.7 (0.32–1.56) | 0.385 |
| Hypothyroidism, n (%) | 17 (18.7) | 4 (11.1) | 0.42 (0.13–1.33) | 0.141 | |
| Baseline lab results (before UDCA) mean (std) | Hemoglobin (g/L) | 12.9 (1.2) | 13.2 (0.9) | 1.19 (0.68–2.07) | 0.545 |
| Platelets (× 109/L) | 236 (74) | 234 (98) | 1 (0.99–1.01) | 0.945 | |
| Creatinine (mg/dL) | 0.77 (0.23) | 0.76 (0.16) | 0.97 (0.03–31.15) | 0.984 | |
| Total bilirubin (mg/dL) | 0.62 (0.26) | 1.16 (1.02) | 5.84 (0.97–35.32) | 0.055 | |
| AST (IU/L) * | 50.4 (32.9) | 76.6 (63.6) | 1.13 (0.98–1.31) | 0.087 | |
| ALT (IU/L) * | 65.0 (69.4) | 101.1 (85.2) | 1.06 (0.98–1.14) | 0.142 | |
| ALP (IU/L) ** | 222 (125) | 518 (309) | 1.38 (1.15–1.66) | <0.001 | |
| GGT (IU/L) * | 275 (284) | 545 (479) | 1.02 (1–1.04) | 0.030 | |
| Albumin (g/L) | 41.2 (3.2) | 38.8 (10.8) | 1.02 (0.82–1.27) | 0.871 | |
| Immune Serology | AMA, n (%) | 75 (88.2) | 26 (78.8) | 0.5 (0.17–1.44) | 0.195 |
| ANA, n (%) | 54 (74.0) | 25 (89.3) | 2.93 (0.79–10.83) | 0.107 | |
| ASMA, n (%) | 3 (4.9) | 8 (29.6) | 8 (1.92–33.26) | 0.004 | |
| Anti-gp210, n (%) | 2 (18.2) | 4 (44.4) | |||
| Anti-sp100, n (%) | 3 (27.2) | 2 (25.0) | |||
| Liver Histology | Interface Hepatitis, n (%) | 20 (57.1) | 16 (94.1) | 12 (1.43–100.8) | 0.022 |
| Advanced Fibrosis, n (%) | 7 (21.2) | 5 (27.8) | 1.43 (0.38–5.38) | 0.598 |
| HR (95% CI) | p-Value | ||
|---|---|---|---|
| Demographics | Female gender | 1.26 (0.17–9.55) | 0.826 |
| Age at diagnosis | 0.96 (0.93–1.00) | 0.046 | |
| Ethnicity—Jewish Ashkenazi | 2.89 (0.66–12.64) | 0.159 | |
| Smoking | 0.59 (0.13–2.63) | 0.487 | |
| Symptoms | Fatigue | 1.37 (0.53–3.53) | 0.521 |
| Severe fatigue | 0.90 (0.26–3.16) | 0.875 | |
| Pruritus | 3.45 (1.29–9.22) | 0.014 | |
| Severe pruritus | 4.29 (1.62–11.34) | 0.003 | |
| Other liver diseases | NASH | 1.72 (0.56–5.24) | 0.342 |
| Cholecystitis/Cholecystectomy | 0.96 (0.22–4.24) | 0.957 | |
| PBC-AIH variant | 1.77 (0.50–6.23) | 0.374 | |
| Other AI diseases | Extrahepatic autoimmune disease | 0.89 (0.35–2.28) | 0.809 |
| Hypothyroidism | 0.86 (0.28–2.60) | 0.790 | |
| Laboratory before UDCA | Hemoglobin | 0.18 (0.02–1.40) | 0.101 |
| Platelets | 0.99 (0.98–1.00) | 0.178 | |
| Creatinine | 16.21 (0.07–3710) | 0.315 | |
| Total bilirubin | 2.30 (0.89–5.96) | 0.086 | |
| AST * | 1.28 (0.94–1.74) | 0.115 | |
| ALT * | 1.17 (1.02–1.35) | 0.026 | |
| ALP ** | 1.28 (1.04–1.58) | 0.022 | |
| GGT * | 1.03 (1.00–1.06) | 0.021 | |
| Albumin | 0.93 (0.86–1.00) | 0.044 | |
| Immune Serology | AMA | 5.40 (0.71–41.21) | 0.104 |
| ANA | 2.42 (0.31–18.7) | 0.396 | |
| ASMA | 2.27 (0.60–8.61) | 0.227 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yehezkel, E.; Israel, I.; Houri, I.; Leshno, M.; Shibolet, O.; Zigmond, E. Real-World Management of Patients with Primary Biliary Cholangitis—A Retrospective Study from a Tertiary Medical Center in Israel. J. Clin. Med. 2021, 10, 4551. https://doi.org/10.3390/jcm10194551
Yehezkel E, Israel I, Houri I, Leshno M, Shibolet O, Zigmond E. Real-World Management of Patients with Primary Biliary Cholangitis—A Retrospective Study from a Tertiary Medical Center in Israel. Journal of Clinical Medicine. 2021; 10(19):4551. https://doi.org/10.3390/jcm10194551
Chicago/Turabian StyleYehezkel, Eyal, Inbal Israel, Inbal Houri, Moshe Leshno, Oren Shibolet, and Ehud Zigmond. 2021. "Real-World Management of Patients with Primary Biliary Cholangitis—A Retrospective Study from a Tertiary Medical Center in Israel" Journal of Clinical Medicine 10, no. 19: 4551. https://doi.org/10.3390/jcm10194551
APA StyleYehezkel, E., Israel, I., Houri, I., Leshno, M., Shibolet, O., & Zigmond, E. (2021). Real-World Management of Patients with Primary Biliary Cholangitis—A Retrospective Study from a Tertiary Medical Center in Israel. Journal of Clinical Medicine, 10(19), 4551. https://doi.org/10.3390/jcm10194551





