Video-Oculography-Assisted Head Impulse Test and Caloric Testing for Detecting Stroke in Acute Vertigo Patients via Modified HINTS Plus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Protocol
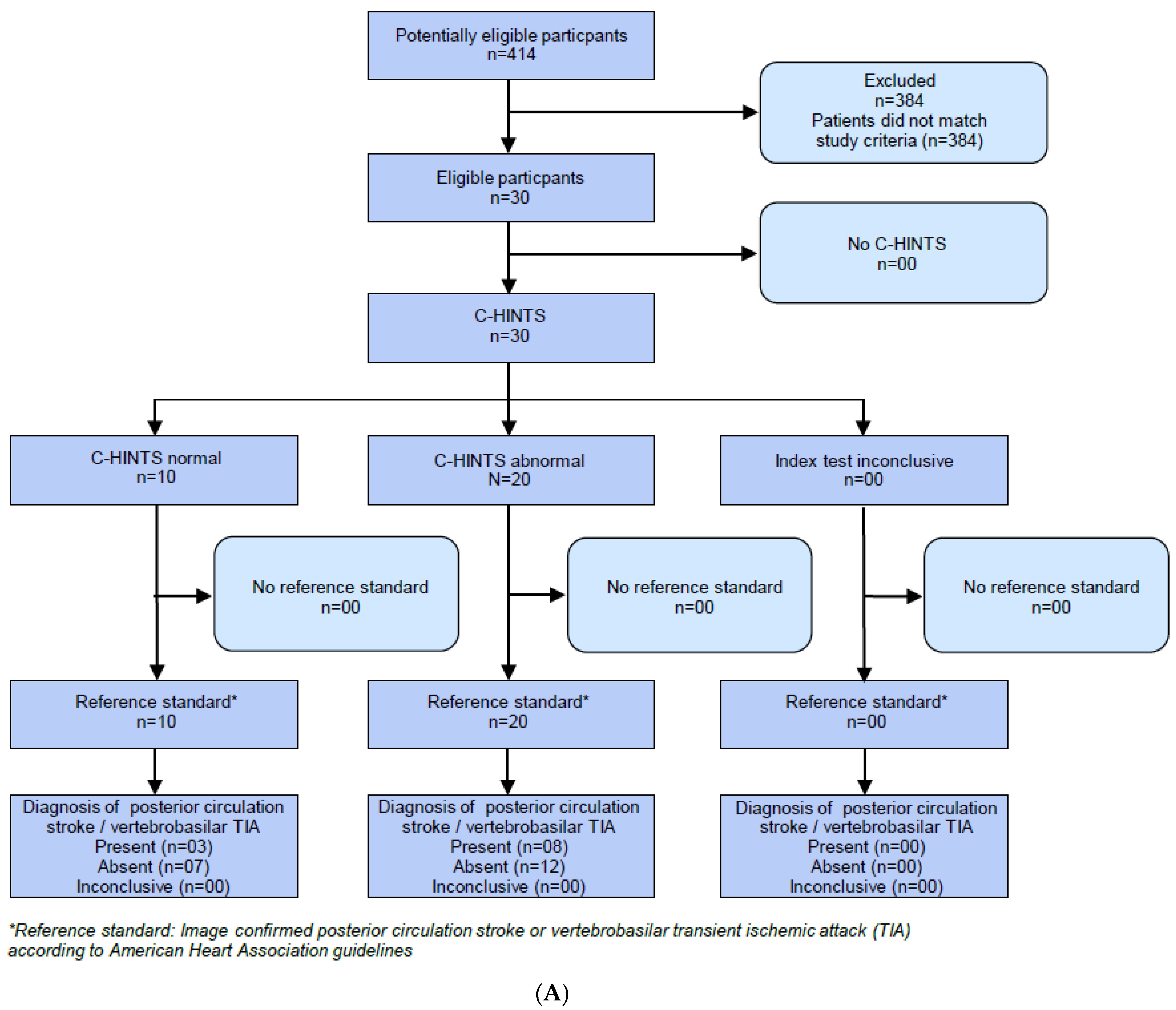
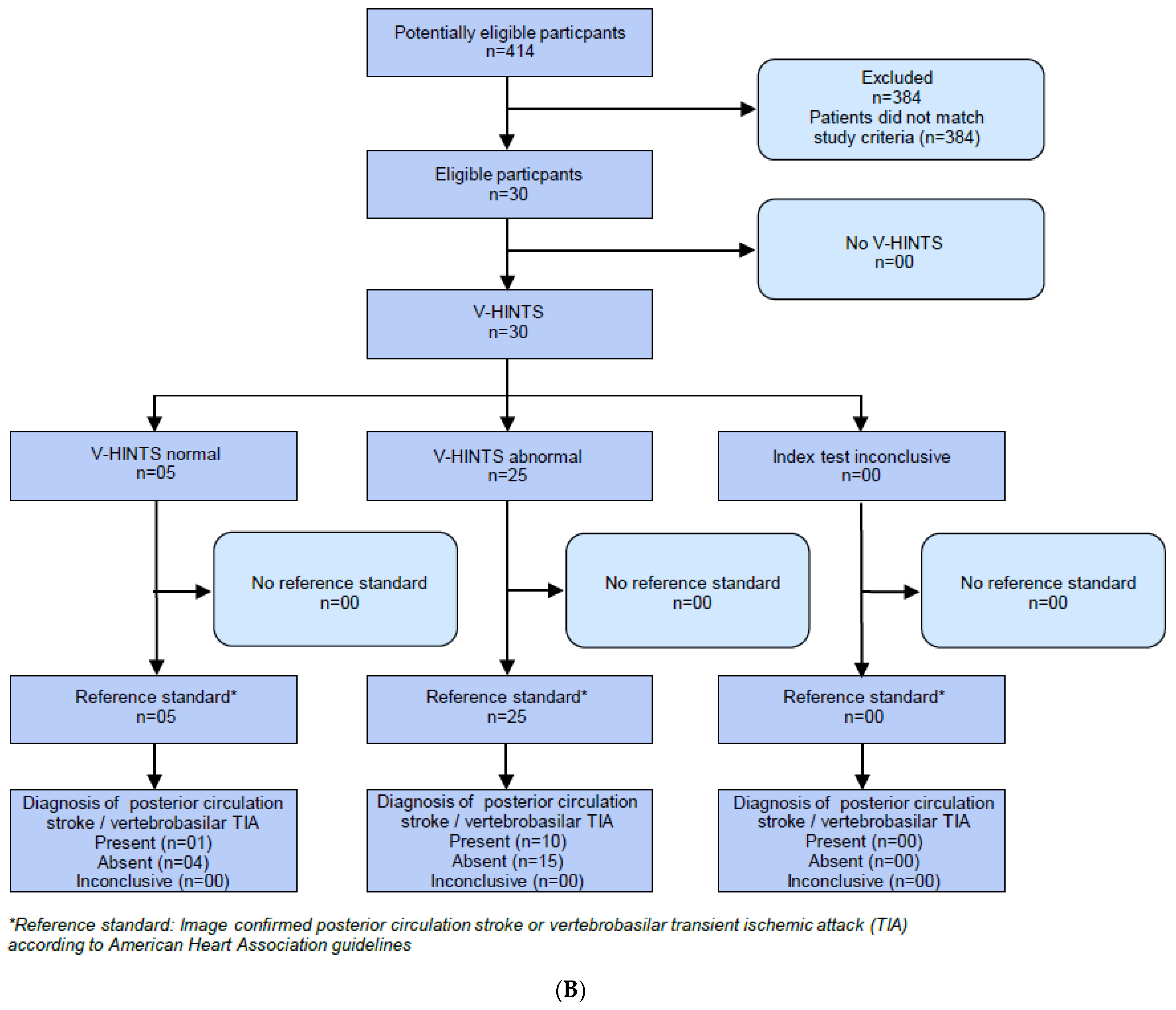
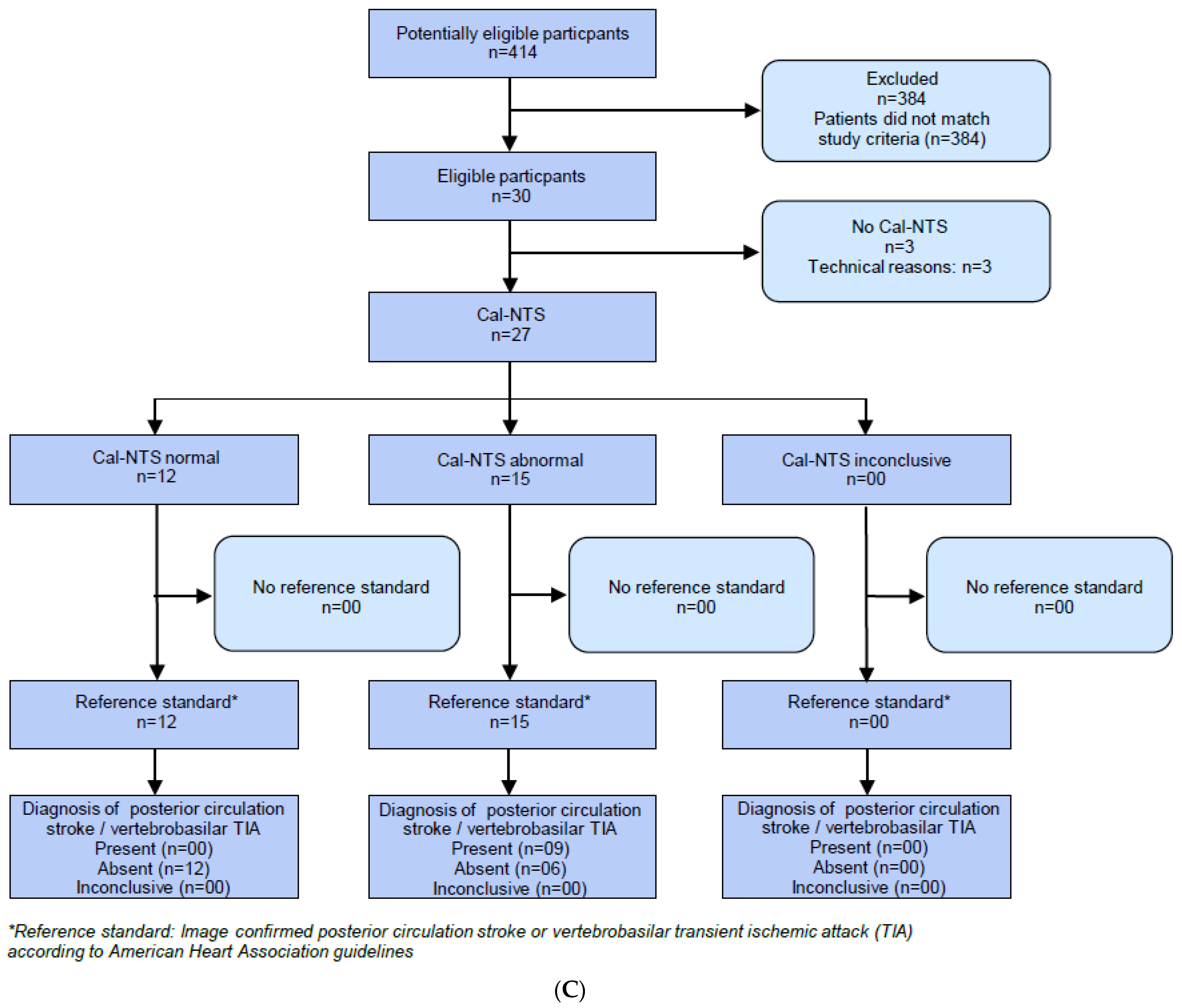
2.2. HINTS Plus
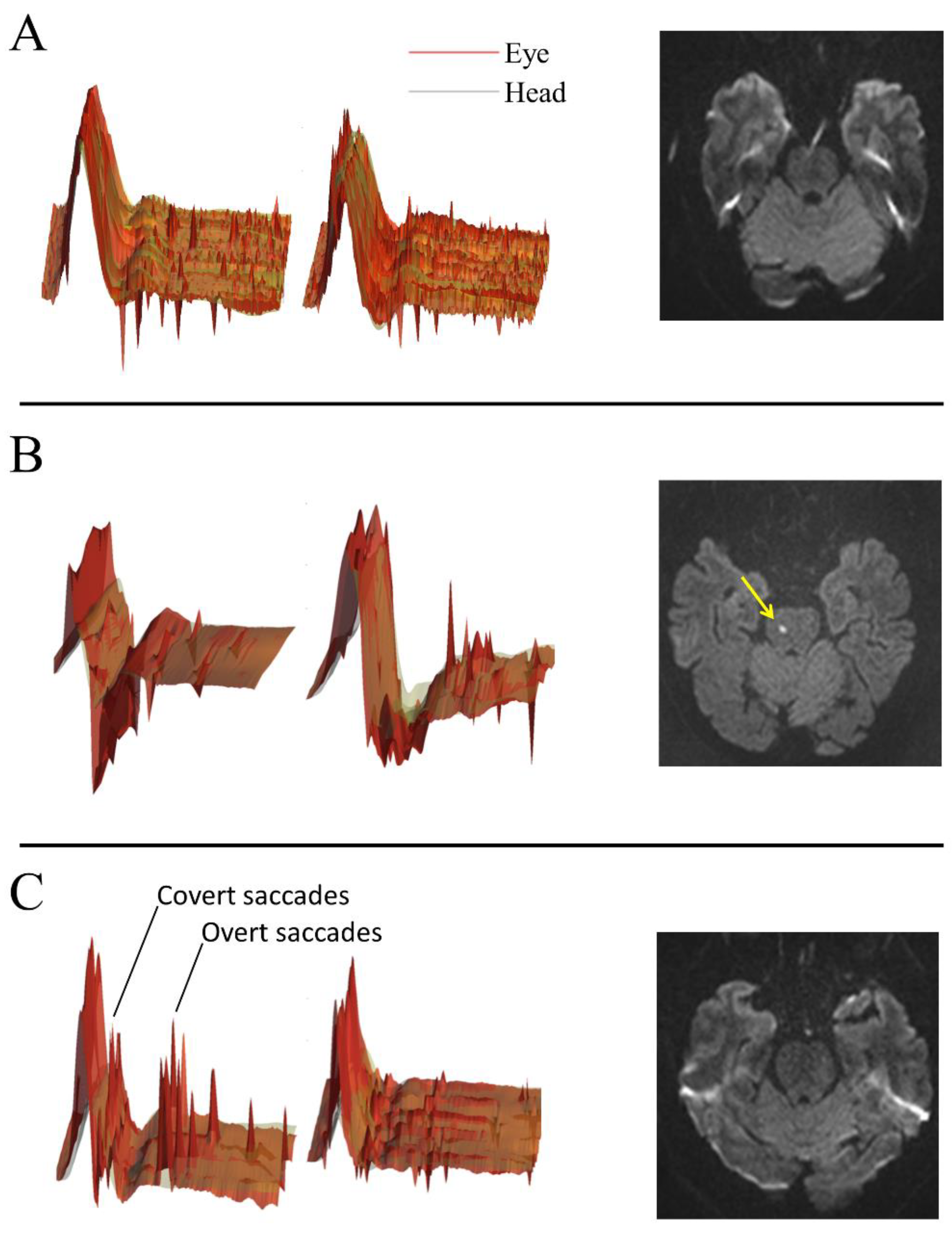
2.3. V-HIT-Assisted HINTS Plus
2.4. Caloric Testing
2.5. Cranial Magnetic Resonance Imaging
2.6. Caloric Testing Assisted NTS Plus
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman-Toker, D.E.; Hsieh, Y.H.; Camargo, C.A., Jr.; Pelletier, A.J.; Butchy, G.T.; Edlow, J.A. Spectrum of dizziness visits to US emergency departments: Cross-sectional analysis from a nationally representative sample. Mayo Clin. Proc. 2008, 83, 765–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saber Tehrani, A.S.; Kattah, J.C.; Mantokoudis, G.; Pula, J.H.; Nair, D.; Blitz, A.; Ying, S.; Hanley, D.F.; Zee, D.S.; Newman-Toker, D.E. Small strokes causing severe vertigo: Frequency of false-negative MRIs and nonlacunar mechanisms. Neurology 2014, 83, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kim, J.S. Differential diagnosis of acute vascular vertigo. Curr. Opin. Neurol. 2020, 33, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Newman-Toker, D.E.; Kerber, K.A.; Hsieh, Y.H.; Pula, J.H.; Omron, R.; Saber Tehrani, A.S.; Mantokoudis, G.; Hanley, D.F.; Zee, D.S.; Kattah, J.C. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad. Emerg. Med. 2013, 20, 986–996. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S. The video head im-pulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 2009, 73, 1134–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [PubMed]
- Navi, B.B.; Kamel, H.; Shah, M.P.; Grossman, A.W.; Wong, C.; Poisson, S.N.; Whetstone, W.D.; Josephson, S.A.; Johnston, S.C.; Kim, A.S. Application of the ABCD2 score to identify cerebrovascular causes of dizziness in the emergency department. Stroke 2012, 43, 1484–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman-Toker, D.E.; Saber Tehrani, A.S.; Mantokoudis, G.; Pula, J.H.; Guede, C.I.; Kerber, K.A.; Blitz, A.; Ying, S.H.; Hsieh, Y.H.; Rothman, R.E.; et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: Toward an ECG for the eyes. Stroke 2013, 44, 1158–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkov, D.; Strupp, M.; Pleshkov, M.; Kingma, H.; van de Berg, R. Diagnosing vestibular hypofunction: An update. J. Neurol. 2021, 268, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ohle, R.; Montpellier, R.A.; Marchadier, V.; Wharton, A.; McIsaac, S.; Anderson, M.; Savage, D. Can Emergency Physicians Accurately Rule Out a Central Cause of Vertigo Using the HINTS Examination? A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2020, 27, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Kerber, K.A.; Meurer, W.J.; Brown, D.L.; Burke, J.F.; Hofer, T.P.; Tsodikov, A.; Hoeffner, E.G.; Fendrick, A.M.; Adelman, E.E.; Morgenstern, L.B. Stroke risk stratification in acute dizziness presentations: A prospective imaging-based study. Neurology 2015, 85, 1869–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman-Toker, D.E. Comment: Diagnosing stroke in acute dizziness–do the “eyes” still have it? Neurology 2015, 85, 1877. [Google Scholar] [CrossRef] [PubMed]
- Jorns-Haderli, M.; Straumann, D.; Palla, A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattah, J.C. Update on HINTS Plus, With Discussion of Pitfalls and Pearls. J. Neurol. Phys. Ther. 2019, 43 (Suppl. 2), S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Schmäl, F.; Lübben, B.; Weiberg, K.; Stoll, W. The minimal ice water caloric test compared with established vestibular caloric test procedures. J. Vestib. Res. 2005, 15, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Dmitriew, C.; Regis, A.; Bodunde, O.; Lepage, R.; Turgeon, Z.; McIsaac, S.; Ohle, R. Diagnostic Accuracy of the HINTS Exam in an Emergency Department: A Retrospective Chart Review. Acad. Emerg. Med. 2021, 28, 387–393. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 30) | Peripheral Lesion (n = 19) | Central Lesion (n = 11) | p | |
|---|---|---|---|---|
| Demographics | ||||
| females, n (%) | 14 (46.7) | 10 (71.4) | 4 (28.6) | 0.47 |
| age (years) | 55.4 ± 17.2 | 50.2 (15.8) | 64.5 (16.2) | 0.02 |
| Cardiovascular risk profile | ||||
| diabetes mellitus type II, n (%) | 5 (16.7) | 2 (10.5) | 3 (27.3) | 0.33 |
| arterial hypertension, n (%) | 21 (70.0) | 12 (63.2) | 9 (81.8) | 0.42 |
| hyperlipidemia, n (%) | 6 (20.0) | 2 (10.5) | 4 (36.4) | 0.16 |
| smoking, n (%) | 8 (26.7) | 6 (31.6) | 2 (18.2) | 0.67 |
| coronary artery disease, n (%) | 1 (3.3) | 0 | 1 (9.1) | 0.37 |
| atrial fibrillation, n (%) | 0 (0) | 0 | 0 | |
| sleep apnea, n (%) | 0 (0) | 0 | 0 | |
| history of stroke/TIA, n (%) | 4 (13.3) | 0 | 4 (36.4) | 0.01 |
| Clinical symptoms | ||||
| dizziness/vertigo | 100 (100) | 19 (100) | 11 (100) | |
| nausea/vomiting, n (%) | 26 (86.7) | 16 (84.2) | 10 (90.9) | 1.0 |
| head motion intolerance, n (%) | 14 (46.7) | 10 (52.6) | 4 (36.6) | 0.47 |
| gait unsteadiness, n (%) | 27 (90) | 16 (84.2) | 11 (100) | 0.28 |
| head/neck pain, n (%) | 3 (10.0) | 2 (10.5) | 1 (9.1) | 1.0 |
| double vision, n (%) | 2 (6.7) | 0 | 2 (18.2) | 0.13 |
| dysphagia, n (%) | 0 (0) | 0 | 0 | |
| slurred speech, n (%) | 1 (3.3) | 1 (5.3) | 0 | 1.0 |
| paresis, n (%) | 0 (0) | 0 | 0 | |
| sensory disturbance, n (%) | 0 (0) | 0 | 0 | |
| new hearing impairment, n (%) | 3 (10) | 2 (10.5) | 1 (9.1) | 1.0 |
| Clinical examination | ||||
| hearing loss, n (%) | 1 (3.3) | 1 (5.3) | 0 | 1.0 |
| gait unsteadiness, n (%) | 26 (86.7) | 15 (78.9) | 11 (100) | 0.27 |
| spontaneous nystagmus, n (%) | 17 (56.7) | 12 (63.2) | 5 (45.5) | 0.45 |
| smooth pursuit abnormality, n (%) | 4 (13.3) | 1 (5.3) | 3 (27.3) | 0.13 |
| saccades abnormality, n (%) | 2 (6.7) | 0 | 1 (9.1) | 0.37 |
| gaze evoked nystagmus, n (%) | 2 (6.7) | 0 | 2 (18.2) | 0.13 |
| abnormal test of skew, n (%) | 0 (0) | 0 | 0 | |
| ocular muscle paresis, n (%) | 0 (0) | 0 | 0 | |
| abnormal conventional HIT, n (%) | 10 (33.3) | 7 (36.8) | 3 (27.3) | 0.7 |
| abnormal V-HIT, n (%) | 8 (26.7) | 6 (31.6) | 2 (18.2) | 0.67 |
| Caloric testing | ||||
| peripheral lesion (total), n (%) | 15 (55.6) * | 13 (72.2) | 0 | 0.001 |
| HINTS plus | ||||
| abnormal C-HINTS plus, n (%) | 20 (66.7) | 12 (63.2) | 8 (72.7) | 0.7 |
| abnormal V-HIT assisted HINTS plus, n (%) | 22 (73.3) | 13 (68.4) | 9 (81.8) | 0.67 |
| abnormal Cal-NTS plus, n (%) | 14 (50) | 6 (33.3) | 9 (100) | 0.001 |
| Cranial MRI | ||||
| vertebrobasilar ischemic lesion, n (%) | 4 (13.3) | 0 | 4 (36.4) | 0.01 |
| Diagnosis | ||||
| vertebrobasilar stroke, n (%) | 4 (13.3) | |||
| posterior TIA/DWI-neg. stroke, n (%) | 7 (23.3) | |||
| vestibular neuritis, n (%) | 12 (40) | |||
| other, n (%) | 7 (23.3) |
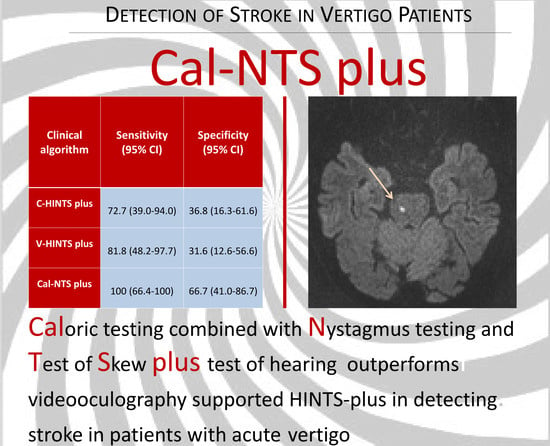
| Clinical Algorithm | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|
| C-HINTS plus | 72.7 (39.0–94.0) | 36.8 (16.3–61.6) | 40.0 (19.1–63.9) | 70.0 (34.8–93.3) | 50.0 (31.3–68.7) |
| V-HIT assisted-HINTS plus | 81.8 (48.2–97.7) | 31.6 (12.6–56.6) | 40.9 (20.7–63.6) | 75.0 (34.9–96.8) | 50.0 (31.3–68.7) |
| Cal-NTS plus | 100 (66.4–100) | 66.7 (41.0–86.7) | 60.0 (32.3–83.7) | 100 (73.5–100) | 77.8 (57.7–91.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siepmann, T.; Gruener, C.; Simon, E.; Sedghi, A.; Kitzler, H.H.; Pallesen, L.-P.; Barlinn, J.; Reichmann, H.; Puetz, V.; Barlinn, K. Video-Oculography-Assisted Head Impulse Test and Caloric Testing for Detecting Stroke in Acute Vertigo Patients via Modified HINTS Plus. J. Clin. Med. 2021, 10, 4471. https://doi.org/10.3390/jcm10194471
Siepmann T, Gruener C, Simon E, Sedghi A, Kitzler HH, Pallesen L-P, Barlinn J, Reichmann H, Puetz V, Barlinn K. Video-Oculography-Assisted Head Impulse Test and Caloric Testing for Detecting Stroke in Acute Vertigo Patients via Modified HINTS Plus. Journal of Clinical Medicine. 2021; 10(19):4471. https://doi.org/10.3390/jcm10194471
Chicago/Turabian StyleSiepmann, Timo, Cosima Gruener, Erik Simon, Annahita Sedghi, Hagen H. Kitzler, Lars-Peder Pallesen, Jessica Barlinn, Heinz Reichmann, Volker Puetz, and Kristian Barlinn. 2021. "Video-Oculography-Assisted Head Impulse Test and Caloric Testing for Detecting Stroke in Acute Vertigo Patients via Modified HINTS Plus" Journal of Clinical Medicine 10, no. 19: 4471. https://doi.org/10.3390/jcm10194471
APA StyleSiepmann, T., Gruener, C., Simon, E., Sedghi, A., Kitzler, H. H., Pallesen, L.-P., Barlinn, J., Reichmann, H., Puetz, V., & Barlinn, K. (2021). Video-Oculography-Assisted Head Impulse Test and Caloric Testing for Detecting Stroke in Acute Vertigo Patients via Modified HINTS Plus. Journal of Clinical Medicine, 10(19), 4471. https://doi.org/10.3390/jcm10194471