High-Frequency Ultrasound Assessment of Skin and Oral Mucosa in Metabolic Syndrome Patients—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
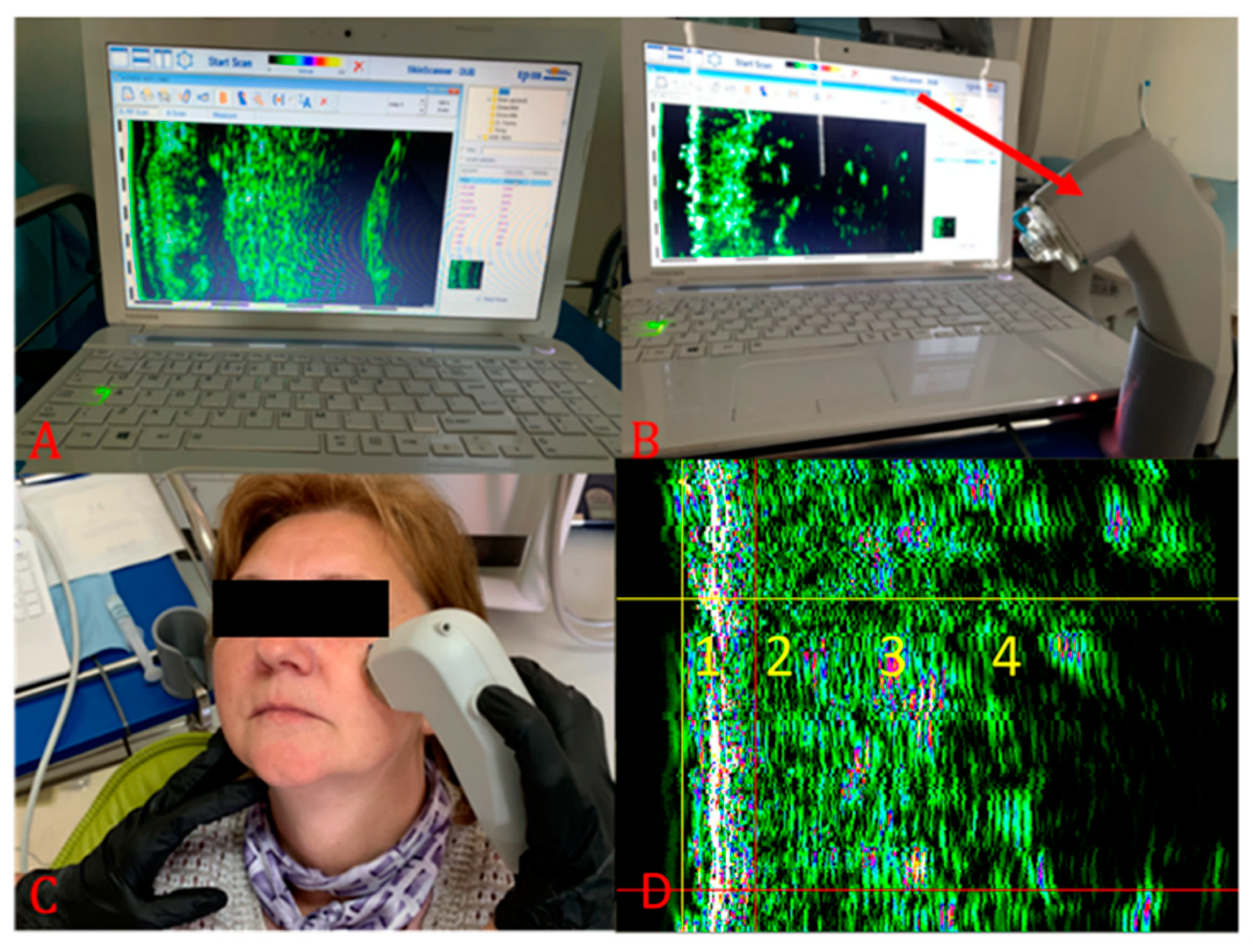
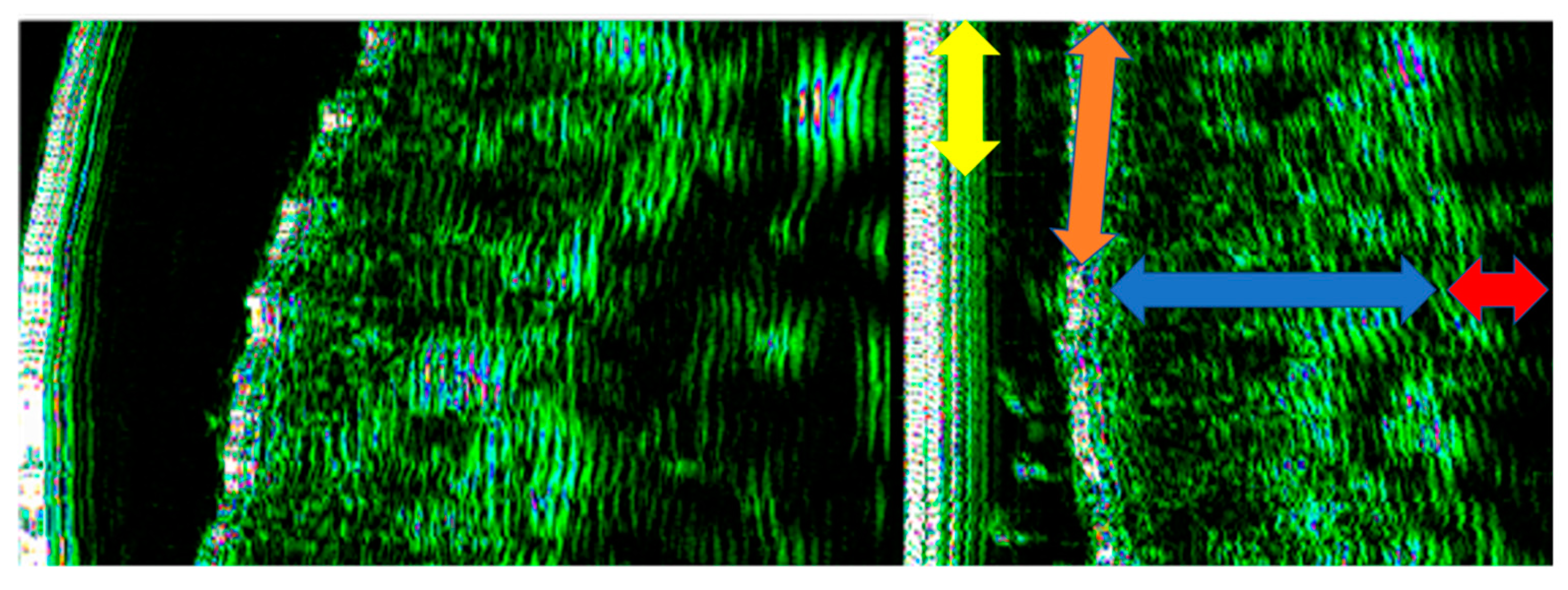
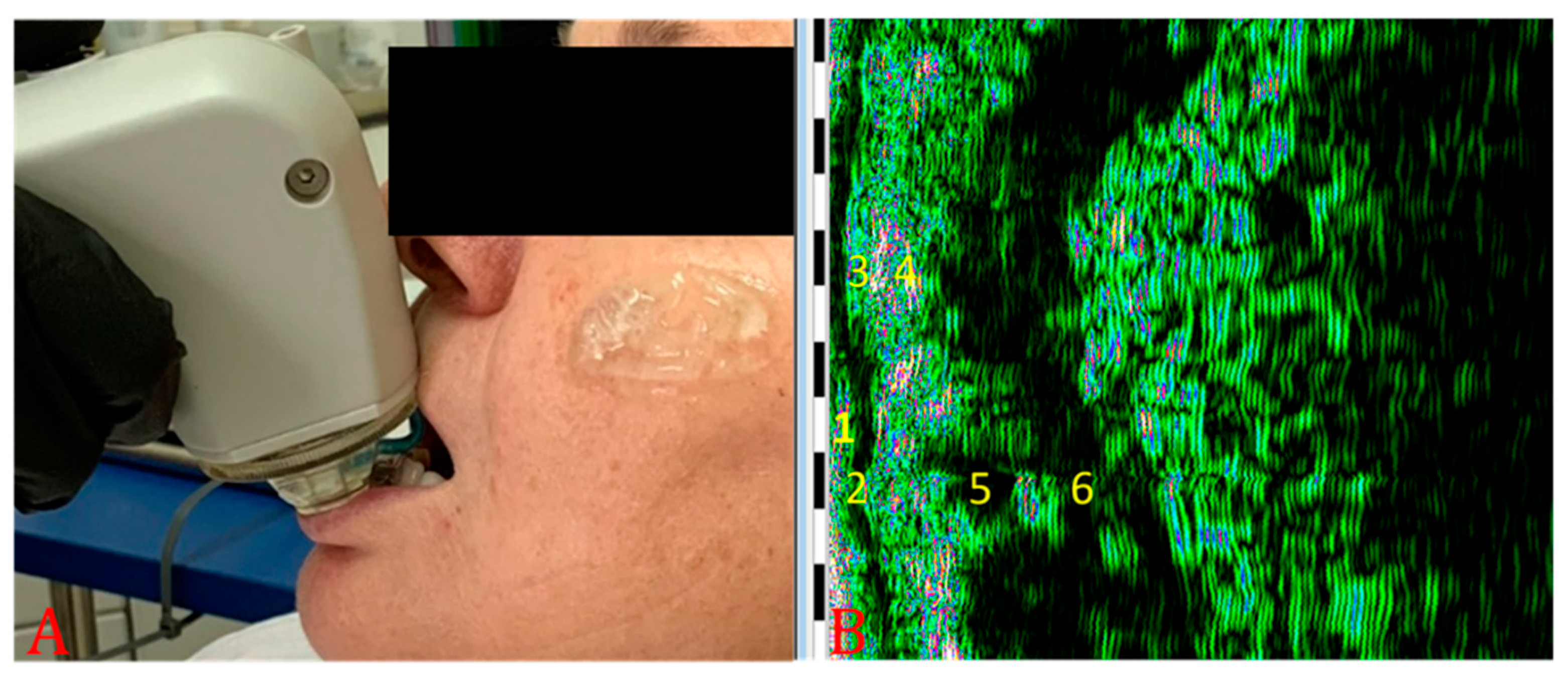
2.2. Ultrasonographic Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moldovan, M.L.; Lahmar, A.; Bogdan, C.; Părăuan, S.; Tomuță, I.; Crișan, M. Formulation and evaluation of a water-in-oil cream containing herbal active ingredients and ferulic acid. Clujul Med. 2017, 90, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Malmgaard-Clausen, N.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of Long-Term Physical Activity and Diet on Skin Glycation and Achilles Tendon Structure. Nutrients 2019, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of glycation inhibitors on aging and age-related diseases. Mech. Ageing Dev. 2016, 160, 1–18. [Google Scholar] [CrossRef]
- Sandby-Moller, J.; Wulf, H.C. Ultrasonographic subepidermal low-echogenic band, dependence of age and body site. Ski. Res. Technol. 2004, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Băbțan, A.; Boșca, B.A.; Petrescu, N.B. Accumulation of N-epsilon carboxymethyl lysine in various tissues and organs related to diet-induced aging process. State of the art and experimental animal study. HVM Bioflux 2017, 11, 106–115. [Google Scholar]
- Moldovan, M.L.; Crisan, D.; Lupsor, M.; Badea, R.; Crisan, M. Ultrasonographic assessment of the cutaneous changes induced by topical flavonoid therapy. Clin. Cosmet. Investig. Dermatol. 2012, 5, 7–13. [Google Scholar] [CrossRef][Green Version]
- Parikh, R.M.; Mohan, V. Changing definitions of metabolic syndrome. Indian J. Endocrinol. Metab. 2012, 16, 7–12. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ibuki, A.; Minematsu, T.; Sugama, J.; Horii, M.; Ogai, K.; Nishizawa, T.; Dai, M.; Sato, A.; Fujimoto, Y.; et al. Structural changes in dermal collagen and oxidative stress levels in the skin of Japanese overweight males. Int. J. Cosmet. Sci. 2014, 36, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Evirgen, Ş.; Kamburoğlu, K. Review on the applications of ultrasonography in dentomaxillofacial region. World J. Radiol. 2016, 8, 50–58. [Google Scholar] [CrossRef]
- Chifor, R.; Badea, A.F.; Chifor, I.; Mitrea, D.-A.; Crisan, M.; Badea, M.E. Periodontal evaluation using a non-invasive imaging method (ultrasonography). Med. Pharm. Rep. 2019, 92, S20–S32. [Google Scholar] [CrossRef]
- Crisan, M.; Crisan, D.; Sannino, G.; Lupsor, M.; Badea, R.; Amzica, F.; Lupsor-Platon, M. Ultrasonographic staging of cutaneous malignant tumors: An ultrasonographic depth index. Arch. Dermatol. Res. 2013, 305, 305–313. [Google Scholar] [CrossRef]
- Vergilio, M.M.; Vasques, L.I.; Leonardi, G.R. Characterization of skin aging through high-frequency ultrasound imaging as a technique for evaluating the effectiveness of anti-aging products and procedures: A review. Ski. Res. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikova, A.N.; Molochkov, V.A.; Selezneva, E.V.; Belova, L.A.; Bezugly, A.; Molochkov, A.V. Ultrasonographic features of superficial and nodular basal cell carcinoma. Med. Ultrason. 2018, 20, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Wortsman, J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Allan, E. Pulsed ultrasound measurements of depth and regression of basal cell carcinomas after photodynamic therapy: Relationship to probability of 1-year local control. Br. J. Dermatol. 2003, 149, 1035–1040. [Google Scholar] [CrossRef]
- Li, X.; Yue, W.; Chen, Y.; Luo, Y.; Gu, P.; Cao, L.; Yang, F.; Huang, D.; Hu, H. Assessments of carotid adventitial remodeling in metabolic syndrome patients using high-frequency ultrasound. Zhonghua Yi Xue Za Zhi 2015, 95, 52–55. [Google Scholar] [PubMed]
- Huzaira, M.; Rius, F.; Rajadhyaksha, M.; Anderson, R.R.; González, S. Topographic Variations in Normal Skin, as Viewed by In Vivo Reflectance Confocal Microscopy. J. Investig. Dermatol. 2001, 116, 846–852. [Google Scholar] [CrossRef]
- Hertzberg, O.; Bauer, A.; Küderle, A.; Pleitez, M.A.; Mäntele, W. Depth-selective photothermal IR spectroscopy of skin: Potential application for non-invasive glucose measurement. Analyst 2017, 142, 495–502. [Google Scholar] [CrossRef]
- Olesen, C.M.; Fuchs, C.S.K.; Philipsen, P.A.; Hædersdal, M.; Agner, T.; Clausen, M.-L. Advancement through epidermis using tape stripping technique and Reflectance Confocal Microscopy. Sci. Rep. 2019, 9, 12217. [Google Scholar] [CrossRef] [PubMed]
- Bridal, S.L.; Fournier, C.; Coron, A.; Leguerney, I.; Laugier, P. Ultrasonic Backscatter and Attenuation (11–27 MHz) Variation with Collagen Fiber Distribution in Ex Vivo Human Dermis. Ultrason. Imaging 2006, 28, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Crişan, M.; Cappare, G.; Neamţiu, L.; Chiorean, I.; Lupşa, L.; Crişan, D.; Badea, R. Multicriteria Optimization Model for the Study of the Efficacy of Skin Antiaging Therapy. Comput. Math. Methods Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.; Rajabi-Estarabadi, A.; Zartab, H.; Pazhohi, N.; Fanian, F.; Janani, L. The influence of gender and age on the thickness and echo-density of skin. Ski. Res. Technol. 2016, 23, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Crisan, D.; Roman, I.; Scharffetter-Kochanek, K.; Crisan, M.; Badea, R. The role of vitamin C in pushing back the boundaries of skin aging: An ultrasonographic approach. Clin. Cosmet. Investig. Dermatol. 2015, 8, 463–470. [Google Scholar] [CrossRef]
- Störchle, P.; Müller, W.; Sengeis, M.; Ahammer, H.; Fürhapter-Rieger, A.; Bachl, N.; Lackner, S.; Mörkl, S.; Holasek, S. Standardized Ultrasound Measurement of Subcutaneous Fat Patterning: High Reliability and Accuracy in Groups Ranging from Lean to Obese. Ultrasound Med. Biol. 2016, 43, 427–438. [Google Scholar] [CrossRef]
- Selkow, N.M.; Pietrosimone, B.G.; Saliba, S.A. Subcutaneous Thigh Fat Assessment: A Comparison of Skinfold Calipers and Ultrasound Imaging. J. Athl. Train. 2011, 46, 50–54. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, Y.Y.; Park, S.G.; Kang, N. Age-dependent facial subcutaneous fat thickness by high-frequency medical diagnostic ultrasound system. Ski. Res. Technol. 2020, 26, 769–771. [Google Scholar] [CrossRef]
- Martin, A.D.; Daniel, M.; Drinkwater, D.T.; Clarys, J.P. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 79–83. [Google Scholar] [CrossRef]
- Murphy, R.A.; Register, T.C.; Shively, C.A.; Carr, J.; Ge, Y.; Heilbrun, M.E.; Cummings, S.R.; Koster, A.; Nevitt, M.C.; Satterfield, S.; et al. Adipose Tissue Density, a Novel Biomarker Predicting Mortality Risk in Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 109–117. [Google Scholar] [CrossRef]
- Serda, R.E.; Ruiz-Esparza, G.U.; Flores-Arredondo, J.H.; Segura-Ibarra, V.; Torre-Amione, G.; Blanco, E.; Ferrari, M. The physiology of cardiovascular disease and innovative liposomal platforms for therapy. Int. J. Nanomed. 2013, 8, 629–640. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Akase, T.; Nagase, T.; Huang, L.; Ibuki, A.; Minematsu, T.; Nakagami, G.; Ohta, Y.; Shimada, T.; Aburada, M.; Sugama, J.; et al. Aging-Like Skin Changes Induced by Ultraviolet Irradiation in an Animal Model of Metabolic Syndrome. Biol. Res. Nurs. 2011, 14, 180–187. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [PubMed]
- Barsh, G.S. What Controls Variation in Human Skin Color? PLoS Biol. 2003, 1, e27. [Google Scholar] [CrossRef]
- Vashi, N.A.; Maymone, M.B.D.C.; Kundu, R.V. Aging Differences in Ethnic Skin. J. Clin. Aesthetic Dermatol. 2016, 9, 31–38. [Google Scholar]


| Characteristic | Without MS (N = 78) | MS (N = 24) | p | |
|---|---|---|---|---|
| Age | 49 (36; 60) | 52 (42; 59) | 0.8 * | |
| Gender | Male | 22 (28.2%) | 10 (41.7%) | 0.3 ** |
| Female | 56 (71.8%) | 14 (58.3%) | ||
| BMI | 26.9 (23.6; 31.4) | 29 (22.6; 32) | 0.01 * | |
| HDL (mg/dL) | 52 (41.3; 64.7) | 45.1 (40.1; 48.2) | 0.04 * | |
| TC (mg/dL) | 178.2 (147.7; 208.1) | 190.8 (163.1; 255.9) | 0.2 * | |
| Tg (mg/dL) | 122.6 (81.5; 170.1) | 160.1 (130.4; 190.5) | 0.001 * | |
| LDL (mg/dL) | 119.8 (89.3; 146.1) | 164 (127.5; 217.1) | 0.02 * | |
| Ischemic Cardiac Diseases | 3 (3.8%) | 5 (20.8%) | 0.006 ** | |
| Arterial Hypertension | 6 (7.7%) | 18 (75%) | <0.001 ** | |
| Diabetes mellitus | 1 (1.3%) | 5 (5.9%) | 0.003 ** | |
| Hypo-HDL | 26 (33.3%) | 20 (83.3%) | <0.001 ** | |
| Hyper-TG | 15 (19.2%) | 18 (75%) | <0.001 ** | |
| Abdominal obesity | 30 (38.5%) | 19 (79,2%) | 0.001 ** | |
| Fitzpatrick skin phenotype | 2 | 4 (5.1%) | - | 0.4 ** |
| 3 | 55 (70.5%) | 19 (79.2%) | ||
| 4 | 19 (24.4%) | 5 (20.8%) | ||
| Smoker | 41 (52.6%) | 8 (33.3%) | 0.1 ** | |
| Variable | Non-MS Patients (N = 78) | MS (N = 24) | p |
|---|---|---|---|
| Epidermis depth (µm) | 289 (266; 334) | 266 (251.5; 299) | 0.008 |
| Epidermis no. of px | 4657 (4173; 5228) | 4182 (4034.5; 4864) | 0.041 |
| Epidermis density | 60.01 (43.68; 78.32) | 53.41 (46.68; 72.89) | 0.813 |
| Aged dermis depth (µm) | 605.5 (497.5; 738.25) | 523.5 (367; 648.75) | 0.037 |
| Aged dermis no. of px | 9475 (8057.25; 11,572.75) | 7941 (5814.75; 10,915.5) | 0.091 |
| Aged dermis density | 12.43 (9.169; 15.86) | 12.375 (7.035; 21.485) | 0.328 |
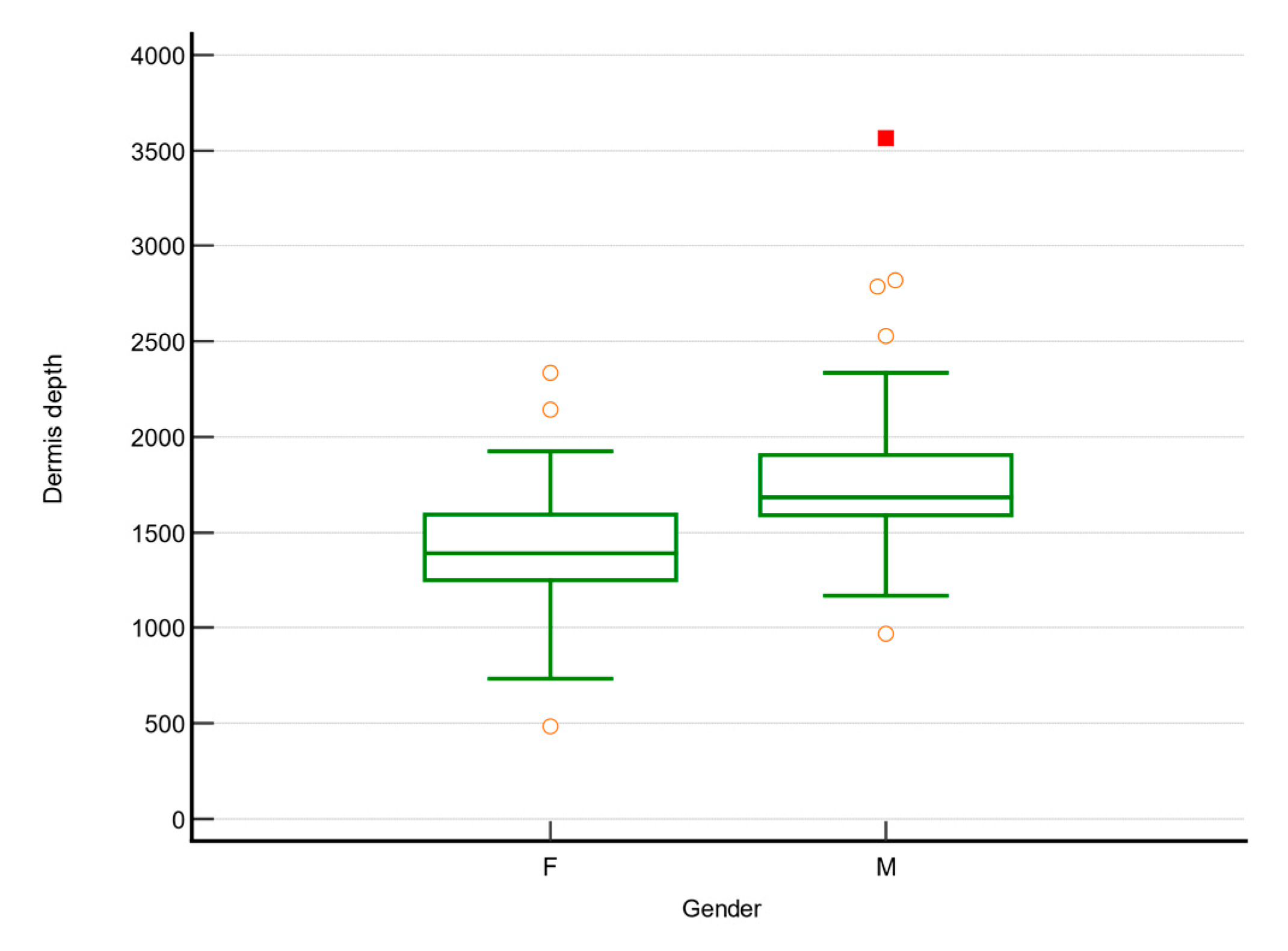
| Dermis depth (µm) | 1586 (1341.75; 1711) | 1375 (1300.75; 1759.5) | 0.731 |
| Dermis no. of px | 24,185 (20,896; 26,433.75) | 21,415 (20,519.75; 27,670.5) | 0.485 |
| Dermis density | 17.95 (13.52; 24.54) | 16.3 (14; 28.95) | 0.944 |
| Subcutaneous tissue depth (µm) | 1332 (999.75; 1894.25) | 1396 (1040.75; 1681.75) | 0.444 |
| Subcutaneous tissue no. of pixels | 20,935.5 (15,903.25; 29,461.5) | 22,245 (16,279.5; 26,468) | 0.711 |
| Subcutaneous tissue density | 8.1 (5.3; 13.2) | 6.8 (5.2; 10.4) | 0.3 |
| Type 2 | Type 3 | Type 4 | p | |
|---|---|---|---|---|
| Nonker. mucosa depth | 586 (404; 781.5) | 352 (305; 422) | 336 (273; 375) | 0.008 |
| Nonker. mucosa no of px | 7373 (6263.5; 11,085) | 5880 (5104; 6966) | 5658 (4284; 6324) | 0.035 |
| Nonker. mucosa density | 12.745 (4.605; 28.992) | 23.07 (14.8; 36.76) | 21.86 (16.33; 47.22) | 0.213 |
| Lamina propriae depth | 1129 (859.25; 1949.25) | 828 (625; 1023) | 875 (594; 1234) | 0.142 |
| Lamina propriae no of px | 18,019.5 (13,855.5; 29,681.25) | 12,980 (9628; 15.776) | 16,214 (9933; 18,802) | 0.084 |
| Lamina propriae density | 21.805 (15.627; 39.937) | 39.49 (29.08; 54.77) | 47.09 (28.26; 58.84) | 0.122 |
| Lip hypodermis depth | 1129 (859.25; 1949.25) | 828 (625; 1023) | 875 (594; 1234) | 0.636 |
| Inf. Lip hypodermis no of px | 5.315 (3.817; 9.917) | 11.93 (9.20; 20.68) | 12.45 (6.69; 31.18) | 0.846 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băbțan, A.M.; Vesa, Ș.C.; Boșca, B.A.; Crișan, M.; Mihu, C.M.; Băciuț, M.F.; Dinu, C.; Crișan, B.; Câmpian, R.S.; Feurdean, C.N.; et al. High-Frequency Ultrasound Assessment of Skin and Oral Mucosa in Metabolic Syndrome Patients—A Cross-Sectional Study. J. Clin. Med. 2021, 10, 4461. https://doi.org/10.3390/jcm10194461
Băbțan AM, Vesa ȘC, Boșca BA, Crișan M, Mihu CM, Băciuț MF, Dinu C, Crișan B, Câmpian RS, Feurdean CN, et al. High-Frequency Ultrasound Assessment of Skin and Oral Mucosa in Metabolic Syndrome Patients—A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(19):4461. https://doi.org/10.3390/jcm10194461
Chicago/Turabian StyleBăbțan, Anida Maria, Ștefan Cristian Vesa, Bianca Adina Boșca, Maria Crișan, Carmen Mihaela Mihu, Mihaela Felicia Băciuț, Cristian Dinu, Bogdan Crișan, Radu Septimiu Câmpian, Claudia Nicoleta Feurdean, and et al. 2021. "High-Frequency Ultrasound Assessment of Skin and Oral Mucosa in Metabolic Syndrome Patients—A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 19: 4461. https://doi.org/10.3390/jcm10194461
APA StyleBăbțan, A. M., Vesa, Ș. C., Boșca, B. A., Crișan, M., Mihu, C. M., Băciuț, M. F., Dinu, C., Crișan, B., Câmpian, R. S., Feurdean, C. N., Ionel, A., Bezugly, A., Bordea, I. R., & Ilea, A. (2021). High-Frequency Ultrasound Assessment of Skin and Oral Mucosa in Metabolic Syndrome Patients—A Cross-Sectional Study. Journal of Clinical Medicine, 10(19), 4461. https://doi.org/10.3390/jcm10194461










