Plasma Levels of Apelinergic System Components in Patients with Chronic and Acute Coronary Syndromes—A Pilot Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients Characteristic
2.2. Ethical Consideration
2.3. Biochemical Determinations
2.4. Echocardiographic Data
2.5. Statistical Analysis
3. Results
3.1. Basic Characteristic of Patients with CCS and ACS
3.2. Circulating Levels of ELA, AP-13, AP-17 and APJ Receptor in CAD Patients and Healthy Controls
3.3. Diagnostic Potential of Circulating ELA and AP-17 Levels in Acute Coronary Syndrome in CAD Patients
4. Discussion
4.1. Levels of Components of the Apelinergis System in Chronic Coronary Syndromes
4.2. Levels of Components of the Apelinergic System in Acute Coronary Syndromes
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piepoli, M.F.; Abreu, A.; Albus, C.; Ambrosetti, M.; Brotons, C.; Catapano, A.L.; Corra, U.; Cosyns, B.; Deaton, C.; Graham, I.; et al. Update on cardiovascular prevention in clinical practice: A position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2020, 27, 181–205. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Van de Voorde, J.; Pauwels, B.; Boydens, C.; Decaluwe, K. Adipocytokines in relation to cardiovascular disease. Metab. Clin. Exp. 2013, 62, 1513–1521. [Google Scholar] [CrossRef]
- Liu, W.; Yan, J.; Pan, W.; Tang, M. Apelin/Elabela-APJ: A novel therapeutic target in the cardiovascular system. Ann. Transl. Med. 2020, 8, 243. [Google Scholar] [CrossRef]
- Marsault, E.; Llorens-Cortes, C.; Iturrioz, X.; Chun, H.J.; Lesur, O.; Oudit, G.Y.; Auger-Messier, M. The apelinergic system: A perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 2019, 1455, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cao, J.; Li, L.; Chen, L. Elabela, a new endogenous ligand of APJ, functions in embryos and adults organisms. Acta. Biochim. Biophys. Sin. 2017, 49, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, K.; Kenward, C.; Rainey, J.K. Apelinergic system structure and function. Compr. Physiol. 2017, 8, 407–450. [Google Scholar] [PubMed]
- Kuba, K.; Sato, T.; Imai, Y.; Yamaguchi, T. Apelin and elabela/toddler; double ligands for APJ/apelin receptor in heart development, physiology, and pathology. Peptides 2019, 111, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Read, C.; Nyimanu, D.; Williams, T.L.; Huggins, D.J.; Sulentic, P.; Macrae, R.G.C.; Yang, P.; Glen, R.C.; Maguire, J.J.; Davenport, A.P. International union of basic and clinical pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacol. Rev. 2019, 71, 467–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Marx, P.; Besserer-Offroy, É.; Longpré, J.M.; Lainé, J.; Reversade, B.; Salvail, D.; et al. discovery and structure-activity relationship of a bioactive fragment of elabela that modulates vascular and cardiac functions. J. Med. Chem. 2016, 59, 2962–2972. [Google Scholar] [CrossRef]
- Perjés, Á.; Kilpiö, T.; Ulvila, J.; Magga, J.; Alakoski, T.; Szabó, Z.; Vainio, L.; Halmetoja, E.; Vuolteenaho, O.; Petäjä-Repo, U.; et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res. Cardiol. 2016, 111, 2. [Google Scholar] [CrossRef]
- Kleinz, M.J.; Davenport, A.P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 2004, 118, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. Elabela: A hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013, 27, 672–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauli, A.; Valen, E.; Schier, A.F. Identifying (non-)coding RNAs and small peptides: Challenges and opportunities. Bioessays 2015, 37, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 2017, 135, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Sunjaya, A.P.; Sunjaya, A.F.; Ferdinal, F. Apela/elabela/toddler: New perspectives in molecular mechanism of heart failure. Glob. Cardiol. Sci. Pract. 2019, 2, e201915. [Google Scholar] [CrossRef]
- Rakhshan, K.; Azizi, Y.; Naderi, N.; Afousi, A.G.; Aboutaleb, N. ELABELA (ELA) peptide exerts cardioprotection against myocardial infarction by targeting oxidative stress and the improvement of heart function. Int. J. Pept. Res. Ther. 2018, 25, 613–621. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Seeman, H.; Montecucco, F.; da Silva, A.R.; Burger, F.; Costa-Fraga, F.P.; Anguenot, L.; Mach, F.; Dos Santos, A.S.R.; Stergiopulos, N. Apelin-13 treatment enhances the stability of atherosclerotic plaques. Eur. J. Clin. Investig. 2018, 48, e12891. [Google Scholar] [CrossRef]
- Akboga, M.K.; Akyel, A.; Sahinarslan, A.; Demirtas, C.Y.; Yayla, C.; Boyaci, B.; Yalcin, R. Relationship between plasma apelin level and coronary collateral circulation. Atherosclerosis 2014, 235, 289–294. [Google Scholar] [CrossRef]
- Liu, X.Y.; Lu, Q.; Ouyang, X.P.; Tang, S.L.; Zhao, G.J.; Lv, Y.C.; He, P.P.; Kuang, H.J.; Tang, Y.Y.; Fu, Y.; et al. Apelin 13 increases expression of ATP-binding cassette transposrter A1 via activatong protein kinase C alpha signalling in THP-1 macrophage-derived foam cell. Atherosclerosis 2013, 226, 398–407. [Google Scholar] [CrossRef]
- Chun, H.J.; Ali, Z.A.; Kojima, Y.; Kundu, R.K.; Sheikh, A.Y.; Agrawal, R.; Zheng, L.; Leeper, N.J.; Patterson, A.J.; Anderson, J.P.; et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Investig. 2008, 118, 3343–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, X.; Ouyang, S.; He, J.; Yu, B.; Lin, X.; Zhang, Q.; Tao, J. Declined circulating elabela levels in patients with essential hypertension and its association with impaired vascular function: A preliminary study. Clin. Exp. Hypertens. 2020, 42, 239–243. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 2015, 5, 8170. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Gatselis, N.K.; Makaritsis, K.; Dalekos, G.N. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013, 227, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Luo, G.; Zheng, Y.; Hu, D.; Peng, F.; Xie, L. Lowered circulating apelin is significantly associated with an increased risk for hypertension: A meta-analysis. Clin. Exp. Hypertens. 2017, 39, 435–440. [Google Scholar] [CrossRef]
- Dönmez, Y.; Acele, A. Increased elabela levels in the acute ST segment elevation myocardial infarction patients. Medicine 2019, 98, e17645. [Google Scholar] [CrossRef]
- Du, S.L.; Yang, X.C.; Zhong, J.C.; Wang, L.F.; Fan, Y.F. Plasma levels of elabela are associated with coronary angiographic severity in patients with acute coronary syndrome. J. Geriatr. Cardiol. 2020, 17, 674–679. [Google Scholar] [PubMed]
- Acele, A.; Bulut, A.; Donmez, Y.; Koc, M. Plasma elabela level significantly increased in patients with complete heart block. Braz. J. Cardiovasc. Surg. 2020, 35, 683–688. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Chen, Y.; Xie, S.; Sng, D.; Reversade, B.; Yang, T. ELABELA antagonizes intrarenal renin-angiotensin system to lower blood pressure and protects against renal injury. Am. J. Physiol. Renal. Physiol. 2020, 318, F1122–F1135. [Google Scholar] [CrossRef]
- Wang, Y. Recombinant Elabela-Fc fusion protein had extended plasma half-life and mitogates post-infarct heart dysfunction in rats. Int. J. Cardiol. 2020, 300, 217–218. [Google Scholar] [CrossRef]
- Gupta, M.D.; Girish, M.P.; Shah, D.; Rain, M.; Mehta, V.; Tyagi, S.; Trehan, V.; Pasha, Q. Biochemical and genetic role of apelin in essential hypertension and acute coronary syndrome. Int. J. Cardiol. 2016, 223, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Chen, M.; Yu, J.; Li, W.J.; Tao, L.; Li, Y.; Guo, W.Y.; Wang, H.C. Plasma apelin level predicts the major adverse cardiac events in patients with ST elevation myocardial infarction receiving percutaneous coronary intervention. Medicine 2015, 94, e449. [Google Scholar] [CrossRef] [PubMed]
- Toczylowski, K.; Hirnle, T.; Harasiuk, D.; Zabielski, P.; Lewczuk, A.; Dmitruk, I.; Ksiazek, M.; Sulik, A.; Gorski, J.; Chabowski, A.; et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J. Transl. Med. 2019, 17, 310. [Google Scholar] [CrossRef] [PubMed]
| Variable | Healthy Control (n = 33) | Chronic Coronary Syndrome (CCS) (n = 30) | Acute Coronary Syndrome (ACS) (n = 84) | p-Value (Fischer Exact Test or ANOVA) |
|---|---|---|---|---|
| Gender: Male Female | 29 (87.9) 4 (12.1) | 22 (73.3) 8 (26.7) | 68 (80.9) 16 (19.1) | 0.336 |
| Age (Years) | 57.91 ± 4.40 | 61.13 ± 8.42 | 59.11 ± 8.96 | 0.278 |
| BMI (kg/m2) | 24.83 ± 2.38 | 27.11 ± 2.72 | 27.17 ± 3.96 | 0.739 |
| Variable | Chronic Coronary Syndrome (CCS) (n = 30) | Acute Coronary Syndrome (ACS) (n = 84) | p-Value (Chi-Square, Fischer Exact or Student t Tests) |
|---|---|---|---|
| Smoking History: | 0.001 * | ||
| No | 24 (80.0) | 37 (44.0) | |
| Yes | 6 (20.0) | 47 (56.0) | |
| Family History of CAD: | 0.633 | ||
| No | 20 (66.7) | 60 (71.4) | |
| Yes | 10 (33.3) | 24 (28.6) | |
| Hg (g/dL) | 13.91 ± 1.38 | 14.32 ± 2.19 | 0.345 |
| Glu (mg/mL) | 122.82 ± 39.69 | 136.68 ± 53.84 | 0.214 |
| TCh (mg/mL) | 146.80 ± 39.76 | 180.84 ± 36.29 | <0.0001 * |
| HDL (mg/mL) | 49.00 ± 11.25 | 44.27 ± 10.82 | 0.051 # |
| LDL (mg/mL) | 71.72 ± 35.55 | 109.16 ± 39.99 | <0.0001 * |
| TG (mg/mL) | 120.26 ± 54.53 | 146.52 ± 80.06 | 0.105 |
| CRP (mg/L) | 2.91 ± 1.28 | 13.72 ± 9.12 | <0.0001 * |
| Hs-Troponin T (ng/L) | 17.25 ± 16.41 | 3081.27 ± 2387.14 | <0.0001 * |
| CK-MB (IU/L) | 15.69 ± 6.51 | 115.82 ± 102.76 | <0.0001 * |
| NT-proBNP (pg/mL) | 169.57 ± 110.05 | 835.76 ± 646.38 | 0.007 * |
| LVEF (%) | 59.63 ± 4.68 | 44.72 ± 7.72 | <0.0001 * |
| STEMI: | - | ||
| No | - | 30 (35.7) | |
| Yes | - | 54 (64.3) |
| Variable | Healthy Control (n = 33) (A) | Chronic CAD (CCS) (n = 30) (B) | Acute Coronary Syndrome (ACS), (n = 84) (C) | p-Value (ANOVA) |
|---|---|---|---|---|
| Elabela (pg/mL) | 1176.00 ± 237.52 B,C | 784.30 ± 371.70 A,C | 1477.82 ± 229.83 A,B | <0.0001 * |
| Apelin-13 (pg/mL) | 59.61 ± 14.35 C | 62.67 ± 10.46 | 69.18 ± 15.97 A | 0.004 * |
| Apelin-17 (pg/mL) | 511.09 ± 85.74 B,C | 341.68 ± 90.44 A,C | 627.34 ± 168.66 A,B | <0.0001 * |
| APJ Receptor (pg/mL) | 1018.77 ± 333.41 B,C | 1510.90 ± 205.90 A,C | 1383.28 ± 204.97 A,B | <0.0001 * |
| Variables | Correlation | ELA (pg/mL) | AP-13 (pg/mL) | AP-17 (pg/mL) | APJ Receptor (pg/mL) |
|---|---|---|---|---|---|
| Hs-Troponin T (ng/mL) | r p-Value | 0.336 0.001 * | −0.042 0.691 | 0.293 0.005 * | −0.069 0.520 |
| CK-MB (IU/L) | r p-Value | 0.387 <0.0001 * | 0.043 0.685 | 0.407 <0.0001 * | 0.050 0.640 |
| NT-proBNP (pg/mL) | r p-Value | 0.143 0.180 | 0.023 0.826 | 0.194 0.069 | −0.151 0.157 |
| LVEF (%) | r p-Value | −0.537 <0.0001 * | −0.097 0.363 | −0.417 <0.0001 * | 0.059 0.582 |
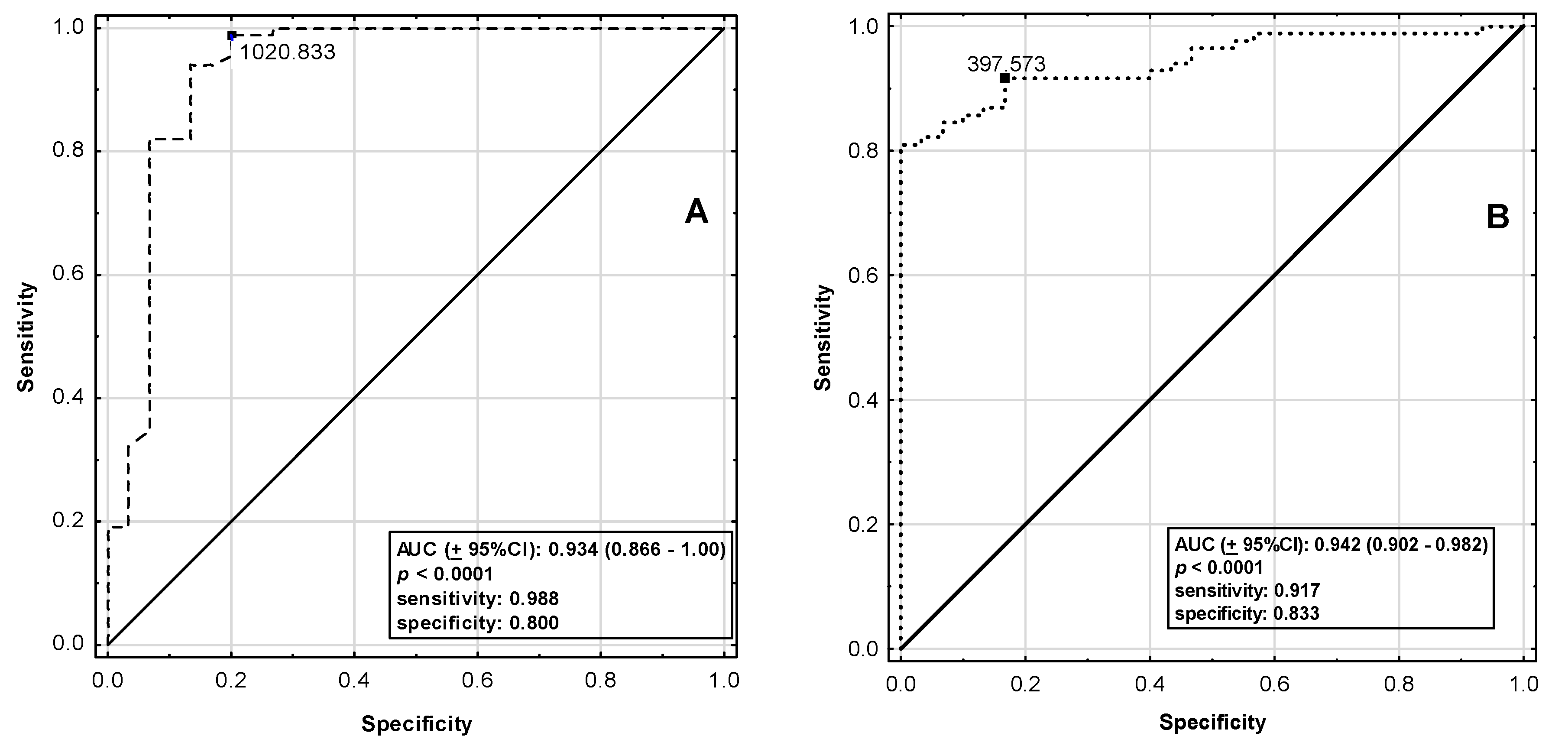
| ELA | AP-17 | |
|---|---|---|
| AUC (±95%CI) | 0.934 (0.866–1.00) | 0.942 (0.902–0.982) |
| p-Value | <0.0001 | <0.0001 |
| SE | 0.035 | 0.020 |
| Cut-Off Point (pg/mL) | 1020.83 | 397.57 |
| Sensitivity | 0.988 | 0.917 |
| Specificity | 0.800 | 0.833 |
| Accuracy | 0.939 | 0.895 |
| LR (+) | 0.200 | 0.167 |
| LR (−) | 0.012 | 0.083 |
| PPV | 0.933 | 0.939 |
| NPV | 0.960 | 0.781 |
| Youden’s Index | 0.788 | 0.750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diakowska, D.; Wyderka, R.; Krzystek-Korpacka, M.; Osuch, Ł.; Leśków, A.; Sołtowska, A.; Stanek, M.; Rosińczuk, J.; Jaroch, J. Plasma Levels of Apelinergic System Components in Patients with Chronic and Acute Coronary Syndromes—A Pilot Study. J. Clin. Med. 2021, 10, 4420. https://doi.org/10.3390/jcm10194420
Diakowska D, Wyderka R, Krzystek-Korpacka M, Osuch Ł, Leśków A, Sołtowska A, Stanek M, Rosińczuk J, Jaroch J. Plasma Levels of Apelinergic System Components in Patients with Chronic and Acute Coronary Syndromes—A Pilot Study. Journal of Clinical Medicine. 2021; 10(19):4420. https://doi.org/10.3390/jcm10194420
Chicago/Turabian StyleDiakowska, Dorota, Rafal Wyderka, Małgorzata Krzystek-Korpacka, Łukasz Osuch, Anna Leśków, Alicja Sołtowska, Marta Stanek, Joanna Rosińczuk, and Joanna Jaroch. 2021. "Plasma Levels of Apelinergic System Components in Patients with Chronic and Acute Coronary Syndromes—A Pilot Study" Journal of Clinical Medicine 10, no. 19: 4420. https://doi.org/10.3390/jcm10194420
APA StyleDiakowska, D., Wyderka, R., Krzystek-Korpacka, M., Osuch, Ł., Leśków, A., Sołtowska, A., Stanek, M., Rosińczuk, J., & Jaroch, J. (2021). Plasma Levels of Apelinergic System Components in Patients with Chronic and Acute Coronary Syndromes—A Pilot Study. Journal of Clinical Medicine, 10(19), 4420. https://doi.org/10.3390/jcm10194420






