Abstract
Severe aplastic anemia (SAA) is a bone marrow failure syndrome that can be treated with hematopoietic cell transplantation (HCT) or immunosuppressive (IS) therapy. A retrospective cohort of 56 children with SAA undergoing transplantation with fludarabine–cyclophosphamide–ATG-based conditioning (FluCyATG) was analyzed. The endpoints were overall survival (OS), event-free survival (EFS), cumulative incidence (CI) of graft versus host disease (GVHD) and CI of viral replication. Engraftment was achieved in 53/56 patients, and four patients died (two due to fungal infection, and two of neuroinfection). The median time to neutrophil engraftment was 14 days and to platelet engraftment was 16 days, and median donor chimerism was above 98%. The overall incidence of acute GVHD was 41.5%, and that of grade III-IV acute GVHD was 14.3%. Chronic GVHD was diagnosed in 14.2% of children. The probability of 2-year GVHD-free survival was 76.1%. In the univariate analysis, a higher dose of cyclophosphamide and previous IS therapy were significant risk factors for worse overall survival. Episodes of viral replication occurred in 33/56 (58.9%) patients, but did not influence OS. The main advantages of FluCyATG include early engraftment with a very high level of donor chimerism, high overall survival and a low risk of viral replication after HCT.
1. Introduction
Severe aplastic anemia (SAA) is a rare but life-threatening hematological disorder with an extremely high risk of fatal infectious complications. The hallmark of SAA is pancytopenia caused by bone marrow (BM) hypoplasia or aplasia as a consequence of direct damage by chemical or physical factors or constitutional or acquired genetic defects, e.g., Fanconi anemia, or telomere biology disorders [1,2,3]. In the majority of SAA patients, the cause cannot be directly identified, but immune-mediated destruction of BM hematopoiesis is the most likely culprit. Autoimmunity can be triggered by alterations in antigens modified by drugs, chemical agents or viral infections and, consequently, can lead to the activation of the immunological cascade and damage to BM cells via activated T lymphocytes [2,4,5,6]. Allogeneic hematopoietic cell transplantation (HCT) from HLA-identical matched sibling donors (MSDs) is the method of choice for the treatment of SAA in children. Patients without sibling donors undergo immunosuppressive (IS) therapy or HCT from matched unrelated donors (MUDs) [6,7,8,9]. In the last 20 years, different protocols have been used in clinical practice in our center, and, at that time, in Polish children with SAA, MSD and MUD HCT were associated with a 5-year probability overall survival of 91 and 64%, respectively [10]. Treosulfan-based conditioning before MUD HCT was associated with 35% treatment-related mortality (Figure 1), and a decision was reached to introduce a combination of fludarabine, cyclophosphamide and antithymocyte globulin (FluCyATG). It must be mentioned here that patients who underwent transplantation after treosulfan had a median time of 19 months from diagnosis and a longer prior history of blood transfusions. The aim of this study was to evaluate the outcome of a conditioning protocol, FluCyATG, in children undergoing transplantation for SAA.
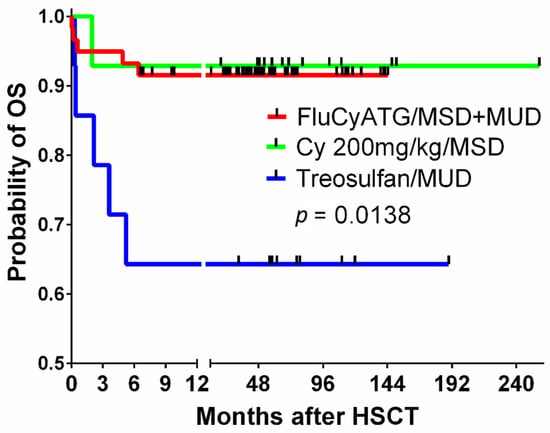
Figure 1.
The overall survival of children with SAA undergoing transplantation after the fludarabine–cyclophosphamide–ATG protocol compared to that of a historic group of MSD recipients conditioned with cyclophosphamide 200 mg/kg BW and ATG and MUD recipients after treosulfan-based conditioning. Legend. SAA, severe aplastic anemia; ATG, antithymocyte globulin, MSD, matched sibling donor; BW, body weight; MUD, matched unrelated donor.
2. Materials and Methods
The group consisted of 56 children (aged 0.8–17.9 years) with a diagnosis of SAA who underwent HCT with transplants from MSDs or MUDs. The patient characteristics are shown in Table 1.

Table 1.
Patient and transplantation characteristics.
Patients with identified constitutional syndromes and those who underwent retransplantation were not included in the analysis. The original conditioning protocol, given in 2008–2011 to 12 patients, consisted of fludarabine at a dose of 40 mg/m2 on days −8 to −5, cyclophosphamide at a dose of 50 mg/kg BW/day on days −5 to −1 and Thymoglobulin (Sanofi) at a dose of 2.5 mg/kg BW daily on days −4 to −1. The modified conditioning protocol, administered after 2011 in 44 patients, consisted of fludarabine at a dose of 30 mg/m2 on days −6 to −3 and cyclophosphamide at a dose of 750 mg/m2/day on days −6 to −3. From day −6 to day −3, antithymocyte globulin Grafalon (Neovii) at 15 mg/kg BW daily or Thymoglobulin (Sanofi) at 2.5 mg/kg BW daily was given. Peripheral blood stem cells (PBSCs) were used as a stem cell source in 31 out of 38 (82%) patients receiving a transplant from MUDs and in 6 of 17 (36%) patients receiving a transplant from MSDs; the remaining patients underwent BM transplantation. Graft versus host disease (GVHD) prophylaxis was based on ciclosporin A started on pretransplantation day –1 and methotrexate 15 mg/m2 given on posttransplantation days +1, +3 and +6. Antimicrobial guidelines are summarized in Appendix A. The patients or legal guardians gave their written informed consent for the treatment and analysis of clinical data. Ethical approval was waived by the local Ethics Committee of Wroclaw Medical University in view of the retrospective nature of the study and because all procedures were performed as a part of routine care.
Statistical Analysis
The endpoints were overall survival (OS), defined as the time from HCT to death or the last report from patients with no events, and event-free survival (EFS), defined as the time from HCT to graft rejection, second malignancy or death. Because no patients experienced events except deaths, the OS and EFS results were the same, and only OS data are presented here. GVHD-free survival (GFS) was defined as the absence of grade III-IV acute GVHD, chronic GVHD that required systemic treatment and death, similar to the composite endpoints proposed by Holtan [11].
Survival curves were estimated using the Kaplan–Meier method and compared between the cohorts by the log-rank test. Cox modeling was adopted to estimate hazard ratios for OS and EFS, considering factors with p < 0.2. Statistical analysis and data formatting for presentation were performed with the GraphPad Prism software (GraphPad Software, La Jolla, CA, USA) and STATISTICA 13.3 (TIBCO Software Inc. 2017, STATISTICA, version 13, Dell, OK, USA).
3. Results
All survival results in the study cohort are presented in Table 2. Three patients died before engraftment on days +1, +5 and +17. Two patients died of uncontrolled invasive fungal infection (one of mucormycosis and one of aspergillosis), and one died as a consequence of acute neurotoxicity of undetermined background.

Table 2.
Impact of factors affecting survival after HCT.
The remaining patients achieved trilineage bone marrow recovery, and no secondary graft failure was found. The median time to absolute neutrophil count over 500/µL was 14 days (range 10–22 days), and the median time to platelet count over 20000/µL was 16 days (range 5–212 days) (Figure 2A).
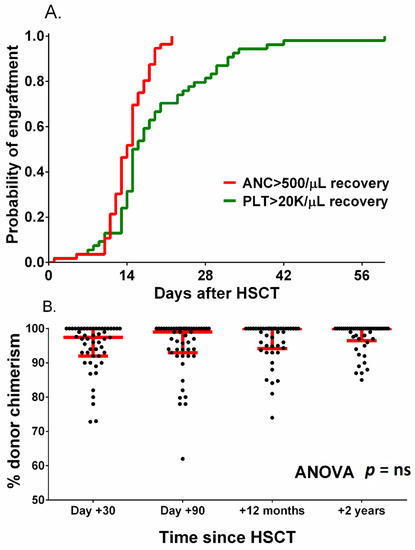
Figure 2.
Probability of neutrophil and platelet engraftment (A) and donor chimerism after HCT (B). Red lines in figure (B) represent the median with interquartile range.
Median donor chimerism at 1, 3, 12 and 24 months after HCT was 98%, 99%, 100% and 100%, respectively (Figure 2B). Among patients whose transplants engrafted, the only observed death was due to neuroinfection.
The overall incidence of acute GVHD was 41.5%, and grade III-IV aGVHD was diagnosed in 14.3% of patients. Chronic GVHD was diagnosed in 14.2% of children within 2 years of HCT, and the grade was moderate to severe in 10.4% (Figure 3B). The probability of 2-year GVHD-free survival was 76.1% (Figure 3C). In the univariate analysis, the antecedent IS protocol was the only factor associated with a significantly lower probability of overall survival (83.3 vs. 100%, p = 0.0017, Figure 3D). The reduction in the intensity of the FluCyATG protocol was associated with improved survival (97.7% vs. 85%, p = 0.0063, Figure 3E). Gender-related differences between donor and recipient affected survival (Figure 3E), with a significantly lower probability of survival with female donors (80% vs. 100%, p = 0.005, Figure 3F). The overall and graft-free survival curves of all studied factors associated with the HCT procedure are presented in Supplementary Figures S1–S3.
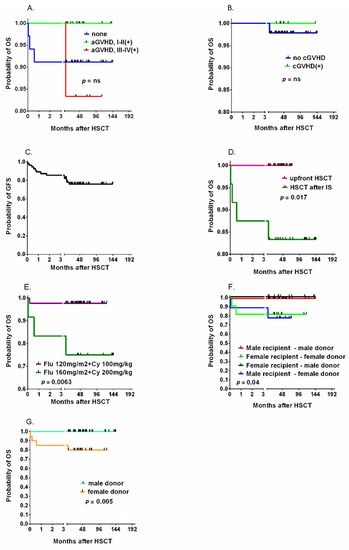
Figure 3.
The cumulative incidence of acute GVHD (A) and chronic GVHD (B) and the probability of graft-free survival (C) after HSCT. The impact of pretransplantation immunosuppressive therapy (IS) (D) and the intensity of the chemotherapy protocol (E) on the probability of OS. The role of recipient and donor genders (F) and of donor genders (G). Legend. OS, overall survival; GFS, graft versus host disease-free survival; HSCT, Allogeneic hematopoietic cell transplantation; Flu, fludarabine; Cy, cyclophosphamide; GVHD, graft versus host disease; aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease.
In addition, the probability of OS was analyzed in the two subgroups: 18 patients received transplants from MSDs after FluCyATG and a historic group of 13 patients received transplants from MSDs after cyclophosphamide 200 mg/kg BW with or without ATG; the probability of 5-year OS was 94.1 and 100%, respectively, but the difference was not statistically significant (Figure 4A). The percentage of donor chimerism between these two groups was not significantly different 1, 3, 12 and 24 months after HCT (Figure 4B).
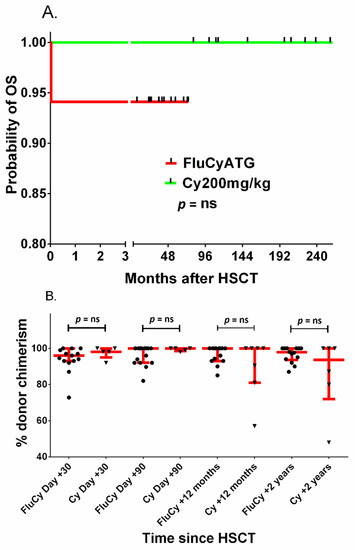
Figure 4.
Probability of OS (A) and donor chimerism (B) after HCT with fludarabine–cyclophosphamide–ATG (FluCyATG) or cyclophosphamide 200 mg/kg BW. Red lines in figure (B) represent the median with interquartile range. Legend. OS, overall survival; GFS, graft versus host disease-free survival; HSCT/HCT, Allogeneic hematopoietic cell transplantation; Flu, fludarabine; Cy, cyclophosphamide; GVHD, graft versus host disease; aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease ATG, antithymocyte globulin.
Posttransplantation viral replications were observed in 33/56 patients (58.9%). ADV viremia was found in 12.5% of patients, BKV in 28.6% of patients, CMV in 28.6% of children and EBV replication in 21.4% of children. In all patients, viral replication was asymptomatic, and preemptive treatment prevented the development of clinical manifestations. The presence of posttransplantation viral infections did not influence OS or GFS (Supplementary Figure S4).
In the Cox multivariate analysis, none of the analyzed factors were significantly associated with either OS or GFS.
4. Discussion
The results of SAA therapy in children are superior to those in the adult population, but the role of IS therapy in recent years has diminished in favor of upfront transplantation from matched unrelated donors due to the high IS failure rate, with an EFS of 33% [12]. Survival in pediatric SAA has improved dramatically for MUD HCT, due to improvements in donor typing, less toxic conditioning regimens with low-dose TBI or TBI free and the use of leukodepleted blood products [13,14,15]. The OS is higher in children who receive a transplant from an MSD than in those who receive a transplant from an MUD (96% vs. 91%, p = ns), but the long-term outcome and freedom from cGVHD have not been properly analyzed [12,16].
In our study, the HCT results in patients with SAA after FluCyATG conditioning were unquestionably good in terms of neutrophil engraftment, predominant donor chimerism and overall survival. The FluCyATG conditioning protocol was associated with an OS comparable to that of the gold-standard cyclophosphamide–ATG (CyATG) in MSD HCTs. The reduction in cyclophosphamide dose in our cohort was balanced by the effect of fludarabine, and no detrimental effect on the probability of OS or donor chimerism level was observed. Among different factors affecting OS, an impact of female donors (independent from recipient sex) was associated with inferior survival. Among non-HLA donor characteristics, sex mismatching (male recipient–female donor) is a proven risk factor for inferior survival associated with cGVHD incidence, but due to the low number of events in our study, this result must be approached with caution [17,18,19].
The introduction of fludarabine into the conditioning regimens of adults with SAA has been seen in the last 20 years [20]. FluCyATG with a cyclophosphamide dose of 200 mg/kg BW is more effective in terms of overall survival and engraftment than CyATG [21]. In the EBMT study, after using fludarabine at a dose of 120 mg/m2, cyclophosphamide at a dose of 1200 mg/m2 (40 mg/kg) and Thymoglobulin at a dose of 15 mg/kg BW, the actuarial 2-year OS was 72%, but graft rejections were observed, and posttransplantation mortality was associated with infections and GVHD [22]. A study by Resnick et al. reported an OS of 84% after fludarabine 180 mg/m2, cyclophosphamide 120 mg/kg and ATG (total dose 40 mg/kg BW) [23]. A Korean study showed improved OS and lower toxicity in patients with SAA who underwent transplantation and received cyclophosphamide at a dose of 120 mg/kg BW than in those who received a dose of 200 mg/kg BW [24].
The influence of the cyclophosphamide dose reduction in our study on the incidence of long-term sequelae was not proven due to the insufficient period of observation, but the reduction in alkylator dose can be expected to be beneficial in terms of short- and long-term toxicity. Impaired spermatogenesis is unlikely when the cyclophosphamide dose is less than 4000 mg/m2, and impaired oocytogeneis is unlikely when the cyclophosphamide is below 6000 mg/m2 [25,26]. However, the effect of fludarabine on fertility outcomes needs to be assessed further [27].
Notably, FluCyATG was highly effective in a subgroup of patients showing PNH-positive clonal disease. This experience encourages transplantation in the pediatric subtype of PNH (+) SAA with less intensive protocols than the treosulfan-based regimens used in the adult population [28]. No patient in our group showed posttransplantation clonal disease. HCT has an advantage over IS therapy through its reduced risk of myelodysplasia and leukemic transformation, although this phenomenon was mostly reported in the adult SAA population [29].
The question of the best GVHD prophylaxis is yet unanswered, but ATG is widely used in SAA. ATG has a narrow therapeutic window and therapeutic drug monitoring of ATG levels helps to optimize dosing to ensure timely T-cell immune reconstitution [30,31]. Exposure to ATG affects survival after HCT in adults, highlighting the importance of optimum ATG dosing. According to these studies, overexposure of ATG delays T-cell reconstitution and is associated with increased relapse rates and viral reactivations, whereas underexposure is associated with the incidence of GvHD and higher mortality [32]. Individualized dosing of ATG, based on lymphocyte counts rather than bodyweight, in adults has been recommended by Admiraal at al., but in children, higher exposure can be observed in patients with a higher bodyweight and/or a lower lymphocyte count pre-Thymoglobulin infusion [30,32]. The results of a mixed pediatric and adult study supported FluCyATG and CyATG as optimal regimens for MSD BMT and the use of rabbit-derived ATG in MUD settings due to a lower risk of acute GVHD [33]. Serotherapy with rabbit ATG, equine ATG or alemtuzumab in SAA transplantation settings was shown to be associated with a survival advantage, but studies also support different approaches [34]. In the randomized study by Champlin, the 5-year overall probabilities of survival after alloHCT from MSD were 74% after cyclophosphamide alone and 80% after cyclophosphamide and equine ATG, but the difference did not reach statistical significance [35]. Another serotherapy option is the administration of alemtuzumab. In a study by Marsh et al., the conditioning regimen consisted of fludarabine at a dose of 30 mg/m2 for 4 days, cyclophosphamide at a dose of 300 mg/m2 for 4 days and alemtuzumab at a median total dose of 60 mg. OS was 95% and 83% in the MSD and MUD subgroups, respectively [36]. In this study, graft failure occurred in 12% of patients, and no evaluated patient achieved full donor chimerism in T lymphocytes, which is suboptimal because idiopathic SAA is caused by oligoclonal T lymphocytes eliminating hematopoietic cells [37,38]. The administration of the same protocol in a study by Shah was associated with 100% survival, and all patients had full donor (>95%) myeloid chimerism from the 3rd month post-HCT until the last follow-up, but one third of children undergoing transplantation showed less than 50% donor cells among their T lymphocytes [39]. In one of the largest studies to date, Dufour et al. reported a 96% probability of survival in MUD patients, 91% in MSD controls and 74% survival after failed IS, which is in line with our results [12]. The conditioning regimen used in the study consisted of fludarabine 150 mg/m2, cyclophosphamide 120 mg/kg BW and alemtuzumab 0.9–1 mg/kg BW. Seventy-two percent of patients received BM transplants, which is different from our group. The 1-year CI of grade II–IV aGVHD was 10 ± 6%. There was only one case of grade III/IV aGVHD (frequency of 3.5%; in one patient receiving MUD HCT) requiring systemic immunosuppression with steroids. The 1-year CI of cGVHD was 19 ± 8% in the Dufour study, and all cases showed only limited grade GVHD with skin involvement. The median whole-blood donor chimerism at the last follow-up was 100% (range 88–100%)11. Viral infections constitute a significant cause of morbidity and mortality after HCT [40]. Dufour et al. highlighted in his study that viral reactivation was common, accounting for 49%, but no fatal outcome was reported [12]. In contrast, Im et al. observed a lower incidence of viral infections (23%), but some of them led to death, and viral reactivation was found to be an independent risk factor for lower OS in this study [41]. Similar results were reported in a Pakistani study, where any viremia was reported in 30% of children after FluCyATG, and mortality was observed in 2.7% of patients [42]. In contrast, Kang et al. recorded that 89% of patients developed viral reactivation, but only two of them died due to viral infection [43]. In our study, the incidence of the replication of any virus was 59%, but there was no impact on survival. This effect can be explained by regular viral surveillance and timely preemptive treatment.
The most common viral reactivations in our cohort were asymptomatic CMV and BKV, both detected in 28.9% of patients. These results are in line with Chaudhry et al., who detected an incidence of 27.6% for CMV infections [42]. In contrast, Im et al. recorded a lower rate of CMV reactivation—20.9%—but CMV reactivation was associated with 3% of fatalities [41]. Kang et al. observed a CMV CI of 69.1% in patients with SAA after HCT but did not describe deaths connected directly to CMV [43]. Dufour et al. observed CMV viremia in 17.2% of patients, and no fatal cases were described [12]. The incidence of BKV seems to be low among children with SAA after HCT [44]. In children with malignancies, the incidence of BKV was shown to be 25–62%, and up to 27% patients developed BKV-hemorrhagic cystitis [45]. Malignant indications were reported in multiple studies to be associated with symptomatic BKV infection, but this can be explained by a more intensive conditioning protocol with uroepithelial damage and intensive immune suppression [45,46,47]. In our cohort, despite BKV replication in 29% of HCT recipients, the infections were asymptomatic and not associated with death. In contrast to our results, Chaudhry observed BKV replication in only 2.7% of patients [42]. Similarly, Giraud et al. observed that hemorrhagic cystitis and BK viruria were less common in patients receiving RIC than in those receiving full conditioning [44].
The risk of ADV infections is increased among pediatric patients, and higher infection rates have been reported over the last few decades [48]. We reported an ADV incidence of 12.5%, which was lower than the 25–50% reported in other studies [49,50]. Moreover, patients were asymptomatic, and ADV viremia and/or viruria did not influence survival. Interestingly, in a study by Dufour et al., in children with SAA who underwent transplantation and received the FCC protocol, the ADV incidence rate was only 3.4% [12]. We found EBV reactivation in 21% of cases but without overt PTLD. A Korean study reported only one case of EBV reactivation followed by PTLD among 43 patients [41]. In a study by Kang, EBV CI was 17.8%, and in 7% of cases, EBV ended with death due to progressive PTLD [43]. In contrast, Dufour et al. reported EBV replication in 10.3% of patients [12]. These differences might result from multiple factors, such as different ages, conditioning regimens, local epidemiologies, seasonal factors and viral testing methods.
Another factor that can have a decisive role in the referral for HCT is the incidence of life-threatening or severely disabling acute or chronic GVHD. In our study, we observed a 76.1% probability of GFS. This result can be influenced by the increasing trend in PBSC transplantations from MUDs due to the convenience of graft processing. However, according to the EBMT analysis, the use of peripheral blood grafts in pediatric HCTs remains the strongest negative predictor of survival [34]. PBSC is not considered the best stem cell source for the first HCT in SAA because of the lower survival and higher risk of aGVHD and cGVHD [34,51,52]. However, more recent studies report better outcomes and similar results among MUDs with PBSC or BM [12]. PBSC has been reported in cases of a second donation by the same donor where previously BM was used to improve engraftment and achieve faster hematopoietic recovery [53]. Horan et al. reported the incidence of graft failure to be 43% in 166 SAA patients who underwent second HCT from MSD using BM or PBSC in 84 and 16% of patients, respectively [54]. Similarly in a pediatric study by Cesaro concerning second allogenic transplantation, use of PBSC was not associated with inferior OS [55].
The observed GFS results can be seen as the main concern challenging the HCT, because, in contrast to adult patients, children’s life expectancy is 50–70 years, and the risk of cGVHD-associated organ complications and metabolic consequences of life-long steroid immunosuppression must not be neglected. The place for GFS improvement can be found in the preferred source, BM, proven to be associated with a lower risk of GVHD in children with acute lymphoblastic leukemia [56]. It must be emphasized that our study did not reveal worse results in patients receiving PBSC transplants than in those receiving BM transplants, and minimal tissue damage by FluCyATG can be suggested as another factor decreasing the induction phase of GVHD [57].
Finally, the conundrum that needs to be resolved in pediatric SAA is the question of when the patient should be referred for HCT and whether upfront HCT is better than IS. Our results show that a median time to transplantation of 4.5 months leaves a window of opportunity for a nontransplant strategy before the availability of the alternative donor. The chance of improving IS results can be seen in using new drugs, such as thrombopoietin receptor antagonists, which have been shown to stimulate the proliferation of autologous hematopoietic stem cells, resulting in the licensing of eltrombopag in SAA in adults and children over 12 years of age, but studies in children have not confirmed the efficacy of SAA upfront therapy [58,59].
5. Conclusions
The benefits of FluCyATG are associated with high overall survival probability, early engraftment with a very high level of donor chimerism and minimal impact of posttransplantation opportunistic infections. Viral monitoring and timely preemptive treatment can reduce the impact of posttransplantation infections to a clinically irrelevant factor. The high curability of SAA raises the issue of long-term sequelae reduction, which can be resolved by less toxic conditioning protocols and prudent referral for alloHCT. The waiting time for alternative-donor HCT in SAA can still be used as a window for pharmacotherapy involving IS and thrombopoietic drugs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10194416/s1. Figure S1: The impact of pretransplantation immunosuppressive therapy (IS) on the probability of OS (A) and GFS (B); the impact of the presence of PNH clone on OS (C) and GFS (D); the impact of the time from diagnosis to transplantation on OS (E) and GFS (F). Figure S2: The impact of recipient sex on OS (A) and GFS (B); the impact of donor sex on OS (C) and GFS (D); the impact of recipient–donor gender combination on OS (E) and GFS (F); the impact of donor and recipient CMV IgG status on OS (G) and GFS (H). Figure S3: The impact of donor type on the probability of OS (A) and GFS (B); the impact of graft source on OS (C) and GFS (D); the impact of the intensity of chemotherapy on OS (E) and GFS (F); and the impact of serotherapy choice on OS (G) and GFS (H). Figure S4: The impact of posttransplantation ADV replication on OS (A) and GFS (B); the impact of posttransplantation BKV replication on OS (C) and GFS (D); the impact of posttransplantation CMV replication on OS (E) and GFS (F); and the impact of posttransplantation EBV replication on OS (G) and GFS (H).
Author Contributions
Data collection, analysis, manuscript preparation and acceptance: M.S.-B. and M.R.; patient care, data collection and manuscript acceptance: J.F., E.G., K.G. (Katarzyna Gul), M.J.-C., T.J., K.K., M.M.-S., I.O., J.O.-L. and A.P.; molecular studies, data collection and manuscript acceptance: K.G. (Kornelia Gajek), B.R. and R.R.-K.; concept, data collection, analysis, manuscript preparation and final acceptance: M.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant number SUB.C200.21.058.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Wroclaw Medical University nr KB-306/2021.
Informed Consent Statement
The patients or legal guardians gave their written informed consent for the treatment and analysis of clinical data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Prophylaxis and Treatment of Infections
Patients undergoing transplantation were screened weekly for the replication of viruses (adenovirus (ADV), polyoma BK virus (BKV), human cytomegalovirus (CMV), Epstein–Barr virus (EBV), stool rotavirus), C. difficile infection (CDI) and multidrug-resistant (MDR) bacteria as a part of routine monitoring. Antimicrobial prophylaxis included oral colistin or rifaximin, and in the case of positivity for C. difficile A or B toxin or binary toxin, oral metronidazole was given. First-line intravenous antibiotic empiric therapy usually included a broad-spectrum beta-lactam and aminoglycoside. In the case of stool colonization with multidrug-resistant Gram-negative rods, carbapenems were used as the first choice. Prophylaxis for viral infections consisted of acyclovir from the beginning of the conditioning regimen to the end of immunosuppression. In the case of CMV replication, preemptive antiviral therapy with ganciclovir was started. In BKV-replicating patients aged >4 years, oral ciprofloxacin was administered to prevent BKV-hemorrhagic cystitis (HC). In the case of symptomatic BKV-HC or ADV replication with or without clinical manifestations, intravenous cidofovir with oral probenecid was used once a week. If EBV-DNA in the blood was positive (≥5 G copies/L), rituximab (375 mg/m2) was administered weekly until replication was negative. Prophylactic trimethoprim/sulfamethoxazole was given to all patients every day before and 3 times/week on consecutive days until at least one month after the end of immunosuppression. Antifungal prophylaxis with oral posaconazole was started at the beginning of the conditioning regimen and continued until discharge, but in patients treated for GVHD, it was continued until the end of immunosuppressive treatment. In the case of invasive fungal disease (IFD), liposomal amphotericin B was administered at a dose of 3–5 mg/kg BW/day.
References
- Keel, S.B.; Scott, A.; Sanchez-Bonilla, M.; Ho, P.A.; Gulsuner, S.; Pritchard, C.C.; Abkowitz, J.L.; King, M.-C.; Walsh, T.; Shimamura, A. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica 2016, 101, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S.; Calado, R.T.; Scheinberg, P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 2006, 108, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S.; Scheinberg, P.; Calado, R.T. Aplastic Anemia. N. Engl. J. Med. 2018, 379, 1643–1656. [Google Scholar] [CrossRef]
- Young, N.S.; Barrett, A.J. The treatment of severe acquired aplastic anemia. Blood 1995, 85, 3367–3377. [Google Scholar] [CrossRef]
- Marsh, J.C. Hematopoietic growth factors in the pathogenesis and for the treatment of aplastic anemia. Semin. Hematol. 2000, 37, 81–90. [Google Scholar] [CrossRef]
- EWOG-SAA-2010, DRKS-ID: DRKS00000610. Available online: https://www.kinderblutkrankheiten.de/content/fachinformationen/studienportal/studien_und_register/ewog_saa_2010/index_ger.html (accessed on 26 September 2021).
- Führer, M.; Rampf, U.; Baumann, I.; Faldum, A.; Niemeyer, C.; Janka-Schaub, G.; Friedrich, W.; Ebell, W.; Borkhardt, A.; Bender-Goetze, C.; et al. Immunosuppressive therapy for aplastic anemia in children: A more severe disease predicts better survival. Blood 2005, 106, 2102–2104. [Google Scholar] [CrossRef]
- Avery, S.; Shi, W.; Lubin, M.; Gonzales, A.M.; Heller, G.; Castro-Malaspina, H.; Giralt, S.; Kernan, N.A.; Scaradavou, A.; Barker, J.N. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood 2011, 117, 3277–3285; quiz 3478. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.C.W. Treatment of acquired aplastic anemia. Haematologica 2007, 92, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Szpecht, D.; Gorczyńska, E.; Kałwak, K.; Owoc-Lempach, J.; Choma, M.; Styczyński, J.; Goździk, J.; Dłużniewska, A.; Wysocki, M.; Kowalczyk, J.; et al. Matched sibling versus matched unrelated allogeneic hematopoietic stem cell transplantation in children with severe acquired aplastic anemia: Experience of the Polish pediatric group for hematopoietic stem cell transplantation. Arch. Immunol. Ther. Exp. 2012, 60, 225–233. [Google Scholar] [CrossRef]
- Holtan, S.G.; DeFor, T.E.; Lazaryan, A.; Bejanyan, N.; Arora, M.; Brunstein, C.G.; Blazar, B.R.; MacMillan, M.; Weisdorf, D.J. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015, 125, 1333–1338. [Google Scholar] [CrossRef]
- Dufour, C.; Veys, P.; Carraro, E.; Bhatnagar, N.; Pillon, M.; Wynn, R.F.; Gibson, B.; Vora, A.J.; Steward, C.G.; Ewins, A.M.; et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working P. Br. J. Haematol. 2015, 171, 585–594. [Google Scholar] [CrossRef]
- Perez-Albuerne, E.D.; Eapen, M.; Klein, J.; Gross, T.J.; Lipton, J.M.; Baker, K.S.; Woolfrey, A.; Kamani, N. Outcome of unrelated donor stem cell transplantation for children with severe aplastic anemia. Br. J. Haematol. 2008, 141, 216–223. [Google Scholar] [CrossRef]
- Deeg, H.J.; O’Donnell, M.; Tolar, J.; Agarwal, R.; Harris, R.E.; Feig, S.A.; Territo, M.C.; Collins, R.H.; McSweeney, P.A.; Copelan, E.A.; et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood 2006, 108, 1485–1491. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Socie’, G.; Lanino, E.; Prete, A.; Locatelli, F.; Locasciulli, A.; Cesaro, S.; Shimoni, A.; Marsh, J.; Brune, M.; et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: A retrospective study from the EBMT-SAA Working Party. Haematologica 2010, 95, 976–982. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Steward, C.; Hiwarkar, P.; Saif, M.A.; Hough, R.; Webb, D.; Norton, A.; Lawson, S.; Qureshi, A.; Connor, P.; et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: A United Kingdom multicentre retrospective experience. Br. J. Haematol. 2012, 157, 339–346. [Google Scholar] [CrossRef]
- Solomon, S.R.; Sizemore, C.A.; Zhang, X.; Brown, S.; Holland, H.K.; Morris, L.E.; Solh, M.; Bashey, A. Impact of Donor Type on Outcome after Allogeneic Hematopoietic Cell Transplantation for Acute Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 1816–1822. [Google Scholar] [CrossRef][Green Version]
- Ayuk, F.; Beelen, D.W.; Bornhäuser, M.; Stelljes, M.; Zabelina, T.; Finke, J.; Kobbe, G.; Wolff, D.; Wagner, E.-M.; Christopeit, M.; et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Gahrton, G. Risk assessment in haematopoietic stem cell transplantation: Impact of donor–recipient sex combination in allogeneic transplantation. Best Pract. Res. Clin. Haematol. 2007, 20, 219–229. [Google Scholar] [CrossRef]
- Viollier, R.; Socie, G.; Tichelli, A.; Bacigalupo, A.; Korthof, E.T.; Marsh, J.; Cornish, J.; Ljungman, P.; Oneto, R.; Békássy, A.N.; et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008, 41, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, J.; Hu, X.; Chen, J.; Gao, L.; Cheng, H.; Tang, G.; Luo, Y.; Zhang, W.; Wang, J. Aplastic Anemia Preconditioned with Fludarabine, Cyclophosphamide, and Anti-Thymocyte Globulin. Ann. Transplant. 2019, 24, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; for the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (SAA WP-EBMT); Locatelli, F.; Lanino, E.; Marsh, J.C.W.; Socie, G.; Maury, S.; Prete, A.; Locasciulli, A.; Cesaro, S.; et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: A report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005, 36, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Resnick, I.B.; Aker, M.; Shapira, M.Y.; Tsirigotis, P.D.; Bitan, M.; Abdul-Hai, A.; Samuel, S.; Ackerstein, A.; Gesundheit, B.; Zilberman, I.; et al. Allogeneic stem cell transplantation for severe acquired aplastic anaemia using a fludarabine-based preparative regimen. Br. J. Haematol. 2006, 133, 649–654. [Google Scholar] [CrossRef]
- Kang, H.J.; Hong, K.T.; Lee, J.W.; Kim, H.; Park, K.D.; Shin, H.Y.; Lee, S.H.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; et al. Improved Outcome of a Reduced Toxicity-Fludarabine, Cyclophosphamide, plus Antithymocyte Globulin Conditioning Regimen for Unrelated Donor Transplantation in Severe Aplastic Anemia: Comparison of 2 Multicenter Prospective Studies. Biol. Blood Marrow Transplant. 2016, 22, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Liu, W.; Kutteh, W.H.; Ke, R.W.; Shelton, K.C.; A Sklar, C.; Chemaitilly, W.; Pui, C.-H.; Klosky, J.L.; Spunt, S.L.; et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014, 15, 1215–1223. [Google Scholar] [CrossRef]
- Levine, J.M.; Whitton, J.A.; Ginsberg, J.P.; Green, D.M.; Leisenring, W.; Stovall, M.; Robison, L.L.; Armstrong, G.T.; Sklar, C.A. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 1044–1052. [Google Scholar] [CrossRef]
- Iftikhar, R.; Chaudhry, Q.U.N.; Anwer, F.; Neupane, K.; Rafae, A.; Mahmood, S.K.; Ghafoor, T.; Shahbaz, N.; Khan, M.A.; Khattak, T.A.; et al. Allogeneic hematopoietic stem cell transplantation in aplastic anemia: Current indications and transplant strategies. Blood Rev. 2021, 47, 100772. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.; Drozd-Sokolowska, J.; Biecek, P.; Dzierzak-Mietla, M.; Boguradzki, P.; Staniak, M.; Piatkowska-Jakubas, B.; Piekarska, A.; Tormanowska, M.; Halaburda, K.; et al. Allogeneic hematopoietic stem cell transplantation for paroxysmal nocturnal hemoglobinuria: Multicenter analysis by Polish Adult Leukemia Group. Biol. Blood Marrow Transplant. 2020, 26, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Drexler, B.; Zurbriggen, F.; Diesch, T.; Viollier, R.; Halter, J.P.; Heim, D.; Holbro, A.; Infanti, L.; Buser, A.; Gerull, S.; et al. Very long-term follow-up of aplastic anemia treated with immunosuppressive therapy or allogeneic hematopoietic cell transplantation. Ann. Hematol. 2020, 99, 2529–2538. [Google Scholar] [CrossRef]
- Admiraal, R.; van Kesteren, C.; Jol-van der Zijde, C.M.; van Tol, M.J.D.; Bartelink, I.H.; Bredius, R.G.M.; Boelens, J.J.; Knibbe, C.A.J. Population Pharmacokinetic Modeling of Thymoglobulin® in Children Receiving Allogeneic-Hematopoietic Cell Transplantation: Towards Improved Survival Through Individualized Dosing. Clin. Pharmacokinet. 2015, 54, 435–446. [Google Scholar] [CrossRef]
- El Amrani, M.; Admiraal, R.; Willaert, L.; Raaij, L.J.C.E.-V.; Lacna, A.M.; Hack, C.E.; Huitema, A.D.R.; Nierkens, S.; Van Maarseveen, E.M. Quantification of T Cell Binding Polyclonal Rabbit Anti-thymocyte Globulin in Human Plasma with Liquid Chromatography Tandem-Mass Spectrometry. AAPS J. 2020, 22, 43. [Google Scholar] [CrossRef]
- Admiraal, R.; van Kesteren, C.; Jol-van der Zijde, C.M.; Lankester, A.C.; Bierings, M.B.; Egberts, T.; van Tol, M.J.D.; Knibbe, C.A.J.; Bredius, R.G.M.; Boelens, J.J. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: A multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015, 2, e194–e203. [Google Scholar] [CrossRef]
- Bejanyan, N.; Kim, S.; Hebert, K.M.; Kekre, N.; Abdel-Azim, H.; Ahmed, I.; Aljurf, M.; Badawy, S.M.; Beitinjaneh, A.; Boelens, J.J.; et al. Choice of conditioning regimens for bone marrow transplantation in severe aplastic anemia. Blood Adv. 2019, 3, 3123–3131. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Socié, G.; Hamladji, R.M.; Aljurf, M.; Maschan, A.; Kyrcz-Krzemien, S.; Cybicka, A.; Sengelov, H.; Unal, A.; Beelen, D.; et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: An EBMT analysis. Haematologica 2015, 100, 696–702. [Google Scholar] [CrossRef]
- Champlin, R.E.; Perez, W.S.; Passweg, J.R.; Klein, J.P.; Camitta, B.M.; Gluckman, E.; Bredeson, C.N.; Eapen, M.; Horowitz, M.M. Bone marrow transplantation for severe aplastic anemia: A randomized controlled study of conditioning regimens. Blood 2007, 109, 4582–4585. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.C.; Gupta, V.; Lim, Z.; Ho, A.Y.; Ireland, R.M.; Hayden, J.; Potter, V.; Koh, M.B.; Islam, M.S.; Russell, N.; et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft-versus-host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood 2011, 118, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Merli, A.; Bacigalupo, A. Analysis at the clonal level of T-cell phenotype and functions in severe aplastic anemia patients. Blood 1991, 78, 1268–1274. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1715221 (accessed on 26 September 2021).
- De Vries, A.C.H.; Langerak, A.W.; Verhaaf, B.; Niemeyer, C.M.; Stary, J.; Schmiegelow, K.; Van Wering, E.R.; Zwaan, C.M.; Beishuizen, A.; Pieters, R.; et al. T-cell receptor Vβ CDR3 oligoclonality frequently occurs in childhood refractory cytopenia (MDS-RC) and severe aplastic anemia. Leukemia 2008, 22, 1170–1174. [Google Scholar] [CrossRef]
- Shah, R.M.; Truong, T.H.; Leaker, M.T.; Wright, N.A.; Le, D.; Steele, M.; Bruce, A.A.; Desai, S.; Guilcher, G.M.; Lewis, V. Fludarabine, Campath, and Low-Dose Cyclophosphamide (FCClow) with or without TBI Conditioning Results in Excellent Transplant Outcomes in Children with Severe Aplastic Anemia. Biol. Blood Marrow Transplant. 2020, 26, 1900–1905. [Google Scholar] [CrossRef]
- Schönberger, S.; Meisel, R.; Adams, O.; Pufal, Y.; Laws, H.; Enczmann, J.; Dilloo, D. Prospective, Comprehensive, and Effective Viral Monitoring in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2010, 16, 1428–1435. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, B.R.; Park, S.M.; Yoon, B.A.; Hwang, T.J.; Baek, H.J.; Kook, H. Better Failure-Free Survival and Graft-versus-Host Disease-Free/Failure Free Survival with Fludarabine-Based Conditioning in Stem Cell Transplantation for Aplastic Anemia in Children. J. Korean Med. Sci. 2020, 35, e46. [Google Scholar] [CrossRef]
- Chaudhry, Q.U.N.; Iftikhar, R.; Satti, T.M.; Mahmood, S.K.; Ghafoor, T.; Shamshad, G.U.; Farhan, M.; Shahbaz, N.; Khan, M.A.; Khattak, T.A.; et al. Outcome of Fludarabine-Based Conditioning in High-Risk Aplastic Anemia Patients Undergoing Matched Related Donor Transplantation: A Single-Center Study from Pakistan. Biol. Blood Marrow Transplant. 2019, 25, 2375–2382. [Google Scholar] [CrossRef]
- Kang, H.J.; Shin, H.Y.; Park, J.E.; Chung, N.G.; Cho, B.; Kim, H.K.; Kim, S.Y.; Lee, Y.H.; Lim, Y.T.; Yoo, K.H.; et al. Successful Engraftment with Fludarabine, Cyclophosphamide, and Thymoglobulin Conditioning Regimen in Unrelated Transplantation for Severe Aplastic Anemia: A Phase II Prospective Multicenter Study. Biol. Blood Marrow Transplant. 2010, 16, 1582–1588. [Google Scholar] [CrossRef]
- Giraud, G.; Bogdanovic, G.; Priftakis, P.; Remberger, M.; Svahn, B.-M.; Barkholt, L.; Ringden, O.; Winiarski, J.; Ljungman, P.; Dalianis, T. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica 2006, 91, 401–404. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16531266 (accessed on 26 September 2021).
- Salamonowicz-Bodzioch, M.; Frączkiewicz, J.; Czyżewski, K.; Zając-Spychała, O.; Gorczyńska, E.; Panasiuk, A.; Ussowicz, M.; Kałwak, K.; Szmit, Z.; Wróbel, G.; et al. Prospective analysis of BKV hemorrhagic cystitis in children and adolescents undergoing hematopoietic cell transplantation. Ann. Hematol. 2021, 100, 1283–1293. [Google Scholar] [CrossRef]
- Riachy, E.; Krauel, L.; Rich, B.S.; McEvoy, M.P.; Honeyman, J.N.; Boulad, F.; Wolden, S.L.; Herr, H.W.; La Quaglia, M.P. Risk Factors and Predictors of Severity Score and Complications of Pediatric Hemorrhagic Cystitis. J. Urol. 2014, 191, 186–192. [Google Scholar] [CrossRef]
- Mori, Y.; Miyamoto, T.; Kato, K.; Kamezaki, K.; Kuriyama, T.; Oku, S.; Takenaka, K.; Iwasaki, H.; Harada, N.; Shiratsuchi, M.; et al. Different Risk Factors Related to Adenovirus- or BK Virus-Associated Hemorrhagic Cystitis following Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 458–465. [Google Scholar] [CrossRef]
- Matthes-Martin, S.; Feuchtinger, T.; Shaw, P.J.; Engelhard, D.; Hirsch, H.H.; Cordonnier, C.; Ljungman, P.; Fourth European Conference on Infections in Leukemia. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011). Transpl. Infect. Dis. 2012, 14, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Feghoul, L.; Chevret, S.; Cuinet, A.; Dalle, J.-H.; Ouachée, M.; Yacouben, K.; Fahd, M.; Khourouj, V.G.-E.; Roupret-Serzec, J.; Sterkers, G.; et al. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: Clues for antiviral pre-emptive treatment. Clin. Microbiol. Infect. 2015, 21, 701–709. [Google Scholar] [CrossRef]
- Mynarek, M.; Ganzenmueller, T.; Mueller-Heine, A.; Mielke, C.; Gonnermann, A.; Beier, R.; Sauer, M.; Eiz-Vesper, B.; Kohstall, U.; Sykora, K.-W.; et al. Patient, Virus, and Treatment-Related Risk Factors in Pediatric Adenovirus Infection after Stem Cell Transplantation: Results of a Routine Monitoring Program. Biol. Blood Marrow Transplant. 2014, 20, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, H.; Passweg, J.R.; Marsh, J.C.W.; Bacigalupo, A.; Bredeson, C.N.; Bullorsky, E.; Camitta, B.M.; Champlin, R.E.; Gale, R.P.; Fuhrer, M.; et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood 2007, 110, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.; Le Rademacher, J.; Antin, J.H.; Champlin, R.E.; Carreras, J.; Fay, J.; Passweg, J.R.; Tolar, J.; Horowitz, M.M.; Marsh, J.C.W.; et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood 2011, 118, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Binder, M.; Schmid, C.; Rutt, C.; Ehninger, G.; Bornhäuser, M. Second donation of hematopoietic stem cells from unrelated donors for patients with relapse or graft failure after allogeneic transplantation. Haematologica 2008, 93, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Horan, J.T.; Carreras, J.; Tarima, S.; Camitta, B.M.; Gale, R.P.; Hale, G.A.; Hinterberger, W.; Marsh, J.; Passweg, J.R.; Walters, M.C.; et al. Risk Factors Affecting Outcome of Second HLA-Matched Sibling Donor Transplantations for Graft Failure in Severe Acquired Aplastic Anemia. Biol. Blood Marrow Transplant. 2009, 15, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, S.; De Latour, R.P.; Tridello, G.; Pillon, M.; Carlson, K.; Fagioli, F.; Jouet, J.-P.; Koh, M.B.C.; Panizzolo, I.S.; Kyrcz-Krzemien, S.; et al. Second allogeneic stem cell transplant for aplastic anaemia: A retrospective study by the severe aplastic anaemia working party of the European society for blood and marrow transplantation. Br. J. Haematol. 2015, 171, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Pdwp-Ebmt, O.B.O.; Dalissier, A.; Labopin, M.; Willasch, A.; Zecca, M.; Mouhab, A.; Chybicka, A.; Balduzzi, A.; Volin, L.; et al. More chronic GvHD and non-relapse mortality after peripheral blood stem cell compared with bone marrow in hematopoietic transplantation for paediatric acute lymphoblastic leukemia: A retrospective study on behalf of the EBMT Paediatric Diseases Working Pa. Bone Marrow Transplant. 2017, 52, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Groarke, E.M.; Patel, B.A.; Gutierrez-Rodrigues, F.; Rios, O.; Lotter, J.; Baldoni, D.; Pierre, A.S.; Shalhoub, R.; Wu, C.O.; Townsley, D.M.; et al. Eltrombopag added to immunosuppression for children with treatment-naïve severe aplastic anaemia. Br. J. Haematol. 2021, 192, 605–614. [Google Scholar] [CrossRef]
- Townsley, D.M.; Scheinberg, P.; Winkler, T.; Desmond, R.; Dumitriu, B.; Rios, O.; Weinstein, B.; Valdez, J.; Lotter, J.; Feng, X.; et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N. Engl. J. Med. 2017, 376, 1540–1550. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).