First-Line Anaplastic Lymphoma Kinase (ALK) Inhibitors for ALK-Positive Lung Cancer in Asian Populations: Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
2.4.1. Pairwise Meta-Analysis
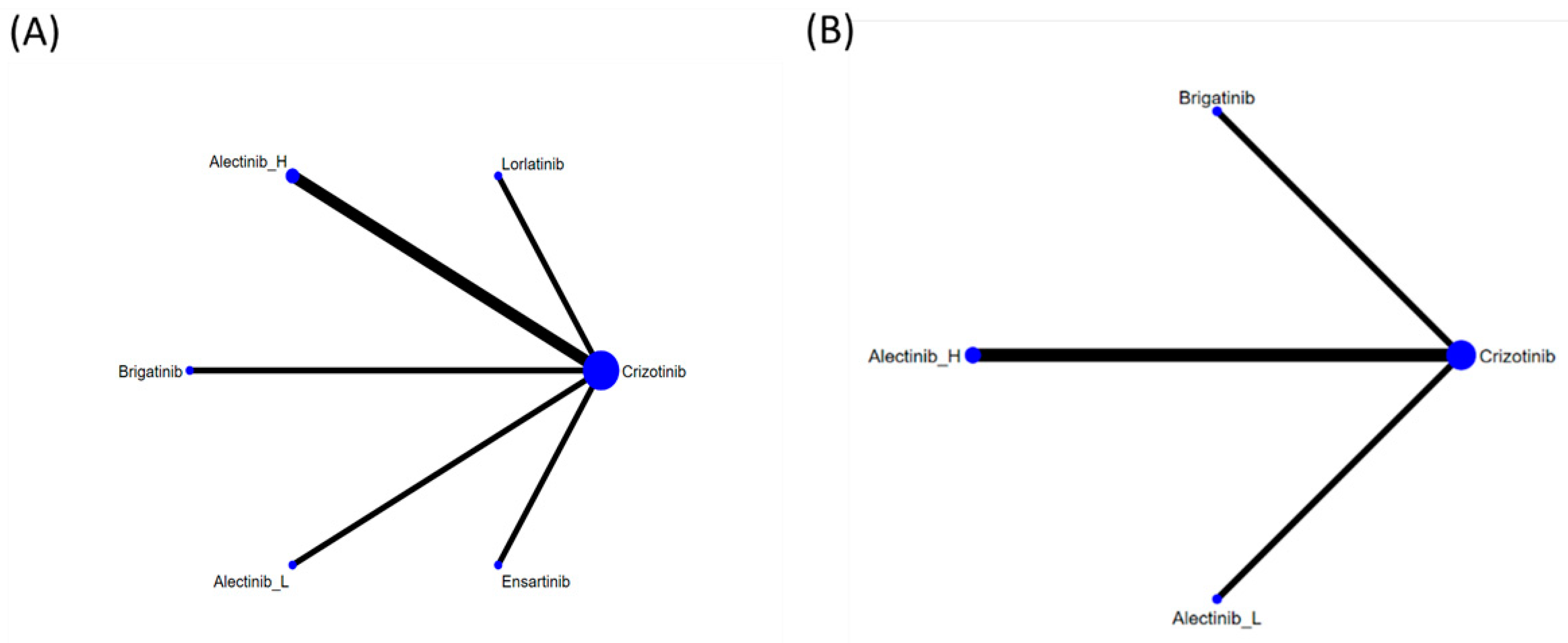
2.4.2. Network Meta-Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics and Quality Evaluation
3.3. Pooled Results for Generation Differences in Efficacy
3.4. Efficacy Evaluation from the Network Meta-Analysis
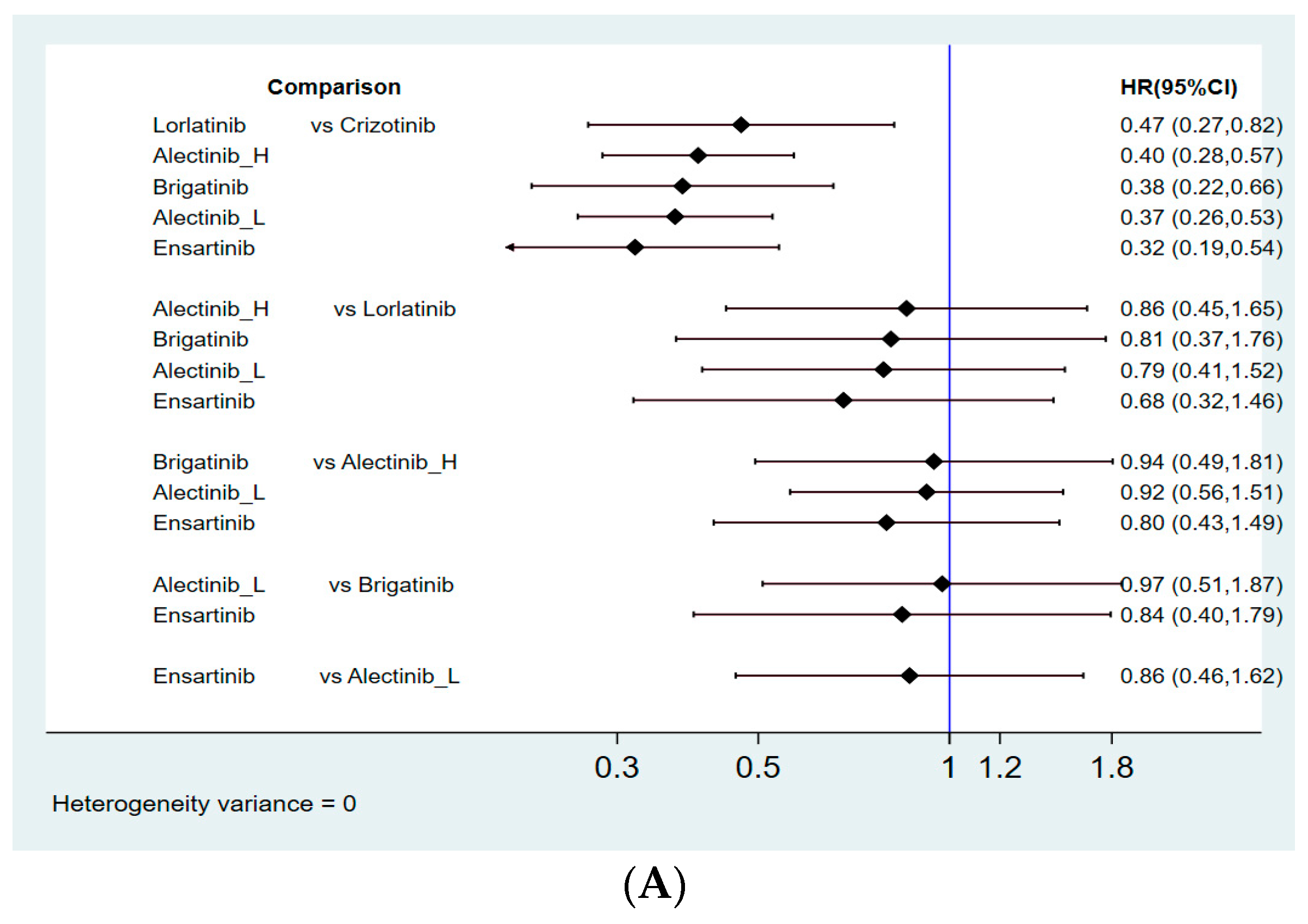
3.4.1. Progression-Free Survival
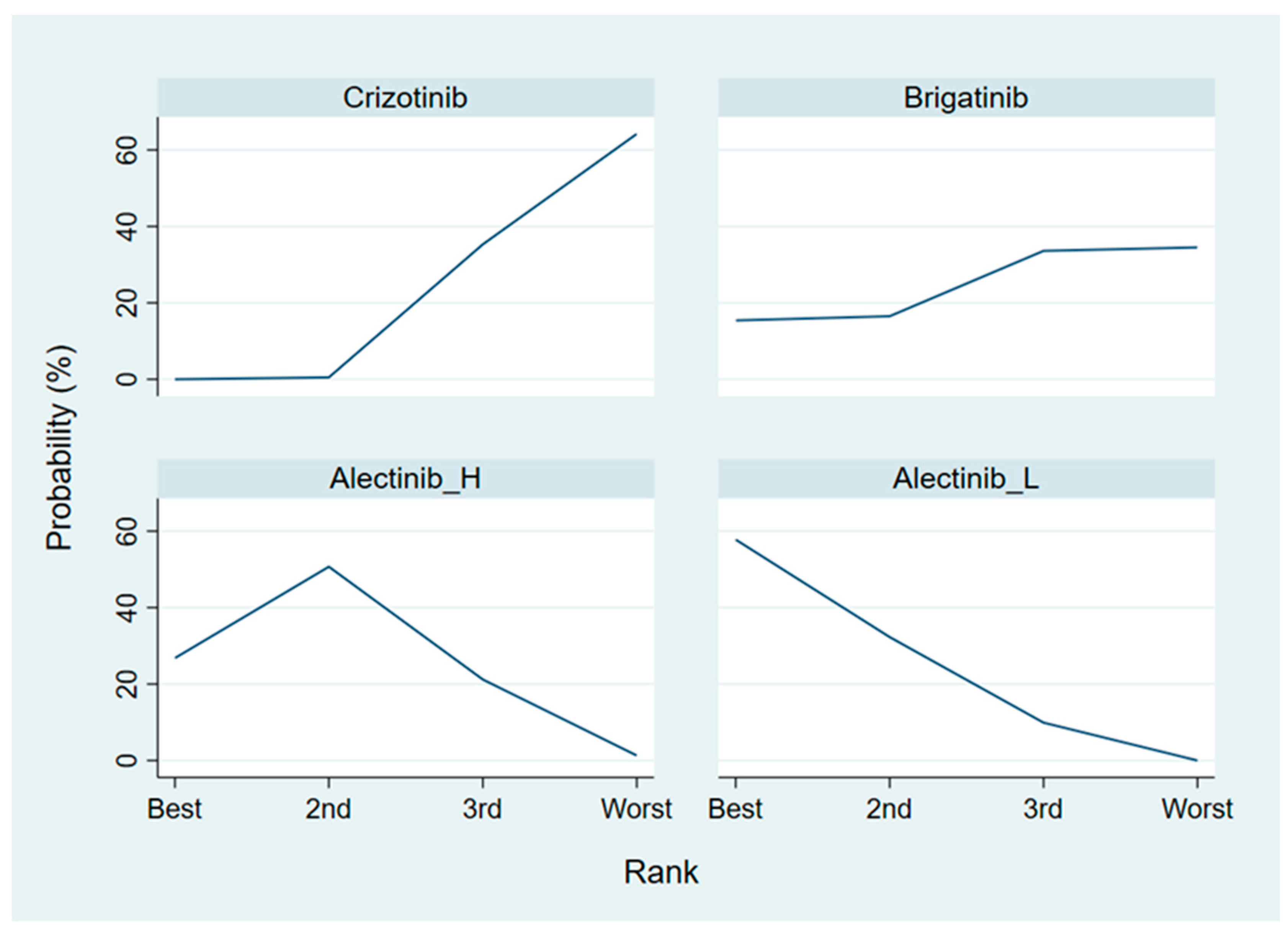
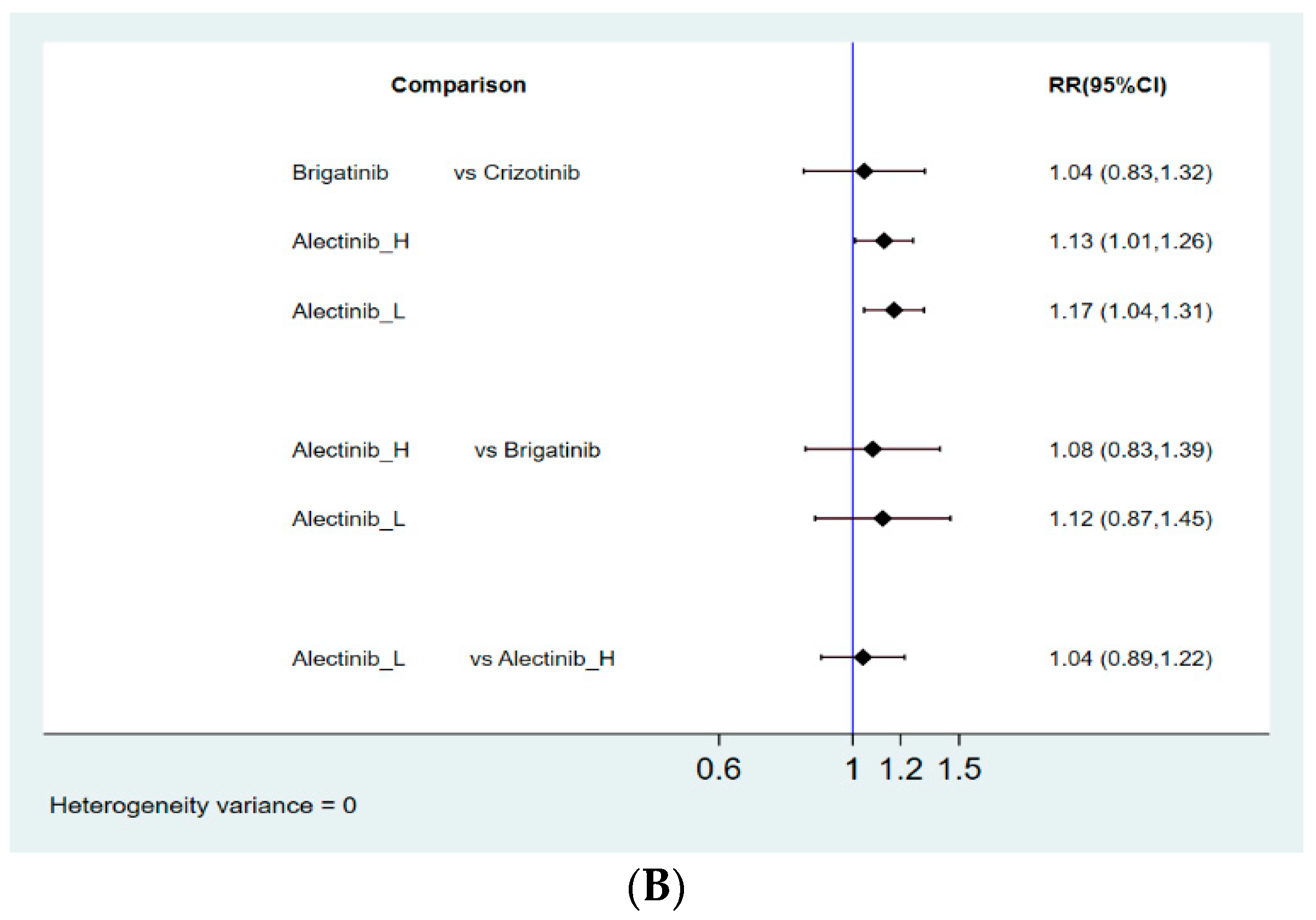
3.4.2. Objective Response Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- The Embase database contains biomedical literature from 1974 to the present.
- MEDLINE and PubMed databases cover journals from 1966 to the present.
- Embase Classic: The Embase back file covers almost 2 million biomedical and pharmacological citations drawn from over 3000 international titles between 1947 and 1973.
| Search | Query | Results |
|---|---|---|
| #1 | ‘non-small cell lung cancer’/exp OR ‘bronchial non-small cell cancer’ OR ‘bronchial non-small cell carcinoma’ OR ‘lung non-small cell cancer’ OR ‘lung non-small cell carcinoma’ OR ‘microcellular lung carcinoma’ OR ‘pulmonary non-small cell cancer’ OR ‘pulmonary non-small cell carcinoma’ OR ‘non-small cell bronchial cancer’ OR ‘non-small cell bronchial carcinoma’ OR ‘non-small cell cancer, lung’ OR ‘non-small cell lung cancer’ OR ‘non-small cell lung carcinoma’ OR ‘non-small cell pulmonary cancer’ OR ‘non-small cell pulmonary carcinoma’ | 171,940 |
| #2 | ‘NSCLC’:ab,ti OR ‘nsclc’:ab,ti OR ((non NEAR/3 ‘small cell ‘ NEAR/3 lung NEAR/3 cancer):ab,ti) OR ((non NEAR/3 ‘small cell’ NEAR/3 lung NEAR/3 carcinoma):ab,ti) OR ((non NEAR/3 ‘small cell ‘ NEAR/3 lung NEAR/3 neoplasm):ab,ti) | 115,898 |
| #3 | ((‘ large cell ‘ NEAR/3 lung NEAR/3 cancer):ab,ti) OR ((‘ large cell ‘ NEAR/3 lung NEAR/3 carcinoma):ab,ti) OR ((‘ large cell ‘ NEAR/3 lung NEAR/3 neoplasm):ab,ti) OR ((squamous NEAR/3 lung NEAR/3 cancer):ab,ti) OR ((squamous NEAR/3 lung NEAR/3 carcinoma):ab,ti) OR ((squamous NEAR/3 lung NEAR/3 neoplasm):ab,ti) OR ((adenocarcinoma NEAR/3 lung NEAR/3 cancer):ab,ti) OR ((adenocarcinoma NEAR/3 lung NEAR/3 carcinoma):ab,ti) OR ((adenocarcinoma NEAR/3 lung NEAR/3 neoplasm):ab,ti) | 11,061 |
| #3 | #1 OR #2 OR #3 | 182,051 |
| #4 | ((‘anaplastic lymphoma kinase ‘ NEAR/3 inhibit*):ab,ti) OR ((‘anaplastic lymphoma kinase ‘ NEAR/3 antagonist*):ab,ti) OR ((‘ALK ‘ NEAR/3 inhibit*):ab,ti) OR ((‘ALK ‘ NEAR/3 antagonist*):ab,ti) OR ((‘alk ‘ NEAR/3 inhibit*):ab,ti) OR ((‘alk ‘ NEAR/3 antagonist*):ab,ti) OR ((‘ALKI’):ab,ti) OR ((‘ALKIS’):ab,ti) | 3841 |
| #5 | crizotinib:ab,ti OR ‘pf 02341066’:ab,ti OR ‘pf 1066’:ab,ti OR ‘pf 2341066’:ab,ti OR pf02341066:ab,ti OR pf1066:ab,ti OR pf2341066:ab,ti OR xalkori:ab,ti OR ceritinib:ab,ti OR ‘ldk 378’:ab,ti OR ldk378:ab,ti OR ‘nvp ldk 378’:ab,ti OR ‘nvp ldk 378 nx’:ab,ti OR ‘nvp ldk378’:ab,ti OR ‘nvp ldk378 nx’:ab,ti OR zykadia:ab,ti OR alectinib:ab,ti OR ‘af 802’:ab,ti OR af802:ab,ti OR alecensa:ab,ti OR ‘alectinib hydrochloride’:ab,ti OR ‘ch 5424802’:ab,ti OR ch5424802:ab,ti OR ‘rg 7853’:ab,ti OR rg7853:ab,ti OR ‘ro 5424802’:ab,ti OR ro5424802:ab,ti OR brigatinib:ab,ti OR ‘ap 26113’:ab,ti OR ap26113:ab,ti OR lorlaninib:ab,ti OR ‘pf 06463922’:ab,ti OR lorlatinib:ab,ti OR ensartinib:ab,ti OR ‘unii sma5zs5b22’:ab,ti OR ‘370651 20 9’:ab,ti OR entrectinib:ab,ti OR ‘nms e 628’:ab,ti OR ‘nms e628’:ab,ti OR ‘rxdx 101’:ab,ti OR rxdx101:ab,ti | 5932 |
| #6 | #4 OR #5 | 7335 |
| #7 | #3 AND #6 | 4922 |
| #8 | ‘clinical trial’/de OR ‘randomized controlled trial’/de OR ‘randomization’/de OR ‘single blind procedure’/de OR ‘double blind procedure’/de OR ‘crossover procedure’/de OR (‘randomi?ed controlled’ NEXT/1 trial*) OR rct OR ‘randomly allocated’ OR ‘allocated randomly’ OR ‘random allocation’ OR (allocated NEAR/2 random) OR (single NEXT/1 blind*) OR (double NEXT/1 blind*) OR ((treble OR triple) NEAR/1 blind*) | 1,760,209 |
| #9 | #7 AND #8 | 793 |
| #10 | #9 NOT ([adolescent]/lim OR [child]/lim OR [preschool]/lim OR [school]/lim) | 786 |
| Search | Query | Results |
|---|---|---|
| #1 | (‘bronchial non-small cell cancer’ OR ‘bronchial non-small cell carcinoma’ OR ‘lung non-small cell cancer’ OR ‘lung non-small cell carcinoma’ OR ‘microcellular lung carcinoma’ OR ‘pulmonary non-small cell cancer’ OR ‘pulmonary non-small cell carcinoma’ OR ‘non-small cell bronchial cancer’ OR ‘non-small cell bronchial carcinoma’ OR ‘non-small cell cancer, lung’ OR ‘non-small cell lung cancer’ OR ‘non-small cell lung carcinoma’ OR ‘non-small cell pulmonary cancer’ OR ‘non-small cell pulmonary carcinoma’):ti,ab,kw | 13,321 |
| #2 | (‘NSCLC’ OR ‘nsclc’ OR ‘large cell lung cancer OR ‘large cell lung carcinoma’ OR ‘large cell lung neoplasm’ OR ‘squamous lung cancer’ OR ‘squamous lung carcinoma’ OR ‘squamous lung neoplasm’ OR ‘adenocarcinoma lung cancer’ OR ‘adenocarcinoma lung carcinoma’ OR ‘adenocarcinoma lung neoplasm’):ti,ab,kw | 11,250 |
| #3 | #1 OR #2 | 15,086 |
| #4 | (‘anaplastic lymphoma kinase’ inhibitor OR ‘anaplastic lymphoma kinase’ antagonist OR ‘ALK inhibitor’ OR ‘ALK antagonist ‘ OR ‘alk inhibitor ‘ OR ‘alk antagonist ‘ OR ‘ALKI’ OR ‘ALKIS’):ti,ab,kw | 429 |
| #5 | (crizotinib OR ‘pf 02341066′ OR ‘pf 1066′ OR ‘pf 2341066′ OR pf02341066 OR pf1066 OR pf2341066 OR xalkori OR ceritinib OR ‘ldk 378′ OR ldk378 OR ‘nvp ldk 378′ OR ‘nvp ldk 378 nx’ OR ‘nvp ldk378′ OR ‘nvp ldk378 nx’ OR zykadia OR alectinib OR ‘af 802′ OR af802 OR alecensa OR ‘alectinib hydrochloride’ OR ‘ch 5424802′ OR ch5424802 OR ‘rg 7853′ OR rg7853 OR ‘ro 5424802′ OR ro5424802 OR brigatinib OR ‘ap 26113′ OR ap26113 OR lorlaninib OR ‘pf 06463922′ OR lorlatinib OR ensartinib OR ‘unii sma5zs5b22′ OR ‘370651 20 9′ OR entrectinib OR ‘nms e 628′ OR ‘nms e628′ OR ‘rxdx 101′ OR rxdx101):ti,ab,kw | 427 |
| #6 | #4 AND #5 | 646 |
| #6 | #3 AND #6 | 521 Source PubMed = 84 Embase = 356 clinicaltrials.gov = 45 ICTRP = 87 |
Appendix B


Appendix C

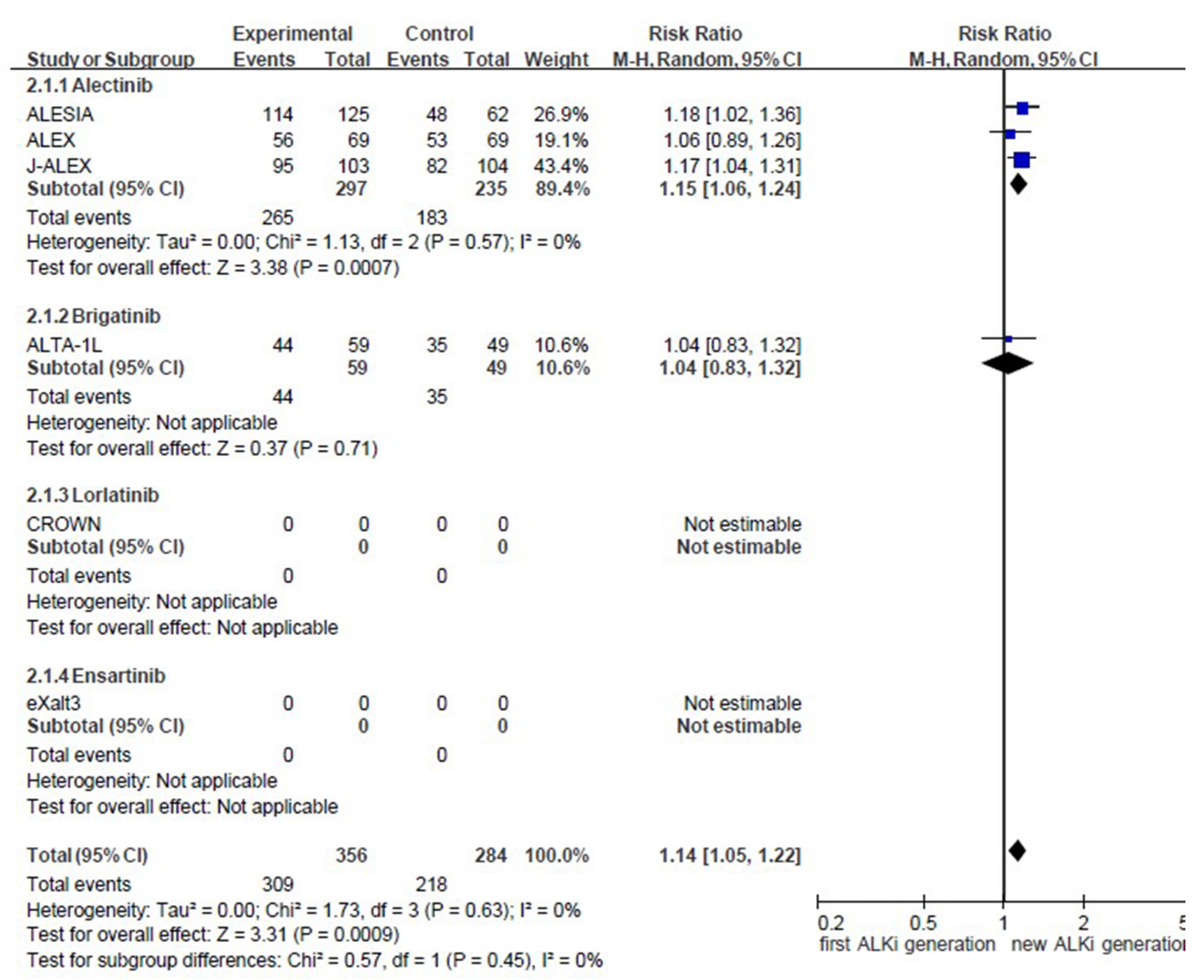
Appendix D
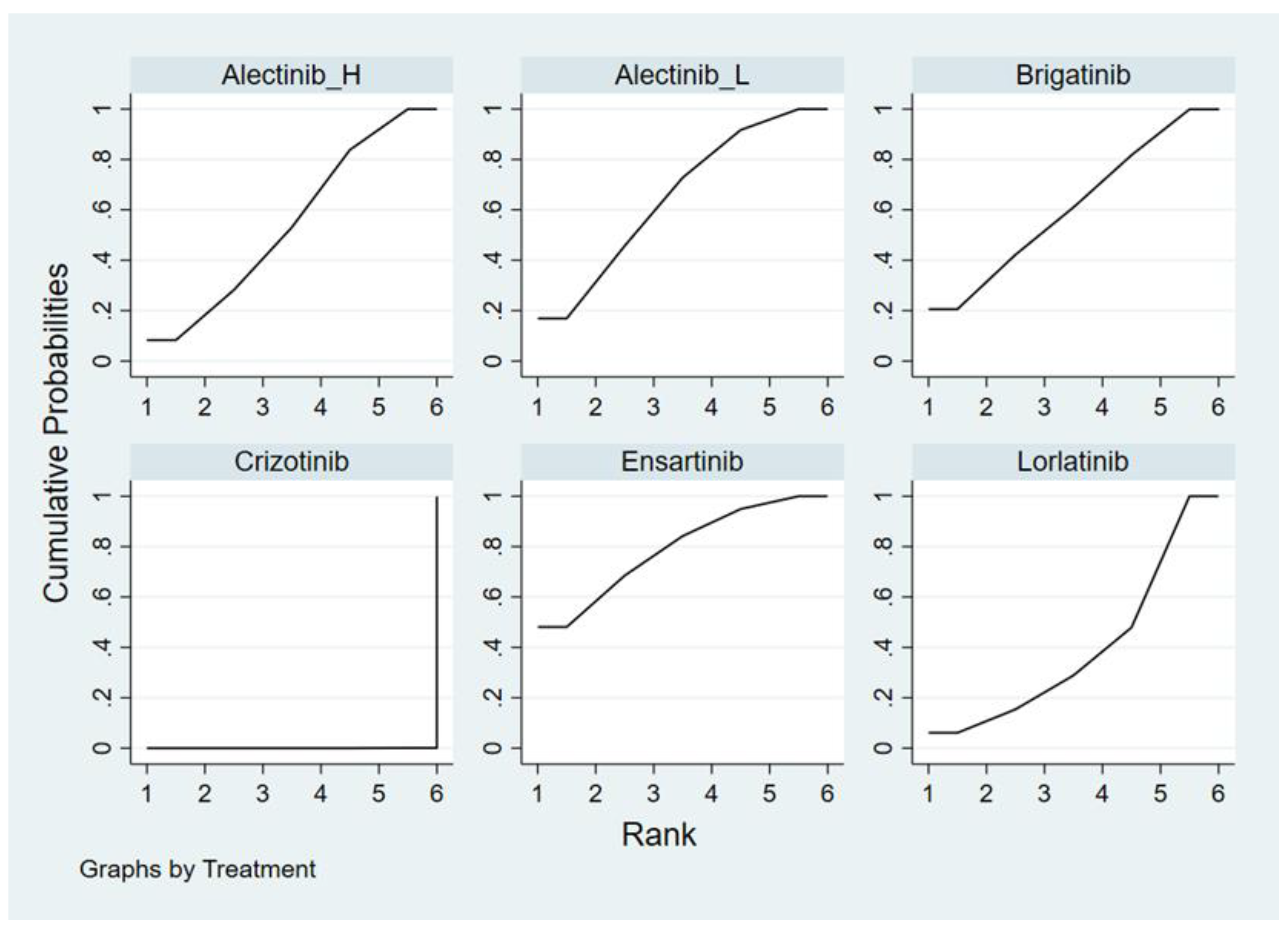
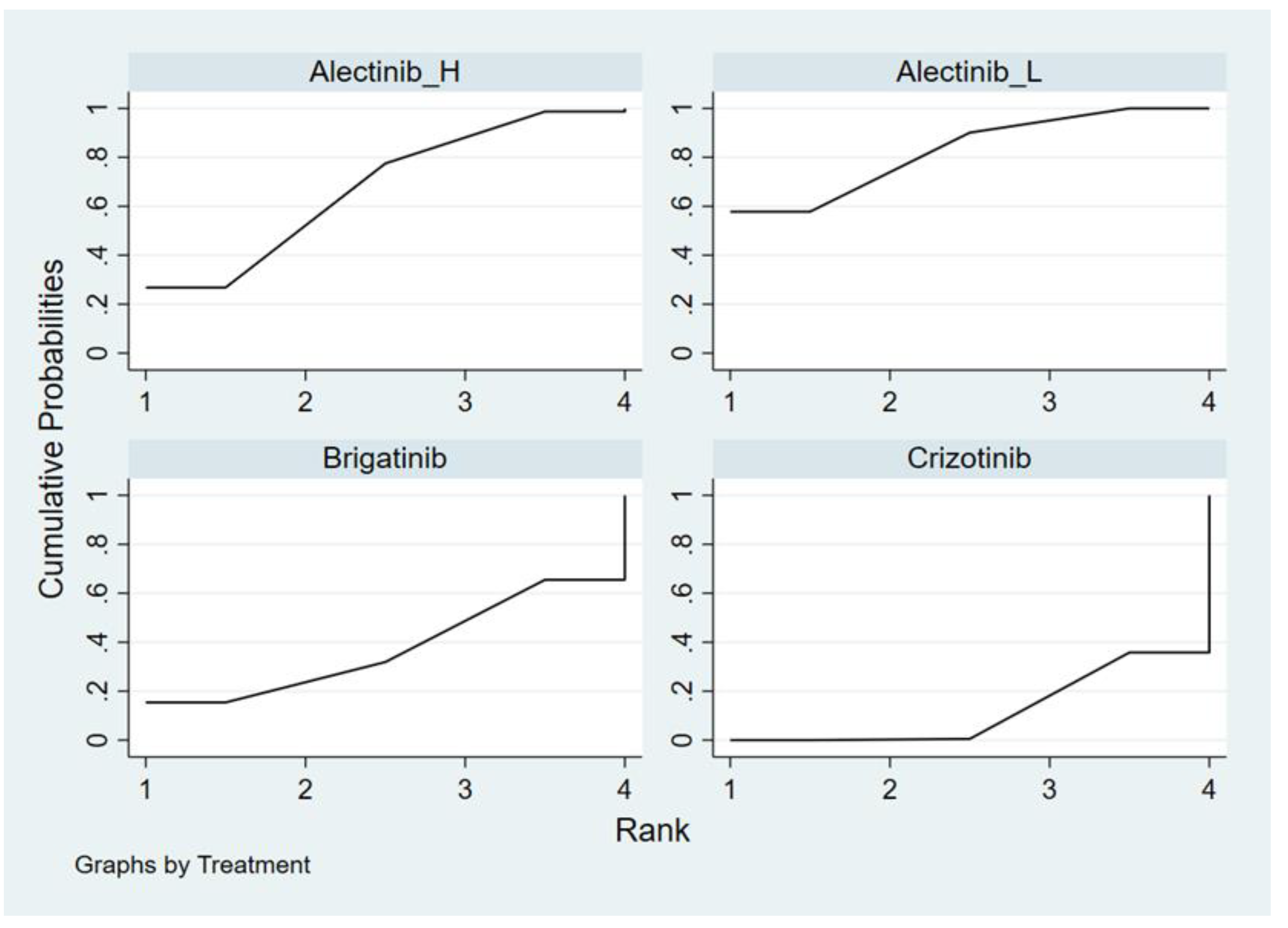
Appendix E
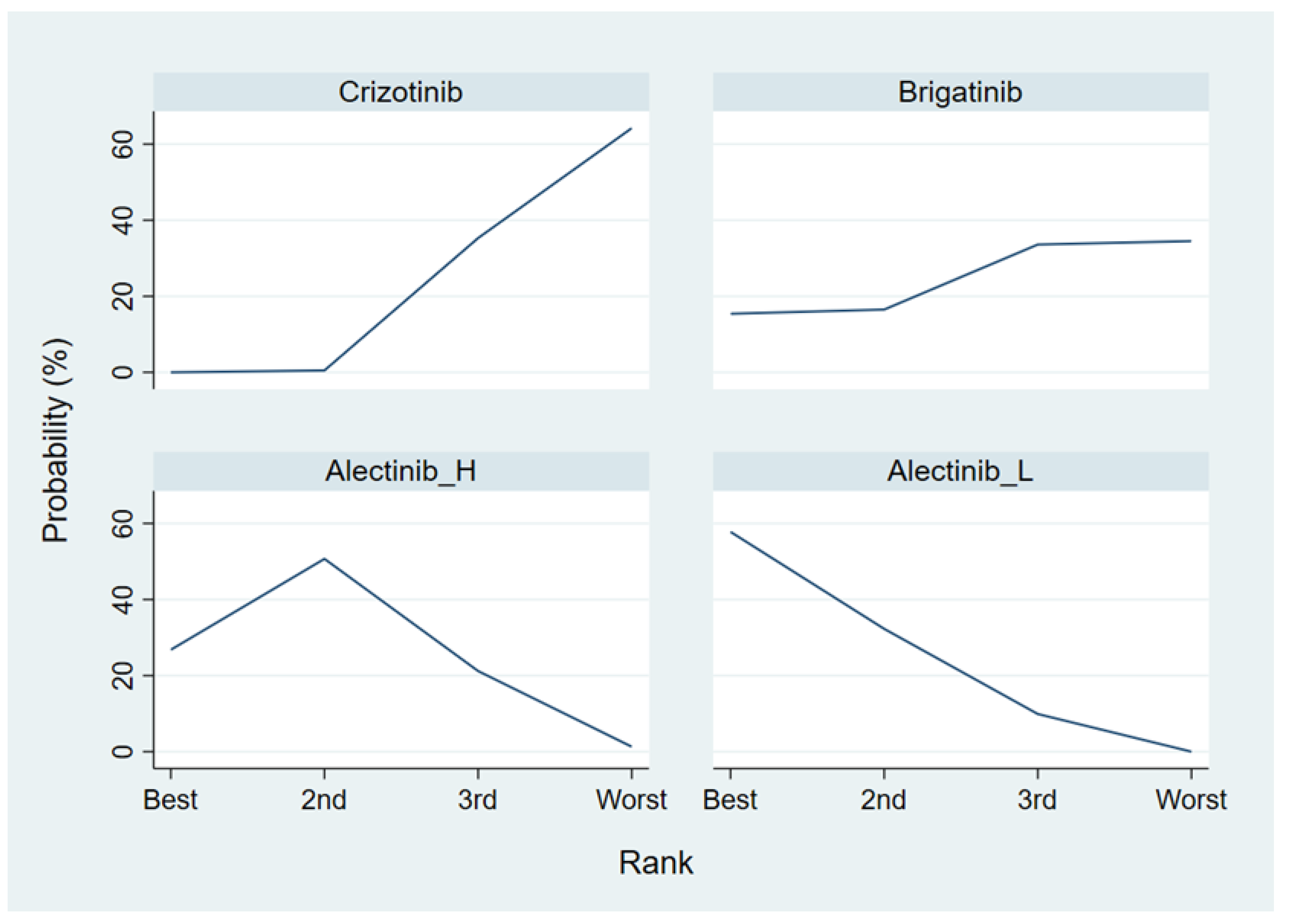
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bar, J.; Urban, D.; Amit, U.; Appel, S.; Onn, A.; Margalit, O.; Beller, T.; Kuznetsov, T.; Lawrence, Y. Long-Term Survival of Patients with Metastatic Non-Small-Cell Lung Cancer over Five Decades. J. Oncol. 2021, 2021, 7836264. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef] [Green Version]
- Zito Marino, F.; Bianco, R.; Accardo, M.; Ronchi, A.; Cozzolino, I.; Morgillo, F.; Rossi, G.; Franco, R. Molecular heterogeneity in lung cancer: From mechanisms of origin to clinical implications. Int. J. Med. Sci. 2019, 16, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Makimoto, G.; Ohashi, K.; Maeda, Y.; Kiura, K. Anaplastic Lymphoma Kinase Fusion: A Review of Therapeutic Drugs and Treatment Strategies. Acta Med. Okayama 2020, 74, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.I.; Shirai, K. Anaplastic Lymphoma Kinase (ALK) Signaling in Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 179–187. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Chen, H.Y.; He, K.; Patel, M.; Justice, R.; Keegan, P.; Pazdur, R. FDA approval summary: Crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014, 19, e5–e11. [Google Scholar] [CrossRef] [Green Version]
- Tabbò, F.; Passiglia, F.; Novello, S. Upfront Management of ALK-Rearranged Metastatic Non-small Cell Lung Cancer: One Inhibitor Fits All? Curr. Oncol. Rep. 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Christiani, D.C. East meets West: Ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin. J. Cancer 2011, 30, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Soo, R.A.; Kawaguchi, T.; Loh, M.; Ou, S.H.; Shieh, M.P.; Cho, B.C.; Mok, T.S.; Soong, R. Differences in outcome and toxicity between Asian and caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012, 8, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 378, 113–125. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Zhou, C.; Kim, S.W.; Reungwetwattana, T.; Zhou, J.; Zhang, Y.; He, J.; Yang, J.J.; Cheng, Y.; Lee, S.H.; Bu, L.; et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir. Med. 2019, 7, 437–446. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38, 3592–3603. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Kim, H.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; Gridelli, C.; et al. Brigatinib (BRG) versus crizotinib (CRZ) in Asian versus non-Asian patients (pts) in the phase III ALTA-1L trial. J. Clin. Oncol. 2019, 37, 9026. [Google Scholar] [CrossRef]
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; de Marinis, F.; et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J. Thorac. Oncol. 2019, 14, 1233–1243. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hida, T.; Nokihara, H.; Morise, M.; Azuma, K.; Kim, Y.H.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020, 139, 195–199. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs. Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 3523. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.K.; Sriuranpong, V.; Ho, J.C.M.; Ong, C.K.; Tsai, C.M.; Chung, C.H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolle, E.; Taucher, V.; Lindenmann, J.; Jost, P.J.; Pichler, M. Current Knowledge about Mechanisms of Drug Resistance against ALK Inhibitors in Non-Small Cell Lung Cancer. Cancers 2021, 13, 699. [Google Scholar] [CrossRef]
- Yanagitani, N.; Uchibori, K.; Koike, S.; Tsukahara, M.; Kitazono, S.; Yoshizawa, T.; Horiike, A.; Ohyanagi, F.; Tambo, Y.; Nishikawa, S.; et al. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci. 2020, 111, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Peters, S.; Camidge, D.; Ou, S.I.; Ahn, J.; Tan, E.; Li, Z.; Lee, J.; Cho, B.; Geater, S. Alectinib (ALC) vs. crizotinib (CRZ) in treatment-naïve ALK+ non-small-cell lung cancer (NSCLC): Asian vs. non-Asian subgroup analysis of the ALEX study. Ann. Oncol. 2017, 28, x191. [Google Scholar] [CrossRef]
- Seto, T.; Kiura, K.; Nishio, M.; Nakagawa, K.; Maemondo, M.; Inoue, A.; Hida, T.; Yamamoto, N.; Yoshioka, H.; Harada, M.; et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013, 14, 590–598. [Google Scholar] [CrossRef]
- Masuda, N.; Ohe, Y.; Gemma, A.; Kusumoto, M.; Yamada, I.; Ishii, T.; Yamamoto, N. Safety and effectiveness of alectinib in a real-world surveillance study in patients with ALK-positive non-small-cell lung cancer in Japan. Cancer Sci. 2019, 110, 1401–1407. [Google Scholar] [CrossRef] [Green Version]

| Trial | CROWN [13] | ALTA-1L [17,18] | ALEX [19] (Asian Subgroup) | J-ALEX [20] | ALESIA [15] | eXalt3 [21] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Shaw et al. | Camidge et al. | Camidge et al. | Nakagawa et al. | Zhou et al. | Horn et al. | ||||||
| Year | 2020 | 2020 | 2019 | 2020 | 2019 | 2021 | ||||||
| Design | Phase III, Open Label, RCT | Phase III, Open Label, RCT | Phase III, Open Label, RCT | Phase III, Open Label, RCT | Phase III, Open Label, RCT | Phase III, Open Label, RCT | ||||||
| Intervention | Lorlatinib 100 mg qd | Crizotinib 250 mg bid | Brigatinib 90 mg qd for 7 Days, then 180 mg qd | Crizotinib 250 mg bid | Alectinib 600 mg bid | Crizotinib 250 mg bid | Alectinib 300 mg bid | Crizotinib 250 mg bid | Alectinib 600 mg bid | Crizotinib 250 mg bid | Ensartinib 225 mg qd | Crizotinib 250 mg bid |
| Sample size | 66 | 65 | 59 | 49 | 69 | 69 | 103 | 104 | 125 | 62 | 73 | 78 |
| Outcome | ||||||||||||
| PFS (month) | NA | NA | NR (NR) | 11.1 (9.2–NR) | 34.8 | 9.6 | 34.1 | 10.2 | NR | 10.7 | NA | NA |
| HR PFS | 0.47 (0.27–0.82) | 0.38 (0.22–0.65) | 0.43 (0.27–0.67) | 0.37 (0.26–0.52) | 0.37 (0.22–0.61) | 0.32 (0.19–0.55) | ||||||
| OS (month) | NA | NA | NA | NA | NA | NA | NR | 43.7 | NR | NR | NA | NA |
| HR OS | NA | NA | 0.74 (0.40–1.36) | 0.80 (0.35–1.82) | 0.28 (0.12–0.68) | NA | ||||||
| ORR (%) | NA | NA | 75% | 71% | 81.20% | 76.8% | 92% | 79% | 91% | 77% | NA | NA |
| CNS PFS (month) | NA | NA | NR | 9.2 | NA | NA | NA | NA | NA | NA | NA | NA |
| DOR (month) | NA | NA | NA | NA | NA | NA | NR | NR | NR | 9.3 | NA | NA |
| Safety | ||||||||||||
| AE ≥ grade 3 | NA | NA | NA | NA | NA | NA | 36.9% | 60.6% | 29% | 48% | NA | NA |
| Discontinuation due to AE (%) | NA | NA | 8.50% | 6.30% | 13.00% | 11.60% | 11.7% | 23.1% | 7% | 10% | NA | NA |
| Patient character | ||||||||||||
| Age (median) | NA | NA | NA | NA | NA | NA | 61 | 59.5 | 51 | 49 | NA | NA |
| Male (%) | NA | NA | NA | NA | NA | NA | 40% | 39% | 51% | 55% | NA | NA |
| ECOG 0~1 (%) | NA | NA | NA | NA | NA | NA | 98% | 98% | 97% | 98% | NA | NA |
| Brain metastasis (%) | NA | NA | NA | NA | NA | NA | 14% | 28% | 35% | 37% | NA | NA |
| Stage IV (%) | NA | NA | NA | NA | NA | NA | 74% | 72% | 90% | 94% | NA | NA |
| Smoking, ever (%) | NA | NA | NA | NA | NA | NA | 46% | 41% | 33% | 27% | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-L.; Chen, H.-L.; Tsai, Y.-M.; Lee, T.-H.; Chang, H.-M.; Tsai, Y.-C.; Chuang, C.-H.; Chang, Y.-C.; Tu, Y.-K.; Yang, C.-J.; et al. First-Line Anaplastic Lymphoma Kinase (ALK) Inhibitors for ALK-Positive Lung Cancer in Asian Populations: Systematic Review and Network Meta-Analysis. J. Clin. Med. 2021, 10, 4376. https://doi.org/10.3390/jcm10194376
Wu K-L, Chen H-L, Tsai Y-M, Lee T-H, Chang H-M, Tsai Y-C, Chuang C-H, Chang Y-C, Tu Y-K, Yang C-J, et al. First-Line Anaplastic Lymphoma Kinase (ALK) Inhibitors for ALK-Positive Lung Cancer in Asian Populations: Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2021; 10(19):4376. https://doi.org/10.3390/jcm10194376
Chicago/Turabian StyleWu, Kuan-Li, Hsiao-Ling Chen, Ying-Ming Tsai, Tai-Huang Lee, Hsiu-Mei Chang, Yu-Chen Tsai, Cheng-Hao Chuang, Yong-Chieh Chang, Yu-Kang Tu, Chih-Jen Yang, and et al. 2021. "First-Line Anaplastic Lymphoma Kinase (ALK) Inhibitors for ALK-Positive Lung Cancer in Asian Populations: Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 10, no. 19: 4376. https://doi.org/10.3390/jcm10194376
APA StyleWu, K.-L., Chen, H.-L., Tsai, Y.-M., Lee, T.-H., Chang, H.-M., Tsai, Y.-C., Chuang, C.-H., Chang, Y.-C., Tu, Y.-K., Yang, C.-J., Hung, J.-Y., & Chong, I.-W. (2021). First-Line Anaplastic Lymphoma Kinase (ALK) Inhibitors for ALK-Positive Lung Cancer in Asian Populations: Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 10(19), 4376. https://doi.org/10.3390/jcm10194376







