Thyroidectomy for Cancer: The Surgeon and the Parathyroid Glands Sparing
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Our Experience
3.2. Literature Examination
- (a)
- (b)
- (c)
- (d)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, L.; Avenia, N.; Bernante, P.; De Palma, M.; Gulino, G.; Nasi, P.G.; Pelizzo, M.R.; Pezzullo, L. Complications of thyroid surgery: Analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J. Surg. 2004, 28, 271–276. [Google Scholar] [CrossRef]
- Perigli, G.; Fiorenza, G.; Badii, B.; Skalamera, I.; Foppa, C.; Cianchi, F. Prevenzione e trattamento della ipocalcemia precoce e tardiva dopotiroidectomia. L’Endocrinologo 2018, 19, 6–9. [Google Scholar]
- Bilezikian, J.P.; Brandi, M.L.; Cusano, N.E.; Mannstadt, M.; Rejnmark, L.; Rizzoli, R.; Rubin, M.R.; Winer, K.K.; Liberman, U.A.; Potts, J.T., Jr. Management of Hypoparathyroidism: Present and Future. J. Clin. Endocrinol. Metab. 2016, 101, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Allas, S.; Ovize, M.; Culler, M.D.; Geraul, C.; van de Wetering, J.; Mannstadt, M. A Single Administration of AZP-3601, a Novel, Long-Acting PTH Analog, Induces a Significant and Sustained Calcemic Response: Preliminary Data From a Randomized, Double-Blind, PlaceboControlled Phase 1 Study. J. Endocr. Soc. 2021, 5, A254. [Google Scholar] [CrossRef]
- Tuttle, R.M. Controversial Issues in Thyroid Cancer Management. J. Nucl Med. 2018, 59, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Puzziello, A.; Rosato, L.; Innaro, N.; Orlando, G.; Avenia, N.; Perigli, G.; Calo, P.G.; De Palma, M. Hypocalcemia following thyroid surgery: Incidence and risk factors. A longitudinal multicenter study comprising 2631 patients. Endocrine 2014, 47, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitges-Serra, A. Etiology and Diagnosis of Permanent Hypoparathyroidism after Total Thyroidectomy. J. Clin. Med. 2021, 10, 543. [Google Scholar] [CrossRef]
- Edafe, O.; Antakia, R.; Laskar, N.; Uttley, L.; Balasubramanian, S.P. Authors’ reply: Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg 2014, 101, 883–884. [Google Scholar] [CrossRef]
- Raffaelli, M.; De Crea, C.; D’Amato, G.; Moscato, U.; Bellantone, C.; Carrozza, C.; Lombardi, C.P. Post-thyroidectomy hypocalcemia is related to parathyroid dysfunction even in patients with normal parathyroid hormone concentrations early after surgery. Surgery 2016, 159, 78–84. [Google Scholar] [CrossRef]
- Pepe, J.; Colangelo, L.; Biamonte, F.; Sonato, C.; Danese, V.C.; Cecchetti, V.; Occhiuto, M.; Piazzolla, V.; De Martino, V.; Ferrone, F.; et al. Diagnosis and management of hypocalcemia. Endocrine 2020, 69, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, M.O.; Yilmaz, A.; Kocaoz, S.; Ozcaglayan, R.; Parlak, O. Risks and prediction of postoperative hypoparathyroidism due to thyroid surgery. Sci. Rep. 2021, 11, 11876. [Google Scholar] [CrossRef]
- Bedi, H.K.; Jedrzejko, N.; Nguyen, A.; Aspinall, S.R.; Wiseman, S.M. Thyroid and parathyroid surgeon case volume influences patient outcomes: A systematic review. Surg. Oncol. 2021, 38, 101550. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Fung, M.M.H.; Lee, C.H.; Fong, C.H.Y.; Woo, Y.C.; Lang, B.H.H. A territory-wide assessment of the incidence of persistent hypoparathyroidism after elective thyroid surgery and its impact on new fracture risk over time. Surgery 2021. [Google Scholar] [CrossRef]
- Lorenz, K.; Raffaeli, M.; Barczynski, M.; Lorente-Poch, L.; Sancho, J. Volume, outcomes, and quality standards in thyroid surgery: An evidence-based analysis-European Society of Endocrine Surgeons (ESES) positional statement. Langenbecks Arch. Surg. 2020, 405, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Anneback, M.; Hedberg, J.; Almquist, M.; Stalberg, P.; Norlen, O. Risk of Permanent Hypoparathyroidism After Total Thyroidectomy for Benign Disease: A Nationwide Population-based Cohort Study From Sweden. Ann. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Cianferotti, L.; Parri, S.; Altieri, P.; Arvat, E.; Benvenga, S.; Betterle, C.; Bondanelli, M.; Boscaro, M.; Camozzi, V.; et al. HypoparaNet: A Database of Chronic Hypoparathyroidism Based on Expert Medical-Surgical Centers in Italy. Calcif. Tissue Int. 2018, 103, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, N.E. Methylene blue for rapid identification of the parathyroids. Br. Med. J. 1971, 3, 680–681. [Google Scholar] [CrossRef] [Green Version]
- Monib, S.; Mohamed, A.; Abdelaziz, M.I. Methylene Blue Spray for Identification of Parathyroid Glands During Thyroidectomy. Cureus 2020, 12, e11569. [Google Scholar] [CrossRef]
- Sari, S.; Aysan, E.; Muslumanoglu, M.; Ersoy, Y.E.; Bektasoglu, H.; Yardimci, E. Safe thyroidectomy with intraoperative methylene blue spraying. Thyroid Res. 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Han, A.Y.; Huang, S.; Pellionisz, P.; Alhiyari, Y.; Krane, J.F.; Shori, R.; Stafsudd, O.; St John, M.A. A Tool to Locate Parathyroid Glands Using Dynamic Optical Contrast Imaging. Laryngoscope 2021, 31, 2391–2397. [Google Scholar] [CrossRef]
- Marsden, M.; Weaver, S.S.; Marcu, L.; Campbell, M.J. Intraoperative Mapping of Parathyroid Glands Using Fluorescence Lifetime Imaging. J. Surg. Res. 2021, 265, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mannoh, E.A.; Thomas, G.; Baregamian, N.; Rohde, S.; Solorzano, C.C.; Mahadevan-Jansen, A. Assessing Intraoperative Laser Speckle Contrast Imaging of Parathyroid Glands in Relation to Thyroidectomy Patient Outcomes. Thyroid 2021. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.T.; Azari, F.S.; Newton, A.D.; Bernstein, E.S.; Fraker, D.L.; Wachtel, H.; Singhal, S. Use of Near-Infrared Molecular Imaging for Localizing Visually Occult Parathyroid Glands in Ectopic Locations. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiangli, W.; Chen, X.; Zhang, J.; Teng, G.; Cui, X.; Idrees, B.S.; Wei, K. Primary study of identification of parathyroid gland based on laser-induced breakdown spectroscopy. Biomed. Opt. Express 2021, 12, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Paras, C.; Keller, M.; White, L.; Phay, J.; Mahadevan-Jansen, A. Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 2011, 16, 067012. [Google Scholar] [CrossRef]
- Aoyama, M.; Takizawa, H.; Yamamoto, K.; Inui, T.; Miyamoto, N.; Sakamoto, S.; Kobayashi, T.; Uehara, H.; Tangoku, A. Effects of excitation light intensity on parathyroid autofluorescence with a novel near-infrared fluorescence imaging system: Two surgical case reports. Gland Surg. 2020, 9, 1584–1589. [Google Scholar] [CrossRef]
- Akbulut, S.; Erten, O.; Gokceimam, M.; Kim, Y.S.; Krishnamurthy, V.; Heiden, K.; Jin, J.; Siperstein, A.; Berber, E. Intraoperative near-infrared imaging of parathyroid glands: A comparison of first- and second-generation technologies. J. Surg. Oncol. 2021, 123, 866–871. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Kang, P.; Choi, J.; Lee, H.S.; Park, S.Y.; Kim, Y.; Ahn, Y.C.; Lee, K.D. Near-Infrared Autofluorescence Imaging May Reduce Temporary Hypoparathyroidism in Patients Undergoing Total Thyroidectomy and Central Neck Dissection. Thyroid 2021, 31, 1400–1408. [Google Scholar] [CrossRef]
- Wiseman, S.M.; Saleh, N.; Tootooni, A.; Eshraghi, P.; Jama, R.; Saleh, S. Parathyroid identification during thyroid and parathyroid operations: A pilot study evaluating a novel low cost autofluorescence based device. Am. J. Surg. 2021, 221, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, C.M.; Thomas, G.; Baregamian, N.; Solomicronrzano, C.C. Initial clinical experiences using the intraoperative probe-based parathyroid autofluorescence identification system-PTeye during thyroid and parathyroid procedures. J. Surg. Oncol. 2021, 124, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mannoh, E.A.; Parker, L.B.; Thomas, G.; Solorzano, C.C.; Mahadevan-Jansen, A. Development of an imaging device for label-free parathyroid gland identification and vascularity assessment. J. Biophotonics 2021, 14, e202100008. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Numata, T.; Shibuya, M. Intraoperative photodynamic detection of normal parathyroid glands using 5-aminolevulinic acid. Laryngoscope 2011, 121, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Dong, Q.; He, Z.; Fan, J.; Liao, K.; Cui, M. Application of a Fluorescence Imaging System with Indocyanine Green to Protect the Parathyroid Gland Intraoperatively and to Predict Postoperative Parathyroidism. Adv. Ther. 2018, 35, 2167–2175. [Google Scholar] [CrossRef]
- Orloff, L.A.; Wiseman, S.M.; Bernet, V.J.; Fahey, T.J., 3rd; Shaha, A.R.; Shindo, M.L.; Snyder, S.K.; Stack, B.C., Jr.; Sunwoo, J.B.; Wang, M.B. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid 2018, 28, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, L.W.; Suliburk, J.; Sidhu, S.; Sywak, M. Parathyroid Cancer: Is There an Epidemic? ANZ J. Surg. 2009, 79, A18. [Google Scholar] [CrossRef]
- Park, I.; Rhu, J.; Woo, J.W.; Choi, J.H.; Kim, J.S.; Kim, J.H. Preserving Parathyroid Gland Vasculature to Reduce Post-thyroidectomy Hypocalcemia. World J. Surg. 2016, 40, 1382–1389. [Google Scholar] [CrossRef]
- Mihai, R.; Thakker, R.V. Management of Endocrine Disease: Postsurgical hypoparathyroidism: Current treatments and future prospects for parathyroid allotransplantation. Eur. J. Endocrinol. 2021, 184, R165–R175. [Google Scholar] [CrossRef]
- Chang, Y.K.; Lang, B.H.H. To identify or not to identify parathyroid glands during total thyroidectomy. Gland Surg. 2017, 6, S20–S29. [Google Scholar] [CrossRef] [Green Version]
- Lang, B.H.; Chan, D.T.; Chow, F.C. Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbecks Arch. Surg. 2016, 401, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Antakia, R.; Edafe, O.; Uttley, L.; Balasubramanian, S.P. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: A systematic review and meta-analysis. Thyroid 2015, 25, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Poch, L.; Sancho, J.; Munoz, J.L.; Gallego-Otaegui, L.; Martinez-Ruiz, C.; Sitges-Serra, A. Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbecks Arch. Surg. 2017, 402, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.Y.; Li, H.; Lin, S.J.; Deng, W.Y.; Li, Q.L.; Chen, Y.F.; Yang, A.K.; Zhang, Q.; Guo, Z.M. Fine-needle aspiration with measurement of parathyroid hormone levels in thyroidectomy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013, 48, 934–938. [Google Scholar] [PubMed]
- Zou, X.; Shi, L.; Zhu, G.; Zhu, L.; Bao, J.; Fan, J.; Hu, Y.; Zhou, B.; Lv, Z. Fine-needle aspiration with rapid parathyroid hormone assay to identify parathyroid gland in thyroidectomy. Medicine 2020, 99, e19840. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, J.; Shen, W.; Zhu, Z.; Yang, Z.; Li, X. A Rapid Intraoperative Parathyroid Hormone Assay Based on the Immune Colloidal Gold Technique for Parathyroid Identification in Thyroid Surgery. Front. Endocrinol. 2020, 11, 594745. [Google Scholar] [CrossRef] [PubMed]
- Coan, K.E.; Yen, T.W.F.; Carr, A.A.; Evans, D.B.; Wang, T.S. Confirmation of Parathyroid Tissue: Are Surgeons Aware of New and Novel Techniques? J. Surg. Res. 2020, 246, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, E.G.; Mittendorf, E.A.; Perrier, N.D.; Lee, J.E. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am. J. Surg. 2008, 196, 931–935, discussion 935–936. [Google Scholar] [CrossRef]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef]
- Solorzano, C.C.; Thomas, G.; Berber, E.; Wang, T.S.; Randolph, G.W.; Duh, Q.Y.; Triponez, F. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery 2021, 169, 868–878. [Google Scholar] [CrossRef]
- Lavazza, M.; Liu, X.; Wu, C.; Anuwong, A.; Kim, H.Y.; Liu, R.; Randolph, G.W.; Inversini, D.; Boni, L.; Rausei, S.; et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg. 2016, 5, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, A.N.; Palazzo, F.F. Near-infrared autofluorescence in thyroid and parathyroid surgery. Gland Surg. 2020, 9, S136–S146. [Google Scholar] [CrossRef]
- Weng, Y.J.; Jiang, J.; Min, L.; Ai, Q.; Chen, D.B.; Chen, W.C.; Huang, Z.H. Intraoperative near-infrared autofluorescence imaging for hypocalcemia risk reduction after total thyroidectomy: Evidence from a meta-analysis. Head Neck 2021, 43, 2523–2533. [Google Scholar] [CrossRef]
- Demarchi, M.S.; Karenovics, W.; Bedat, B.; Triponez, F. Intraoperative Autofluorescence and Indocyanine Green Angiography for the Detection and Preservation of Parathyroid Glands. J. Clin. Med. 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, L.N.; van den Hoven, P.; van Schaik, J.; Leeuwenburgh, L.; Hendricks, C.H.F.; Verduijn, P.S.; van der Bogt, K.E.A.; van Rijswijk, C.S.P.; Schepers, A.; Vahrmeijer, A.L.; et al. Perfusion Parameters in Near-Infrared Fluorescence Imaging with Indocyanine Green: A Systematic Review of the Literature. Life 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Dip, F.; Falco, J.; Verna, S.; Prunello, M.; Loccisano, M.; Quadri, P.; White, K.; Rosenthal, R. Randomized Controlled Trial Comparing White Light with Near-Infrared Autofluorescence for Parathyroid Gland Identification During Total Thyroidectomy. J. Am. Coll. Surg. 2019, 228, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.; Solorzano, C.C.; Baregamian, N.; Mannoh, E.A.; Gautam, R.; Irlmeier, R.T.; Ye, F.; Nelson, J.A.; Long, S.E.; Gauger, P.G.; et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection—A surgeon-blinded multi-centric study. Am. J. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Solorzano, C.C.; Thomas, G.; Baregamian, N.; Mahadevan-Jansen, A. Detecting the Near Infrared Autofluorescence of the Human Parathyroid: Hype or Opportunity? Ann. Surg. 2020, 272, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; De Leeuw, F.; Breuskin, I.; Casiraghi, O.; Lakhdar, A.B.; Ghanem, W.; Laplace-Builhe, C.; Hartl, D. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral. Oncol. 2018, 87, 186–196. [Google Scholar] [CrossRef]
- Wong, A.; Wong, J.C.Y.; Pandey, P.U.; Wiseman, S.M. Novel techniques for intraoperative parathyroid gland identification: A comprehensive review. Expert Rev. Endocrinol. Metab. 2020, 15, 439–457. [Google Scholar] [CrossRef]
- Perigli, G.; Cortesini, C.; Qirici, E.; Boni, D.; Cianchi, F. Clinical benefits of minimally invasive techniques in thyroid surgery. World J. Surg. 2008, 32, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Perigli, G.; Qirici, E.; Badii, B.; Kokomani, A.; Staderini, F.; Luconi, M.; Crescioli, C.; Mannelli, M.; Maggi, M.; Cianchi, F. Feasibility and safety of minimal-incision thyroidectomy for Graves’ disease: A prospective, single-center study. Head Neck 2013, 35, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, T.H.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Kim, Y.N.; Kim, H.; Kim, S.W.; Chung, J.H. Surgeon volume and prognosis of patients with advanced papillary thyroid cancer and lateral nodal metastasis. Br. J. Surg. 2018, 105, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Onder, C.E.; Kuskonmaz, S.M.; Koc, G.; Firat, S.F.; Omma, T.; Culha, C. Evaluation of management of patients with postoperative permanent hypoparathyroidism. How close are we to the targets? Minerva Endocrinol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
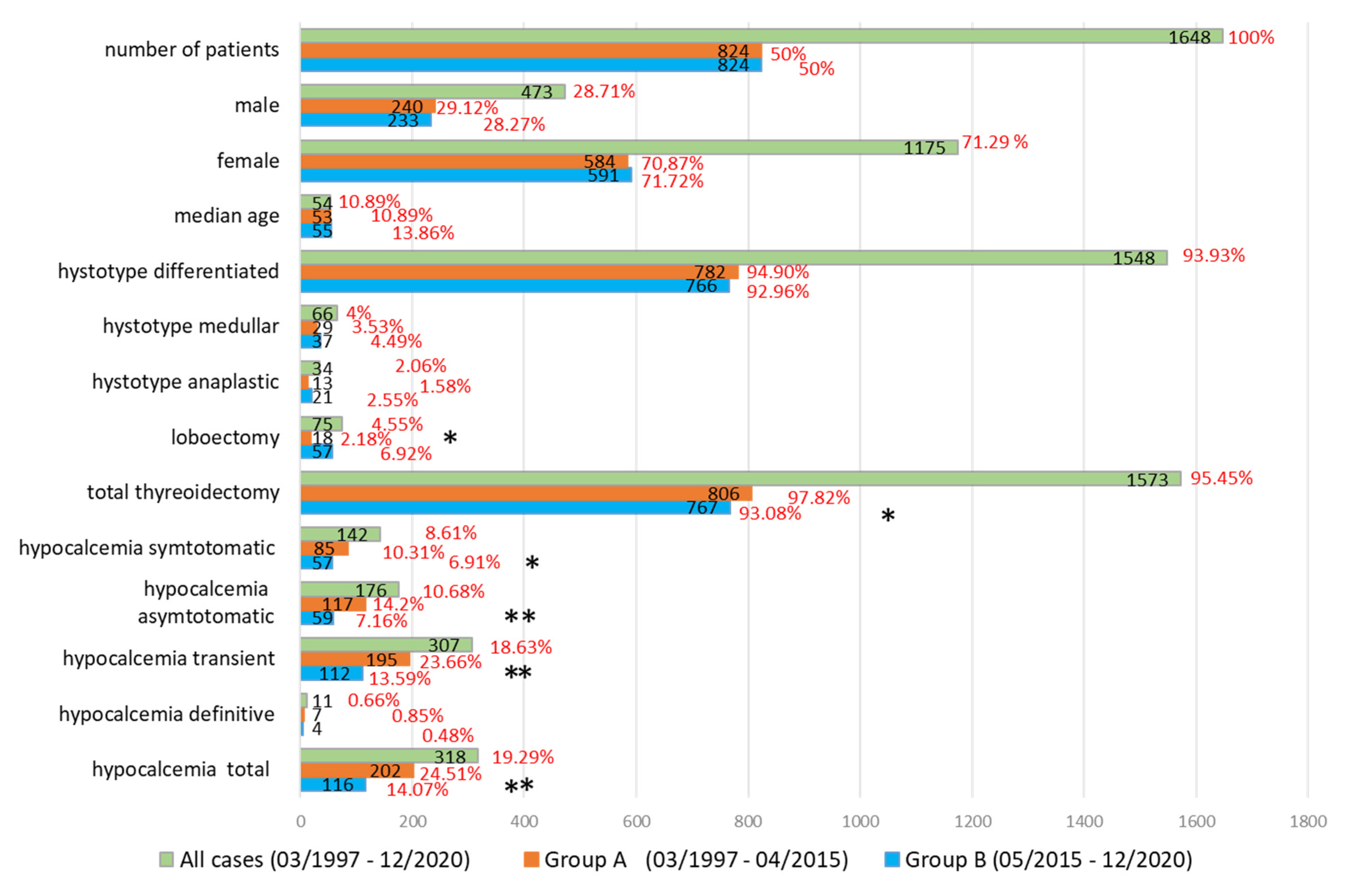
| Group A | Group B | p Value | Group A | Group B | p Value | ||||||
| Type of Surgery | Minimally Invasive | Conventional | |||||||||
| cases | 403 | 257 | 421 | 567 | |||||||
| N. | % | N. | % | N. | % | N. | % | ||||
| hypocalcemia | symptomatic | 38 | 9.43 | 14 | 5.44 | 0.0752 | 47 | 11.16 | 43 | 7.58 | 0.0577 |
| asymptomatic | 56 | 13.89 | 24 | 9.34 | 0.0875 | 61 | 14.49 | 35 | 6.17 | <0.0001 | |
| transient | 91 | 22.58 | 37 | 14.39 | 0.01 | 104 | 24.7 | 76 | 13.4 | <0.0001 | |
| definitive | 3 | 0.74 | 1 | 0.39 | 1 | 4 | 0.95 | 2 | 0.35 | 0.4106 | |
| total | 94 | 23.32 | 38 | 14.78 | 0.003 | 108 | 25.65 | 78 | 13.75 | <0.0001 | |
| Type of Surgery | Cclnd | No Cclnd | |||||||||
| cases | 216 | 185 | 608 | 639 | |||||||
| N. | % | N. | % | N. | % | N. | % | ||||
| hypocalcemia | symptomatic | 44 | 20.37 | 32 | 17.29 | 0.4464 | 45 | 7.4 | 28 | 4.38 | 0.0293 |
| asymptomatic | 40 | 18.51 | 29 | 15.68 | 0.5077 | 73 | 12.01 | 27 | 4.22 | <0.0001 | |
| transient | 79 | 36.57 | 59 | 31.89 | 0.3 | 116 | 19.08 | 53 | 8.29 | <0.0001 | |
| definitive | 5 | 2.31 | 2 | 1.08 | 0.4589 | 2 | 0.33 | 2 | 0.31 | 1 | |
| total | 84 | 38.88 | 61 | 32.97 | 0.2 | 118 | 19.41 | 55 | 8.6 | <0.0001 | |
| Reference | Technology | Article Type | Nb pt | Parathyroid Identification | Postoperative Hypo-PTH /Hypoca | Conclusions |
|---|---|---|---|---|---|---|
| Dudley et al. [19] 1971 | Intravenous infusion of methylene blue | original/humans | 17 | 41/68 | / | Could help to reduce the high incidence of clinical hypoparathyroidism after total thyroidectomy. |
| Monib et al. [20] 2020 | Intraoperative methylene blue spray | original/humans | 50 | 82% accuracy | 18% | Safe, feasible, and effective to identify parathyroid glands |
| Sari et al. [21] 2012 | Intraoperative methylene blue spray | original/humans | 56 | / | 5% transient | Identification of parathyroid glands within three minutes and also of recurrent laryngeal nerves and inferior thyroid arteries. |
| Hu et al. [22] 2021 | Dynamic optical contrast imaging (DOCI) | original/animals and humans ex vivo | / | / | / | Facilitates specific parathyroid gland localization |
| Marsden et al. [23] 2021 | Fluorescence lifetime imaging (FLIm) | original/humans | 21 | 100% sensitivity 93% specificity | / | Good sensitivity and specificity for the rapid identification of PG. |
| Mannoh et al. [24] 2021 | Laser speckle contrast imaging (LSCI) | original/humans | 72 | / | 8.3% temporary 1.4% permanent | Promising technique for assessing parathyroid gland vascularity |
| Kennedy et al. 2021 [25] | Near-infrared molecular Imaging (IMI) | original/humans | 5 | 9/9 | 1/9 asymptomatic | Accurate and reproducible method of localizing parathyroid glands |
| Wang et al. [26] 2021 | Laser-induced breakdown spectroscopy (LIBS) | original/animals ex vivo | / | / | / | Can discriminate between smear samples of PG and NPG |
| Paras et al. [27] 2011 | Near-infrared (NIR) autofluorescence | original/humans | 21 | / | / | Parathyroid fluorescence was two to eleven times higher than that of the thyroid tissues with peak fluorescence occurring at 820 to 830 nm. |
| Aoyama et al. [28] 2020 | Near-infrared (NIR) autofluorescence | original/humans | 2 | / | / | The autofluorescence of diseased glands was weaker than that of normal glands, even with the excitation light of NIR. |
| Akbulut et al. [29] 2021 | Near-infrared (NIR) autofluorescence | original/humans | 300 | 25% * | / | Second-generation NIFI (CMOS) displayed higher detection rates and AF intensity. |
| Kim et al. [30] 2021 | Near-infrared (NIR) autofluorescence | original/humans | 542 | / | 4.2% permanent | May reduce temporary hypoparathyroidism and the risk of inadvertent resection of PGs in CND. |
| Wiseman et al. [31] 2021 | Near-infrared (NIR) autofluorescence | original/humans in vivo and ex vivo | / | / | / | Can successfully intraoperatively identify both normal and pathological PGs. |
| Kiernan et al. [32] 2021 | Near-infrared (NIR) autofluorescence | original/humans | 83 | 94.3% accuracy | / | Probe-based NIRAF detection can be a valuable adjunct device to intraoperatively identify PGs. |
| Mannoh et al. [33] 2021 | ParaSPAI a device that combines NIRAF imaging with LSCI | original/humans | / | / | / | Capable of label-free parathyroid gland identification and vascularity assessment through the combination of NIRAF imaging with LSCI. |
| Suzuki et al. [34] 2011 | 5-Aminolevulinic Acid | original/humans | 13 | In all patients at least one | / | Useful to localize the normal parathyroid glands during thyroid surgery |
| Jin et al. [35] 2018 | Indocyanine green | original/humans | 26 | / | 7.69% transient | Safe, easy and effective method to protect the parathyroid and predict postoperative hypoparathyroidism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perigli, G.; Cianchi, F.; Giudici, F.; Russo, E.; Fiorenza, G.; Petrone, L.; Sparano, C.; Staderini, F.; Badii, B.; Morandi, A. Thyroidectomy for Cancer: The Surgeon and the Parathyroid Glands Sparing. J. Clin. Med. 2021, 10, 4323. https://doi.org/10.3390/jcm10194323
Perigli G, Cianchi F, Giudici F, Russo E, Fiorenza G, Petrone L, Sparano C, Staderini F, Badii B, Morandi A. Thyroidectomy for Cancer: The Surgeon and the Parathyroid Glands Sparing. Journal of Clinical Medicine. 2021; 10(19):4323. https://doi.org/10.3390/jcm10194323
Chicago/Turabian StylePerigli, Giuliano, Fabio Cianchi, Francesco Giudici, Edda Russo, Giulia Fiorenza, Luisa Petrone, Clotilde Sparano, Fabio Staderini, Benedetta Badii, and Alessio Morandi. 2021. "Thyroidectomy for Cancer: The Surgeon and the Parathyroid Glands Sparing" Journal of Clinical Medicine 10, no. 19: 4323. https://doi.org/10.3390/jcm10194323
APA StylePerigli, G., Cianchi, F., Giudici, F., Russo, E., Fiorenza, G., Petrone, L., Sparano, C., Staderini, F., Badii, B., & Morandi, A. (2021). Thyroidectomy for Cancer: The Surgeon and the Parathyroid Glands Sparing. Journal of Clinical Medicine, 10(19), 4323. https://doi.org/10.3390/jcm10194323







