Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS—Results from an Open Label Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Methods of Laboratory Testing
2.2.1. Determination of Specific IgG Antibodies Titers against Selected Foods (GROUP 2-G2-IP)
2.2.2. Determination of Fecal Calprotectin Concentration
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ono, M.; Kato, M.; Miyamoto, S.; Tsuda, M.; Mizushima, T.; Ono, S.; Nakagawa, M.; Mabe, K.; Nakagawa, S.; Muto, S.; et al. Multicenter observational study on functional bowel disorders diagnosed using Rome III diagnostic criteria in Japan. J. Gastroenterol. 2018, 53, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Whitehead, W.E.; Tilburg, M.A. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.L. Irritable bowel syndrome: A clinical review. World J. Gastroenterol. 2014, 20, 12144–12160. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Törnblom, H. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenerology 2014, 146, 67–75. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K.; Irving, P.M. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gibson, P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Ther. Adv. Gastroenterol. 2012, 5, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Nawawi, K.N.M.; Belov, M.; Goulding, C. Low FODMAP diet significantly improves IBS symptoms: An Irish retrospective cohort study. Eur. J. Nutr. 2020, 59, 2237–2248. [Google Scholar] [CrossRef]
- De Roest, R.H.; Dobbs, B.R.; Chapman, B.A. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: A prospective study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef]
- Atkinson, W.; Sheldon, T.A.; Shaath, N. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.S. Treatment of delayed food allergy based on specific immunoglobulin G RAST testing. Otolaryngol. Head Neck Surg. 2000, 123, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Li, Y.Q. The therapeutic effects of eliminating allergic foods according to food specific IgG antibodies in irritable bowel syndrome. Zhonghua Nei Ke Za Zhi 2007, 46, 641–643. [Google Scholar] [PubMed]
- Drisko, J.; Bischoff, B.; Hall, M.; McCallum, R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J. Am. Coll. Nutr. 2006, 25, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Andresen, V.; Pehl, C.; Allescher, H.; Bischoff, S.C.; Classen, M.; Enck, P.; Frieling, T.; Haag, S.; Holtmann, G.; et al. Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten; Deutschen Gesellschaft für Neurogastroenterologie und Motilität (Irritable bowel syndrome: German consensus guidelines on definition, pathophysiology and management). Gastroenterol 2011, 49, 237–293. [Google Scholar]
- Xie, Y.; Zhou, G.; Xu, Y.; He, B.; Wang, Y.; Ma, R.; Chang, Y.; He, D.; Xu, C.; Xiao, Z. Effects of Diet Based on IgG Elimination Combined with Probiotics on Migraine Plus Irritable Bowel Syndrome. Pain Res. Manag. 2019, 21, 7890461. [Google Scholar] [CrossRef] [Green Version]
- Aydinlar, E.I.; Dikmen, P.Y.; Tiftikci, A. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013, 53, 514–525. [Google Scholar] [CrossRef]
- önsson, F.; Mancardi, D.A.; Kita, Y.; Karasuyama, H.; Iannascoli, B.; Van Rooijen, N.; Shimizu, T.; Daëron, M.; Bruhns, P. Mouse and human neutrophils induce anaphylaxis. J. Clin. Invest. 2011, 1, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.L.; Li, Y.Q.; Li, W.J. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy 2007, 37, 823–830. [Google Scholar] [CrossRef]
- Kalliomaki, M.A. Food allergy and irritable bowel syndrome. Curr. Opin. Gastroenterol. 2005, 21, 708–711. [Google Scholar] [CrossRef]
- Bentz, S.; Hausmann, M.; Piberger, H.; Kellermeier, S.; Paul, S.; Held, L.; Falk, W.; Obermeier, F.; Fried, M.; Schölmerich, J.; et al. Clinical relevance of IgG antibodies against food antigen in Crohn’s disease—A double blind cross over diet intervention study. Digestion 2017, 81, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzunısmaıl, H.; Cengız, M.; Uzun, H.; Ozbakir, F.; Göksel, S.; Demırdağ, F.; Can, G.; Balci, H. The effects of provocation by foods with raised IgG antibodies and additives on the course of Crohn’s disease: A pilot study. Turk. J. Gastroenterol. 2012, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Alpay, K.; Ertas, M.; Orhan, E.K.; Ustay, D.K.; Lieners, C.; Baykan, B. Diet restriction in migraine, based on IgG against foods: A clinical double-blind, randomised, cross-over trial. Cephalalgia 2010, 30, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Fedewa, A.; Rao, S.S.C. Dietary fructose intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, M.J.; Behrouz, V.; Azimi, M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. J. Gastroenterol. Hepatol. 2018, 33, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [Green Version]
- Traczyk, I.; Jarosz, M.; Tomasiuk, R. Concentration of IgG antibodies against food allergens in patients with irritable bowel syndrome and healthy individuals. Przegląd Gastroenterol. 2011, 6, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Koloski, N.; Jones, M.; Walker, M.M.; Veysey, M.; Zala, A.; Keely, S.; Holtmann, G.; Talley, N.J. Population based study: Atopy and autoimmune diseases are associated with functional dyspepsia and irritable bowel syndrome, independent of psychological distress. Aliment. Pharmacol. Ther. 2019, 49, 546–555. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Talley, N.J. Evidence that independent gut-to-brain and brain-to-gutpathways operate in the irritable bowel syndrome and functionaldyspepsia: A 1-year population-based prospective study. Aliment. Pharmacol. Ther. 2016, 44, 592–600. [Google Scholar] [CrossRef]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 2002, 51, 41–44. [Google Scholar] [CrossRef]
- Sinagra, E.; Pompei, G.; Tomasello, G.; Cappello, F.; Morreale, G.C.; Amvrosiadis, G.; Rossi, F.; Lo Monte, A.I.; Rizzo, A.G.; Raimondo, D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J. Gastroenterol. 2016, 22, 2242–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiho, H.; Ihara, E.; Nakamura, K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J. Gastrointest. Pathophysiol. 2010, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Maes, M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [PubMed]
- Teuber, S.S.; Beyer, K. IgG to foods: A test not ready for prime time. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 257–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.S.; van Swieten, P.; Aalberse, R.C. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 1986, 64, 415–422. [Google Scholar]
- Stapel, S.O.; Asero, R.; Ballmer-Weber, B.K.; Knol, E.F.; Strobel, S.; Vieths, S.; Kleine-Tebbe, J. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy 2008, 63, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2012, 8, 477–512. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome dependent regulation of Tregs and Th17 cells in mucosa. Front. Immunol. 2019, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. Department of Medicine, Cincinnati Veterans Affairs Medical Center, Ohio, USA. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef]
- Ishikawa, R.; Tsujimura, Y.; Obata, K.; Kawano, Y.; Minegishi, Y.; Karasuyama, H. IgG-mediated systemic anaphylaxis to protein antigen can be induced even under conditions of limited amounts of antibody and antigen. Biochem. Biophys. Res. Commun. 2010, 26, 742–746. [Google Scholar] [CrossRef]
- Strait, R.T.; Morris, S.C.; Yang, M.; Qu, X.W.; Finkelman, F.D. Pathways of anaphylaxis in the mouse. J. Allergy Clin. Immunol. 2002, 109, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Olender, K.; Bergmann, K.; Odrowąż-Sypniewska, G. Faecal calprotectin as an inflammatory marker in inflammatory bowel diseases. J. Lab. Diagn. 2012, 48, 433–439. [Google Scholar]
- Burri, E.; Beglinger, C. Faecal calprotectin in the diagnosis of inflammatory bowel disease. Biochem. Med. 2011, 21, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Meucci, G.; D’Incà, R.; Maieron, R. Diagnostic value of faecal calprotectin in unselected outpatients referred for colonoscopy: A multicenter prospective study. Dig. Liver Dis. 2010, 42, 191–195. [Google Scholar] [CrossRef]
- Von Roon, A.C.; Karamountzos, L.; Purkayastha, S.; Reese, G.E.; Darzi, A.W.; Teare, J.P.; Paraskeva, P.; Tekkiset, P.P. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am. J. Gastroenterol. 2007, 102, 803–813. [Google Scholar] [CrossRef]
- Łykowska-Szuber, L.; Eder, P.; Klimczak, K. Przydatność diagnostyczna kopromarkerów w wybranych chorobach jelit. Gastroenterol. Prakt. 2014, 4, 37–42. [Google Scholar]
- Dhaliwal, A.; Zeino, Z.; Tomkins, C.; Cheung, M.; Nwokolo, C.; Smith, S.; Harmston, C.; Arasaradnam, R.P. Utility of faecal calprotectin in inflammatory bowel disease (IBD): What cut-offs should we apply? Frontline Gastroenterol. 2015, 6, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Fengming, Y.; Jianbing, W. Biomarkers of inflammatory bowel disease. Dis. Markers 2014, 710915. [Google Scholar] [CrossRef]
- Däbritz, J.; Musci, J.; Foell, D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J. Gastroenterol. 2014, 14, 363–375. [Google Scholar] [CrossRef]
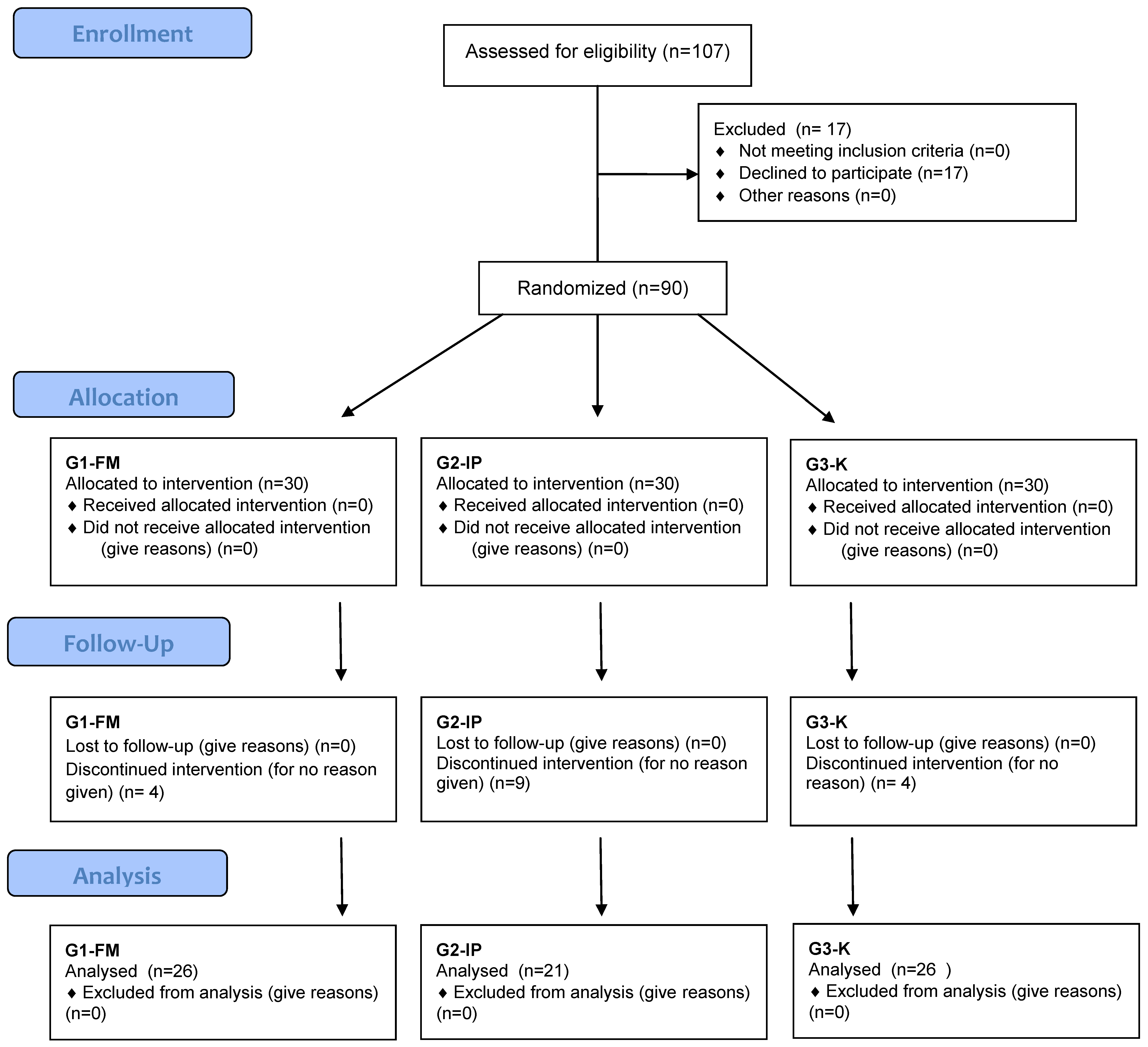
| Selected Demographic Features | G1-FM (n= 26) | G2-IP (n = 21) | G3-K (n = 26) | p |
|---|---|---|---|---|
| AGE | ||||
| Mean age | 42.70 ± 16.70 | 40.60 ± 14.50 | 41.70 ± 13.40 | 0.931 * |
| Median | 46.00 | 46.00 | 43.50 | |
| Total mean age | 41.70 ± 14.80 | |||
| BODY WEIGHT (kg) | ||||
| Mean 1st examination | 66.73 ± 13.35 | 63.14 ± 9.13 | 73.77 ± 19.85 | 0.126 * |
| Mean 2nd examination | 66.73 ± 13.35 | 63.14 ± 9.13 | 73.77 ± 19.85 | 0.090 * |
| p | 0.531 | 0.055 | 0.680 | |
| BMI (kg/m2) | ||||
| Mean 1st examination | 24.12 ± 4.58 | 23.81 ± 3.72 | 26.50 ± 6.93 | 0.341 * |
| Mean 2nd examination | 24.09 ± 4.43 | 23.54 ± 3.59 | 26.40 ± 6.86 | 0.324 * |
| p | 0.918 *** | 0.038 *** | 0.684 *** | |
| SMOKING TOBACCO | ||||
| Smokes | 0 | 4 (19.00%) | 4 (15.40%) | 0.071 * |
| Does not smoke | 19 (73.10%) | 10 (47.60%) | 19 (73.10%) | |
| Expeller | 7 (26.90%) | 7 (33.30%) | 3 (11.50%) | |
| LEVEL OF EDUCATION | ||||
| Vocational | 2 (7.70%) | 2 (9.50%) | 2 (7.70%) | 0.078 ** |
| Secondary | 7 (26.90%) | 11 (52.40%) | 4 (15.40%) | |
| Higher | 17 (65.40%) | 8 (38.10%) | 20 (76.90%) | |
| PLACE OF RESIDENCE | ||||
| Village | 3 (11.50%) | 3 (14.30%) | 1 (3.80%) | 0.155 ** |
| Town | 0 | 3 (14.30%) | 1 (3.80%) | |
| City | 23 (88.50%) | 15 (71.40%) | 24 (92.30%) | |
| IBS DURATION | ||||
| <5 years | 11 (42.30%) | 12 (57.10%) | 11 (42.30%) | 0.849 ** |
| 6–10 years | 9 (34.60%) | 5 (23.80%) | 9 (34.60%) | |
| >11 years | 6 (23.10%) | 4 (19.00%) | 6 (23.10%) | |
| Symptoms | G1-FM | G2-IP | G3-K | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | ||
| Idiopathic abdominal pain | N | 15 | 11 | 0.125 | 16 | 2 | <0.001 | 16 | 14 | 0.500 |
| % | 57.7 | 42.3 | 76.2 | 9.5 | 61.5 | 53.8 | ||||
| Abdominal pain after a meal | N | 11 | 6 | 0.063 | 14 | 2 | <0.001 | 14 | 12 | 0.625 |
| % | 42.3 | 23.1 | 66.7 | 9.5 | 53.8 | 46.2 | ||||
| Abdominal pain during defecation | N | 5 | 2 | 0.250 | 9 | 1 | 0.008 | 6 | 6 | 1.000 |
| % | 19.2 | 7.7 | 42.9 | 4.8 | 23.1 | 23.1 | ||||
| Sensation of incomplete defecation | N | 13 | 10 | 0.250 | 13 | 2 | 0.001 | 14 | 15 | 1.000 |
| % | 50.0 | 38.5 | 61.9 | 9.5 | 53.8 | 57.7 | ||||
| Mucus in stool | N | 8 | 2 | 0.031 | 6 | 0 | * | 5 | 6 | 1.000 |
| % | 30.8 | 7.7 | 28.6 | 0.0 | 19.2 | 23.1 | ||||
| Blood in stool | N | 3 | 0 | * | 2 | 0 | * | 2 | 2 | 1.000 |
| % | 11.5 | 0.0 | 9.5 | 0.0 | 7.7 | 7.7 | ||||
| Difficulty to defecate (constipations) | N | 11 | 7 | 0.219 | 14 | 4 | 0.002 | 19 | 17 | 0.500 |
| % | 42.3 | 26.9 | 66.7 | 19.0 | 73.1 | 65.4 | ||||
| Bloating | N | 22 | 7 | <0.001 | 19 | 2 | <0.001 | 24 | 22 | 0.500 |
| % | 84.6 | 26.9 | 90.5 | 9.5 | 92.3 | 84.6 | ||||
| Gurgling sensation | N | 17 | 4 | <0.001 | 18 | 2 | <0.001 | 21 | 19 | 0.500 |
| % | 65.4 | 15.4 | 85.7 | 9.5 | 80.8 | 73.1 | ||||
| Gastric fullness | N | 15 | 3 | <0.001 | 19 | 2 | <0.001 | 22 | 19 | 0.250 |
| % | 57.7 | 11.5 | 90.5 | 9.5 | 84.6 | 73.1 | ||||
| Symptoms | G1-FM | G2-IP | G3-K | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | ||
| Nausea | N | 6 | 0 | * | 7 | 0 | * | 9 | 9 | 1.000 |
| % | 23.1 | 0.0 | 33.3 | 0.0 | 34.6 | 34.6 | ||||
| Heartburn | N | 2 | 2 | 1.000 | 7 | 1 | 0.031 | 5 | 4 | 1.000 |
| % | 7.7 | 7.7 | 33.3 | 4.8 | 19.2 | 15.4 | ||||
| Belching | N | 5 | 4 | 1.000 | 6 | 0.0 | * | 7 | 7 | 1.000 |
| % | 19.2 | 15.4 | 28.6 | 0.0 | 26.9 | 26.9 | ||||
| Symptoms | G1-FM | G2-IP | G3-K | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | 1st Examination | 2nd Examination | p | ||
| Constant tiredness and weakness | N | 5 | 4 | 1.000 | 7 | 1 | 0.031 | 9 | 8 | 1.000 |
| % | 19.2 | 15.4 | 33.3 | 4.8 | 34.6 | 30.8 | ||||
| Skin conditions | N | 0 | 0 | * | 4 | 0 | * | 1 | 1 | 1.000 |
| % | 0.0 | 0.0 | 19.0 | 0.0 | 3.8 | 3.8 | ||||
| Headaches/migraines | N | 3 | 3 | 1.000 | 3 | 0 | * | 5 | 4 | 1.000 |
| % | 11.5 | 11.5 | 14.3 | 0.0 | 19.2 | 15.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, L.; Wasiluk, D.; Lieners, C.F.J.; Gałęcka, M.; Bartnicka, A.; Tveiten, D. Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS—Results from an Open Label Study. J. Clin. Med. 2021, 10, 4317. https://doi.org/10.3390/jcm10194317
Ostrowska L, Wasiluk D, Lieners CFJ, Gałęcka M, Bartnicka A, Tveiten D. Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS—Results from an Open Label Study. Journal of Clinical Medicine. 2021; 10(19):4317. https://doi.org/10.3390/jcm10194317
Chicago/Turabian StyleOstrowska, Lucyna, Diana Wasiluk, Camille F. J. Lieners, Mirosława Gałęcka, Anna Bartnicka, and Dag Tveiten. 2021. "Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS—Results from an Open Label Study" Journal of Clinical Medicine 10, no. 19: 4317. https://doi.org/10.3390/jcm10194317
APA StyleOstrowska, L., Wasiluk, D., Lieners, C. F. J., Gałęcka, M., Bartnicka, A., & Tveiten, D. (2021). Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS—Results from an Open Label Study. Journal of Clinical Medicine, 10(19), 4317. https://doi.org/10.3390/jcm10194317







