Patients with Common Variable Immunodeficiency Complicated by Autoimmune Phenomena Have Lymphopenia and Reduced Treg, Th17, and NK Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Compliance with Research Ethics Standards
2.3. Flow Cytometry Analysis
- transitional B cells: IgM++ IgD++ CD38++ CD27− CD19+ CD45+
- naïve B cells: IgM+ IgD++ CD38+ CD27− CD19+ CD45+
- nonswitched memory B cells (marginal zone-like B cells): IgM++ IgD+ CD38+ CD27+ CD19+ CD45+
- class-switched memory B cells: IgM− IgD− CD38+ CD27+ CD19+ CD45+
- plasmablasts: IgM−/+ IgD− CD38+++ CD27++ CD19+ CD45+
- CD21low B cells: IgM+ IgD+ CD38+low CD27− CD21+low CD19+ CD45+
- RTE T cells: CD45RA+ CD62L+ CD31+ CD3+ CD45+
- naïve T cells: CD45RA+ CD197+ CD3+ CD45+
- effector T cells: CD45RA+ CD197− CD3+ CD45+
- central memory T cells: CD45RO+ CD197+ CD3+ CD45+
- effector memory T cells: CD45RO+ CD197− CD3+ CD45+
- RTE T cells: CD45RA+ CD62L+ CD31+
- Bregs: CD19+ CD5+ CD1dhigh
- Tregs: CD3+ CD4+ CD25high FoxP3+ CD127−
- Th17: CD3+ CD4+ CD45RO+ CD196+
2.4. Statistical Analysis
3. Results
3.1. The Clinical Characteristics of Patients
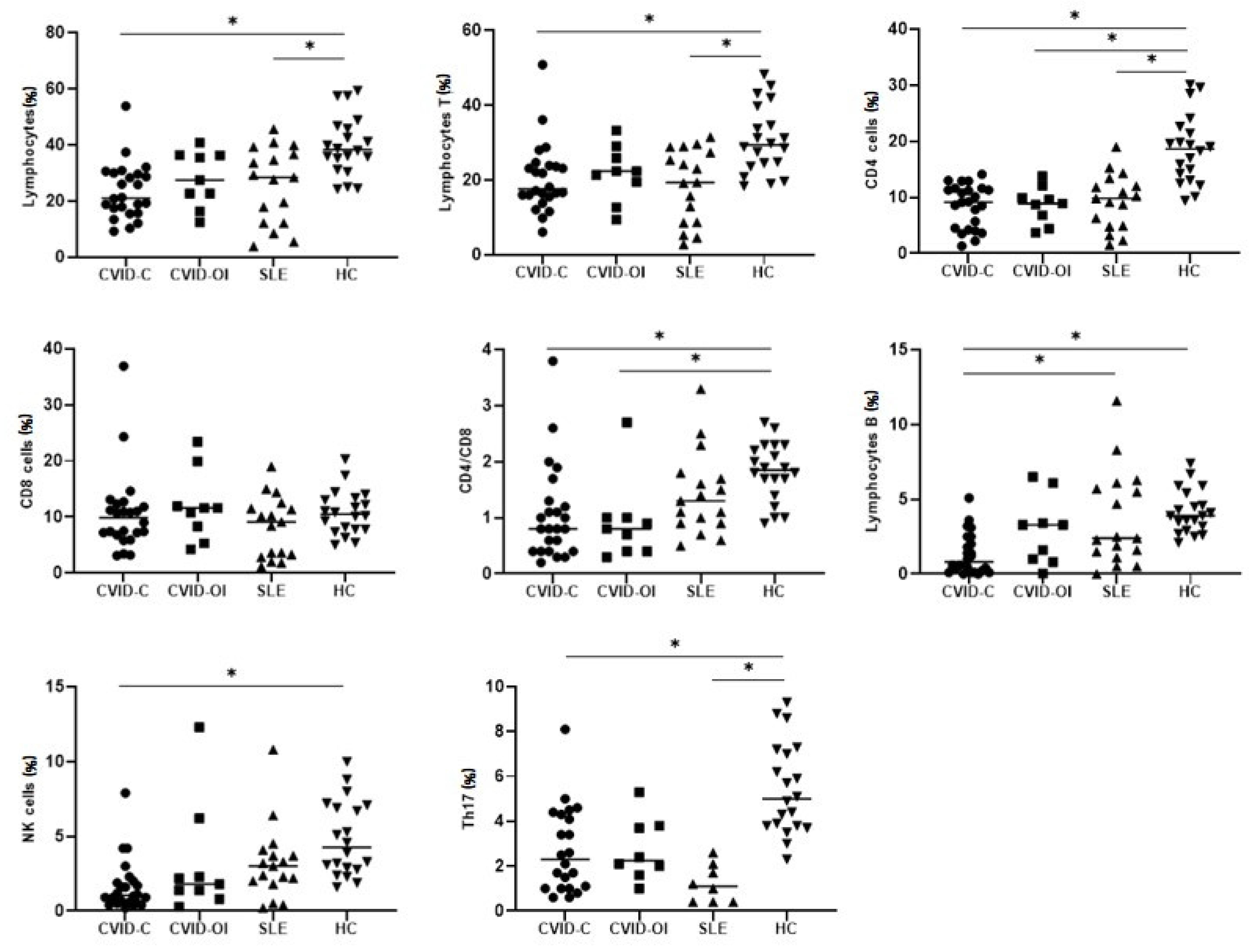
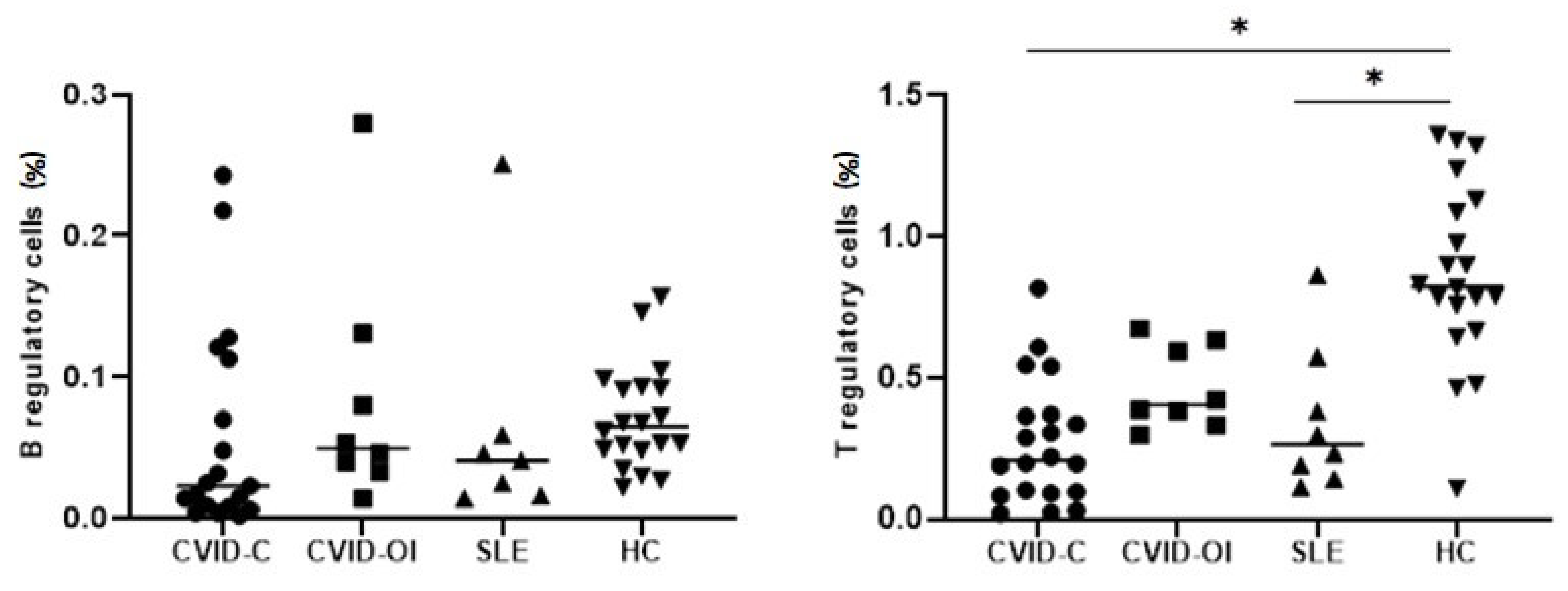
3.2. Peripheral Main Lymphocyte Subsets, Tregs, Bregs, and Th17 Cells
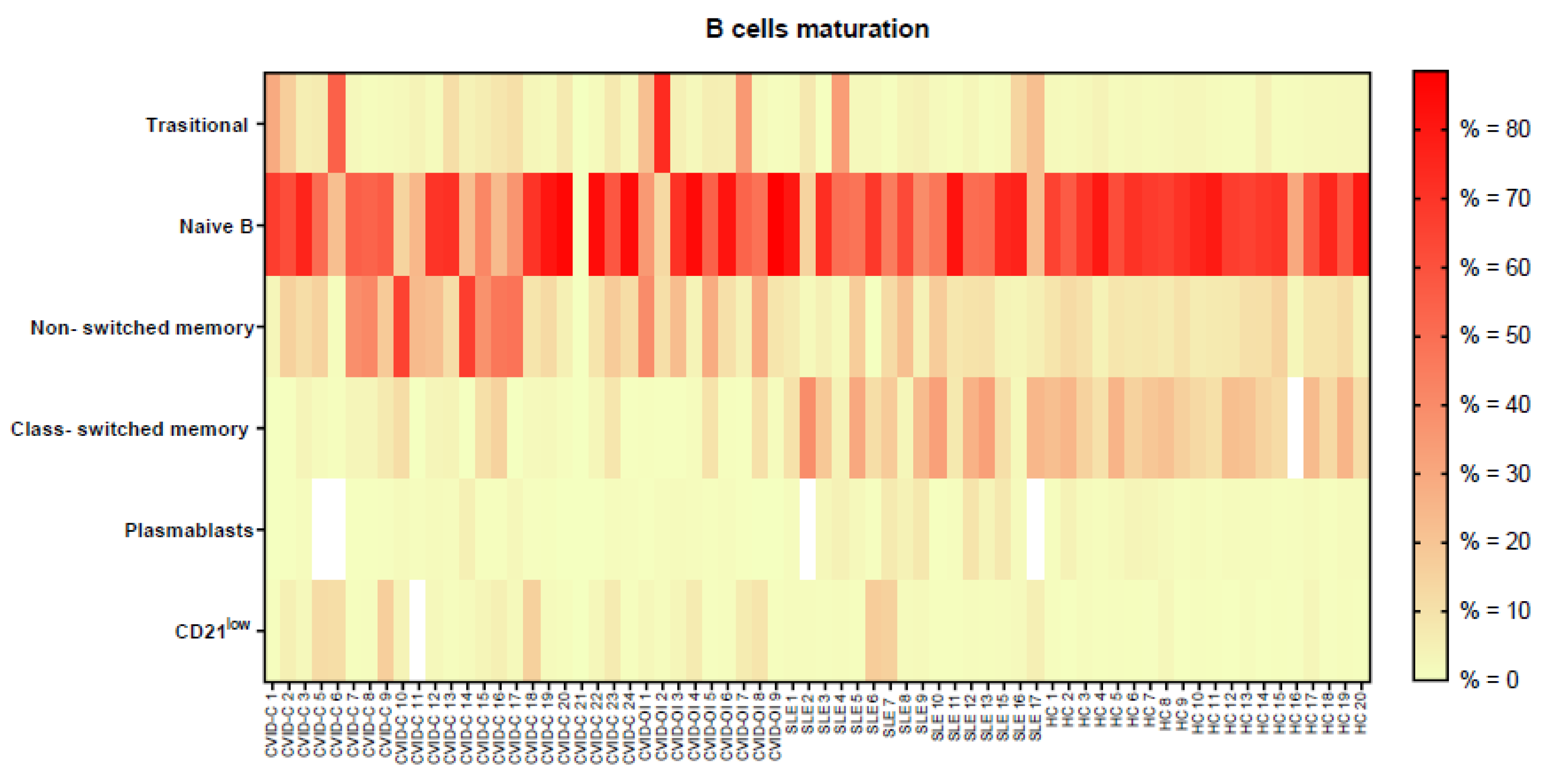
3.3. B Lymphocyte Maturation
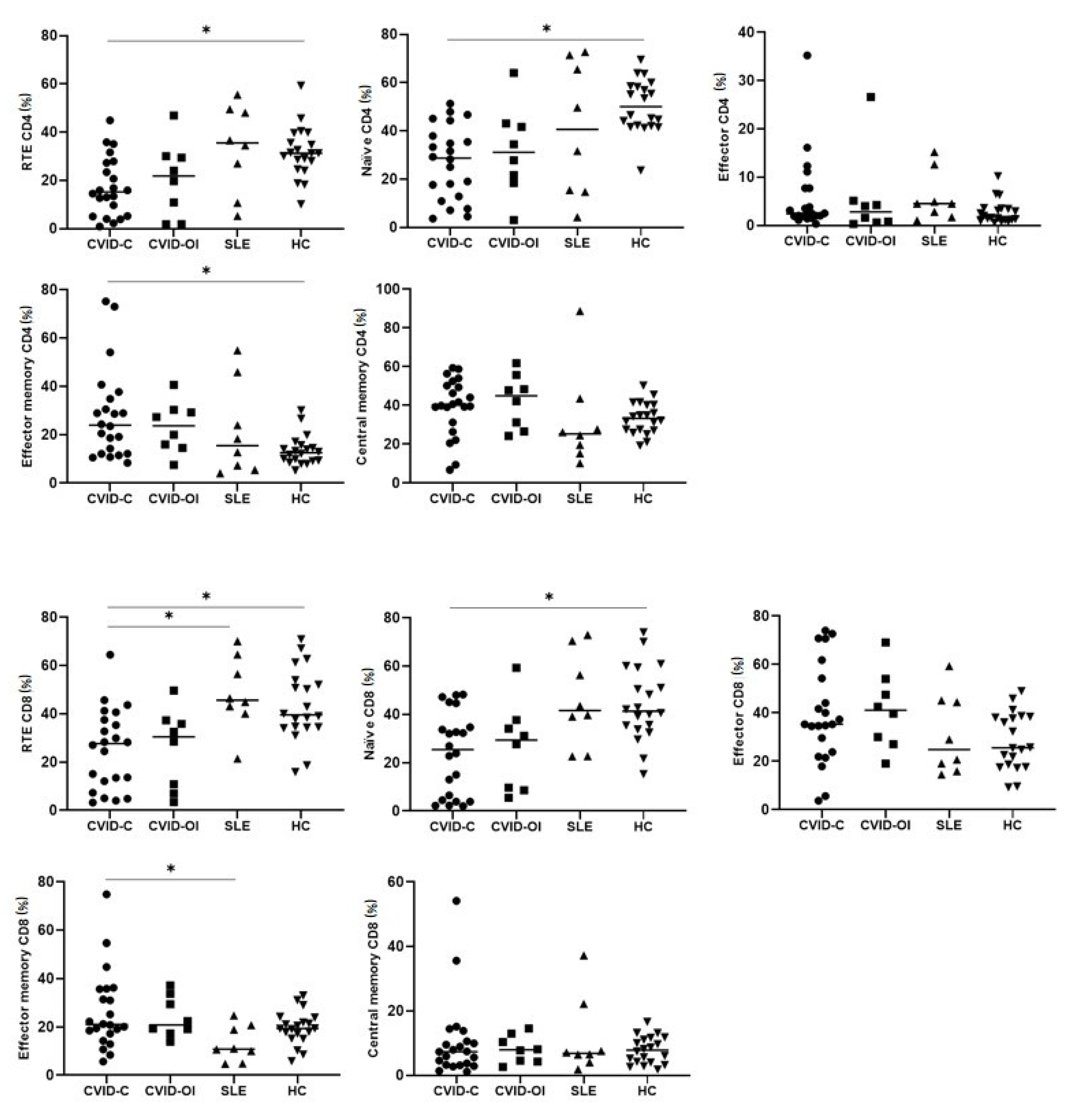
3.4. T Lymphocyte Maturation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Więsik-Szewczyk, E.; Jahnz-Różyk, K. From Infections to Autoimmunity: Diagnostic Challenges in Common Variable Immunodeficiency. World J. Clin. Cases 2020, 8, 3942–3955. [Google Scholar] [CrossRef]
- Ziętkiewicz, M.; Więsik-Szewczyk, E.; Matyja-Bednarczyk, A.; Napiórkowska-Baran, K.; Zdrojewski, Z.; Jahnz-Różyk, K. Shorter Diagnostic Delay in Polish Adult Patients With Common Variable Immunodeficiency and Symptom Onset After 1999. Front. Immunol. 2020, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, Y.; Vosughi, A.; Azizi, G.; Yazdani, R.; Kiaee, F.; Hafezi, N.; Alimorad, S.; Khoshmirsafa, M.; Seif, F.; Hassanpour, G.; et al. Comparison of Clinical and Immunological Features and Mortality in Common Variable Immunodeficiency and Agammaglobulinemia Patients. Immunol. Lett. 2019, 210, 55–62. [Google Scholar] [CrossRef]
- Ho, H.E.; Cunningham-Rundles, C. Non-infectious Complications of Common Variable Immunodeficiency: Updated Clinical Spectrum, Sequelae, and Insights to Pathogenesis. Front. Immunol. 2020, 11, 149. [Google Scholar] [CrossRef]
- Costagliola, G.; Consolini, R. Lymphadenopathy at the crossroad between immunodeficiency and autoinflammation: An intriguing challenge. Clin. Exp. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.S.; Schäffer, A.A.; Grüning, B.A.; et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar] [CrossRef]
- Lo, B.; Zhang, K.; Lu, W.; Zheng, L.; Zhang, Q.; Kanellopoulou, C.; Zhang, Y.; Liu, Z.; Fritz, J.M.; Marsh, R.; et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 2015, 349, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Klemann, C.; Camacho-Ordonez, N.; Yang, L.; Eskandarian, Z.; Rojas-Restrepo, J.L.; Frede, N.; Bulashevska, A.; Heeg, M.; Al-Ddafari, M.S.; Premm, J.; et al. Clinical and Immunological Phenotype of Patients With Primary Immunodeficiency Due to Damaging Mutations in NFKB2. Front. Immunol. 2019, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Tuijnenburg, P.; Lango Allen, H.; Burns, S.O.; Greene, D.; Jansen, M.H.; Staples, E.; Stephens, J.; Carss, K.J.; Biasci, D.; Baxendale, H.; et al. Loss-of-function nuclear factor κB subunit 1 (NFKB1) variants are the most common monogenic cause of common variable immunodeficiency in Europeans. J. Allergy Clin. Immunol. 2018, 142, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Elgizouli, M.; Lowe, D.M.; Speckmann, C.; Schubert, D.; Hülsdünker, J.; Eskandarian, Z.; Dudek, A.; Schmitt-Graeff, A.; Wanders, J.; Jørgensen, S.F.; et al. Activating PI3Kδ mutations in a cohort of 669 patients with primary immunodeficiency. Clin. Exp. Immunol. 2016, 183, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sogkas, G.; Dubrowinskaja, N.; Adriawan, I.R.; Anim, M.; Witte, T.; Schmidt, R.E.; Atschekzei, F. High Frequency of Variants in Genes Associated with Primary Immunodeficiencies in Patients with Rheumatic Diseases with Secondary Hypogammaglobulinaemia. Ann. Rheum. Dis. 2020, 80, 392–399. [Google Scholar] [CrossRef]
- Perazzio, S.F.; Granados, Á.; Salomão, R.; Silva, N.P.; Carneiro-Sampaio, M.; Andrade, L.E.C. High Frequency of Immunodeficiency-like States in Systemic Lupus Erythematosus: A Cross-Sectional Study in 300 Consecutive Patients. Rheumatology 2016, 55, 1647–1655. [Google Scholar] [CrossRef]
- Errante, P.R.; Perazzio, S.F.; Frazão, J.B.; da Silva, N.P.; Andrade, L.E.C. Primary Immunodeficiency Association with Systemic Lupus Erythematosus: Review of Literature and Lessons Learned by the Rheumatology Division of a Tertiary University Hospital at São Paulo, Brazil. Rev. Bras. Reumatol. 2016, 56, 58–68. [Google Scholar] [CrossRef]
- Almaghlouth, I.; Su, J.; Johnson, S.R.; Pullenayegum, E.; Gladman, D.; Urowitz, M. Acquired Low Immunoglobulin Levels and Risk of Clinically Relevant Infection in Adult Patients with Systemic Lupus Erythematosus: A Cohort Study. Rheumatol. Oxf. Engl. 2020, 60, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Warnatz, K.; Denz, A.; Dräger, R.; Braun, M.; Groth, C.; Wolff-Vorbeck, G.; Eibel, H.; Schlesier, M.; Peter, H.H. Severe Deficiency of Switched Memory B Cells (CD27(+)IgM(-)IgD(−)) in Subgroups of Patients with Common Variable Immunodeficiency: A New Approach to Classify a Heterogeneous Disease. Blood 2002, 99, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Wehr, C.; Kivioja, T.; Schmitt, C.; Ferry, B.; Witte, T.; Eren, E.; Vlkova, M.; Hernandez, M.; Detkova, D.; Bos, P.R.; et al. The EUROclass Trial: Defining Subgroups in Common Variable Immunodeficiency. Blood 2008, 111, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Amirkashani, D.; Parvaneh, N.; Mohammadinejad, P.; Gharib, B.; Shahinpour, S.; Hirbod-Mobarakeh, A.; Mirghorbani, M.; Movahedi, M.; Gharagozlou, M.; et al. Autoimmune Phenotype in Patients with Common Variable Immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2013, 23, 323–329. [Google Scholar] [PubMed]
- Edwards, E.S.J.; Bosco, J.J.; Aui, P.M.; Stirling, R.G.; Cameron, P.U.; Chatelier, J.; Hore-Lacy, F.; O’Hehir, R.E.; van Zelm, M.C. Predominantly Antibody-Deficient Patients With Non-Infectious Complications Have Reduced Naive B, Treg, Th17, and Tfh17 Cells. Front. Immunol. 2019, 10, 2593. [Google Scholar] [CrossRef]
- Warnatz, K.; Schlesier, M. Flowcytometric Phenotyping of Common Variable Immunodeficiency. Cytom. B Clin. Cytom. 2008, 74, 261–271. [Google Scholar] [CrossRef]
- Giovannetti, A.; Pierdominici, M.; Mazzetta, F.; Marziali, M.; Renzi, C.; Mileo, A.M.; De Felice, M.; Mora, B.; Esposito, A.; Carello, R.; et al. Unravelling the Complexity of T Cell Abnormalities in Common Variable Immunodeficiency. J. Immunol. 2007, 178, 3932–3943. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.A.L.; Ayers, L.; Sadler, R.; Lucas, M.; Roberts, C.; Woods, A.; Packwood, K.; Burden, J.; Harrison, D.; Kaenzig, N.; et al. T Cell Phenotypes in Patients with Common Variable Immunodeficiency Disorders: Associations with Clinical Phenotypes in Comparison with Other Groups with Recurrent Infections. Clin. Exp. Immunol. 2012, 170, 202–211. [Google Scholar] [CrossRef]
- von Spee-Mayer, C.; Koemm, V.; Wehr, C.; Goldacker, S.; Kindle, G.; Bulashevska, A.; Proietti, M.; Grimbacher, B.; Ehl, S.; Warnatz, K. Evaluating Laboratory Criteria for Combined Immunodeficiency in Adult Patients Diagnosed with Common Variable Immunodeficiency. Clin. Immunol. 2019, 203, 59–62. [Google Scholar] [CrossRef]
- Azizi, G.; Rezaei, N.; Kiaee, F.; Tavakolinia, N.; Yazdani, R.; Mirshafiey, A.; Aghamohammadi, A. T-Cell Abnormalities in Common Variable Immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2016, 26, 233–243. [Google Scholar] [CrossRef]
- Unger, S.; Seidl, M.; van Schouwenburg, P.; Rakhmanov, M.; Bulashevska, A.; Frede, N.; Grimbacher, B.; Pfeiffer, J.; Schrenk, K.; Munoz, L.; et al. The TH1 Phenotype of Follicular Helper T Cells Indicates an IFN-γ-Associated Immune Dysregulation in Patients with CD21low Common Variable Immunodeficiency. J. Allergy Clin. Immunol. 2018, 141, 730–740. [Google Scholar] [CrossRef]
- Le Saos-Patrinos, C.; Loizon, S.; Blanco, P.; Viallard, J.-F.; Duluc, D. Functions of Tfh Cells in Common Variable Immunodeficiency. Front. Immunol. 2020, 11, 6. [Google Scholar] [CrossRef]
- Turpin, D.; Furudoi, A.; Parrens, M.; Blanco, P.; Viallard, J.-F.; Duluc, D. Increase of Follicular Helper T Cells Skewed toward a Th1 Profile in CVID Patients with Non-Infectious Clinical Complications. Clin. Immunol. 2018, 197, 130–138. [Google Scholar] [CrossRef]
- Robinson, G.A.; Peng, J.; Dönnes, P.; Coelewij, L.; Naja, M.; Radziszewska, A.; Wincup, C.; Peckham, H.; Isenberg, D.A.; Ioannou, Y.; et al. Disease-Associated and Patient-Specific Immune Cell Signatures in Juvenile-Onset Systemic Lupus Erythematosus: Patient Stratification Using a Machine-Learning Approach. Lancet Rheumatol. 2020, 2, e485–e496. [Google Scholar] [CrossRef]
- Jin, W.; Luo, Z.; Yang, H. Peripheral B Cell Subsets in Autoimmune Diseases: Clinical Implications and Effects of B Cell-Targeted Therapies. J. Immunol. Res. 2020, 2020, 9518137. [Google Scholar] [CrossRef]
- Wehr, C.; Eibel, H.; Masilamani, M.; Illges, H.; Schlesier, M.; Peter, H.-H.; Warnatz, K. A New CD21low B Cell Population in the Peripheral Blood of Patients with SLE. Clin. Immunol. 2004, 113, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, A.; Etemadi, H.; Adriawan, I.R.; Ernst, D.; Jacobs, R.; Buyny, S.; Witte, T.; Schmidt, R.E.; Atschekzei, F.; Sogkas, G. Peripheral Blood Lymphocyte Phenotype Differentiates Secondary Antibody Deficiency in Rheumatic Disease from Primary Antibody Deficiency. J. Clin. Med. 2020, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Wirsum, C.; Glaser, C.; Gutenberger, S.; Keller, B.; Unger, S.; Voll, R.E.; Vach, W.; Ness, T.; Warnatz, K. Secondary Antibody Deficiency in Glucocorticoid Therapy Clearly Differs from Primary Antibody Deficiency. J. Clin. Immunol. 2016, 36, 406–412. [Google Scholar] [CrossRef]
- ESID. Registry—Working Definitions for Clinical Diagnosis. Available online: https://Esid.Org/Education/Diagnostic-Criteria-Pid (accessed on 23 July 2021).
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Common Variable Immunodeficiency Disorders: Division into Distinct Clinical Phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.-M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Boldt, A.; Borte, S.; Fricke, S.; Kentouche, K.; Emmrich, F.; Borte, M.; Kahlenberg, F.; Sack, U. Eight-color immunophenotyping of T-, B-, and NK-cell subpopulations for characterization of chronic immunodeficiencies. Cytom. B Clin. Cytom. 2014, 86, 191–206. [Google Scholar] [CrossRef]
- Fevang, B.; Yndestad, A.; Sandberg, W.J.; Holm, A.M.; Müller, F.; Aukrust, P.; Frøland, S.S. Low Numbers of Regulatory T Cells in Common Variable Immunodeficiency: Association with Chronic Inflammation in Vivo. Clin. Exp. Immunol. 2007, 147, 521–525. [Google Scholar] [CrossRef]
- Kofod-Olsen, E.; Jørgensen, S.E.; Nissen, S.K.; Westh, L.; Møller, B.K.; Østergaard, L.; Larsen, C.S.; Mogensen, T.H. Altered Fraction of Regulatory B and T Cells Is Correlated with Autoimmune Phenomena and Splenomegaly in Patients with CVID. Clin. Immunol. 2016, 162, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.; Mirshafiey, A.; Jeddi-Tehrani, M.; Abolhassani, H.; Sadeghi, B.; Mirminachi, B.; Shaghaghi, M.; Aghamohammadi, A. Evaluation of CD4+CD25+FOXP3+ Regulatory T Cells Function in Patients with Common Variable Immunodeficiency. Cell. Immunol. 2013, 281, 129–133. [Google Scholar] [CrossRef]
- Kutukculer, N.; Azarsiz, E.; Aksu, G.; Karaca, N.E. CD4+CD25+Foxp3+ T Regulatory Cells, Th1 (CCR5, IL-2, IFN-γ) and Th2 (CCR4, IL-4, Il-13) Type Chemokine Receptors and Intracellular Cytokines in Children with Common Variable Immunodeficiency. Int. J. Immunopathol. Pharmacol. 2016, 29, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Gérard, L.; Carpentier, S.; Vély, F.; Cypowyj, S.; Farnarier, C.; Vince, N.; Malphettes, M.; Fieschi, C.; Oksenhendler, E.; et al. Low Circulating Natural Killer Cell Counts Are Associated With Severe Disease in Patients With Common Variable Immunodeficiency. EBioMedicine 2016, 6, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wehr, C. Trying to Understand NK Cell Function in Vivo Points towards a Severity Score for CVID Patients. EBioMedicine 2016, 6, 18–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haymore, B.R.; Mikita, C.P.; Tsokos, G.C. Common Variable Immune Deficiency (CVID) Presenting as an Autoimmune Disease: Role of Memory B Cells. Autoimmun. Rev. 2008, 7, 309–312. [Google Scholar] [CrossRef]
- Barbosa, R.R.; Silva, S.P.; Silva, S.L.; Melo, A.C.; Pedro, E.; Barbosa, M.P.; Pereira-Santos, M.C.; Victorino, R.M.M.; Sousa, A.E. Primary B-Cell Deficiencies Reveal a Link between Human IL-17-Producing CD4 T-Cell Homeostasis and B-Cell Differentiation. PLoS ONE 2011, 6, e22848. [Google Scholar] [CrossRef] [PubMed]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Bosma, A.; Abdel-Gadir, A.; Isenberg, D.A.; Jury, E.C.; Mauri, C. Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity 2012, 36, 477–490. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Asmamaw Dejenie, T.; Mengie Ayele, T.; Dagnew Baye, N.; Agegnehu Teshome, A.; Tilahun Muche, Z. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yesillik, S.; Agrawal, S.; Gollapudi, S.V.; Gupta, S. Phenotypic Analysis of CD4+ Treg, CD8+ Treg, and Breg Cells in Adult Common Variable Immunodeficiency Patients. Int. Arch. Allergy Immunol. 2019, 180, 150–158. [Google Scholar] [CrossRef]
- Barsotti, N.S.; Almeida, R.R.; Costa, P.R.; Barros, M.T.; Kalil, J.; Kokron, C.M. IL-10-Producing Regulatory B Cells Are Decreased in Patients with Common Variable Immunodeficiency. PLoS ONE 2016, 11, e0151761. [Google Scholar] [CrossRef] [PubMed]
- Stuchlý, J.; Kanderová, V.; Vlková, M.; Heřmanová, I.; Slámová, L.; Pelák, O.; Taraldsrud, E.; Jílek, D.; Králíc Ková, P.; Fevang, B.; et al. Common Variable Immunodeficiency Patients with a Phenotypic Profile of Immunosenescence Present with Thrombocytopenia. Sci. Rep. 2017, 7, 39710. [Google Scholar] [CrossRef]
- Koga, T.; Ichinose, K.; Kawakami, A.; Tsokos, G.C. Current Insights and Future Prospects for Targeting IL-17 to Treat Patients With Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 624971. [Google Scholar] [CrossRef]
- Osnes, L.T.; Nakken, B.; Bodolay, E.; Szodoray, P. Assessment of Intracellular Cytokines and Regulatory Cells in Patients with Autoimmune Diseases and Primary Immunodeficiencies—Novel Tool for Diagnostics and Patient Follow-Up. Autoimmun. Rev. 2013, 12, 967–971. [Google Scholar] [CrossRef] [PubMed]

| Clinical Phenotypes and Organ Complications in CVID Patients (n = 33) | |
|---|---|
| No disease-related complications | 9 (27%) |
| Bronchiectasis | 4 (12%) |
| Splenomegaly | 7 (21%) |
| Autoimmunity | 20 (60%) |
| Thrombocytopenia | 10 (33%) |
| Hemolytic anemia | 6 (18%) |
| Addison–Biermer disease | 2 (6%) |
| Vitiligo | 1 (3%) |
| Chronic seronegative polyarthritis | 2 (6%) |
| Alopecia areata | 1 (3%) |
| Nonspecific inflammatory bowel disease | 1 (3%) |
| Psoriasis | 3 (6%) |
| Polyclonal lymphocytic infiltration | |
| Generalized lymphadenopathy | 19 (57%) |
| Granulomatous lesions (histopathological confirmation) | 9 (27%) |
| Immunoglobulin replacement therapy | 29 |
| Immunoglobulin naïve | 3 |
| Prednisolone | 2; dose 5 mg/day |
| Methotrexate and etanercept | 1 |
| Rituximab in anamnesis | 2 |
| Clinical data of SLE patients (n = 17) | |
| SLEDAI2K | 3.6 (min 0–max 9) |
| Treatment | 16/17 |
| Prednisolone | 10 (58%); dose: 9 mg/day (min 5–max 15 mg) |
| Antimalarials | 16 (94%) |
| Immunosuppressive medication | |
| Methotrexate | 2 (11%) |
| Rituximab in anamnesis | 1 (5%) |
| As Median (Q1–Q3) | CVID-C (a) n = 24 | CVID-OI (b) n = 9 | SLE (c) n = 17 | HC (d) n = 20 | p < 0.05 Group a-b-c-d ANOVA, Kruskal–Wallis | p < 0.05 between Groups Post Hoc Test |
|---|---|---|---|---|---|---|
| % of all cells | ||||||
| Lymphocytes | 21.0 (16.6–29.8) | 27.5 (22.7–36.3) | 28.4 (12.1–36.6) | 38.3 (33.2–46.3) | p = 0.0002 | *a-d, *c-d |
| Lymphocytes T | 17.6 (15.7–23.7) | 22.4 (19.5–25.9) | 19.3 (8.7–27.4) | 29.5 (24.0–37.2) | p = 0.0011 | *a–d, *c–d |
| CD4 cells | 9.1 (4.3–11.4) | 8.9 (6.8–9.9) | 9.8 (4.9–12.0) | 18.6 (13.6–22.0) | p < 0.0001 | *a–d, *b–d, *c–d |
| CD8 cells | 9.8 (7.0–12.0) | 11.6 (8.3–11.9) | 9.1 (3.3–11.5) | 10.5 (7.8–13.2) | - | - |
| Lymphocytes B | 0.8 (0.1–2.2) | 3.3 (1.0–3.4) | 2.4 (1.5–5.7) | 3.9 (3.0–5.0) | p < 0.0001 | *a–c, *a–d |
| NK cells | 1.0 (0.5–1.9) | 1.8 (1.4–2.3) | 3.0 (2.0–3.7) | 4.2 (2.8–7.0) | p = 0.0001 | *a–d |
| Bregs | 0.023 (0.008–0.113 | 0.050 (0.037–0.106) | 0.041 (0.016–0.059) | 0.065 (0.049–0.093) | - | - |
| Tregs | 0.209 (0.093–0.366) | 0.404 (0.356–0.613) | 0.265 (0.167–0.478) | 0.824 (0.711–1.109) | p < 0.0001 | *a–d, *c–d |
| Th17 | 2.3 (1.0–4.3) | 2.2 (1.8–3.7) | 1.1 (0.4–1.9) | 5.0 (3.8–7.1) | p < 0.0001 | *a–d, *c–d |
| (cells/µL) | ||||||
| WBC | 5575 (4605–7555) | 6600 (5440–7370) | 5690 (3630–8310) | 6555 (4930–7535) | - | - |
| Lymphocytes | 1201 (755–2145) | 1986 (1119–2402) | 1115 (1005–1576) | 2037 (1838–2934) | p = 0.0002 | *a–d, *c–d |
| Lymphocytes T | 1071 (701–1614) | 1457 (961–2093) | 887 (570–1125) | 1660 (1409–2292) | p = 0.0004 | *a–d, *c–d |
| CD4 cells | 458 (305–553) | 574 (372–680) | 418 (288–499) | 978 (756–1559) | p < 0.0001 | *a–d, *c–d |
| CD8 cells | 580 (305–809) | 814 (374–1089) | 319 (162–582) | 624 (457–791) | p = 0.0269 | *c–d |
| Ratio CD4/CD8 | 0.8 (0.4–1.2) | 0.8 (0.4–1.0) | 1.3 (0.9–1.7) | 1.8 (1.5–2.5) | p = 0.0003 | *a–d, *b–d |
| Lymphocytes B | 47 (12–127) | 212 (117–332) | 145 (69–222) | 216 (190–284) | p < 0.0001 | *a–d |
| NK cells | 54 (32–100) | 152 (96–488) | 126 (87–234) | 245 (204–447) | p = 0.0001 | *a–d |
| Bregs | 1 (0–4) | 4 (2–9) | 2 (1–3) | 4 (3–7) | - | - |
| Tregs | 15 (4–21) | 26 (22–30) | 20 (12–28) | 55 (37–82) | p < 0.0001 | *a–d, *c–d |
| Th17 | 130 (73–190) | 131 (94–284) | 77 (35–113) | 256 (209–494) | p < 0.0001 | *a–d, *c–d |
| As Median (Q1–Q3) | CVID-C (a) n = 24 | CVID-OI (b) n = 9 | SLE (c) n = 17 | HC (d) n = 20 | p < 0.05 Group a-b-c-d ANOVA, Kruskal–Wallis | p < 0.05 between Groups Post Hoc Test |
|---|---|---|---|---|---|---|
| % of B cells | ||||||
| Transitional B | 4.3 (1.9–8.4) | 5.5 (2.4–21.6) | 2.1 (1.0–6.2) | 1.8 (1.4–2.3) | p = 0.0149 | *b–d |
| Naïve B | 56.3 (24.6–71.5) | 54.8 (48.8–81.3) | 51.0 (46.0–73.4) | 68.0 (63.5–73.1) | - | - |
| Nonswitched memory | 15.9 (8.9–38.7) | 11.0 (8.8–28.7) | 6.0 (3.7–11.1) | 8.6 (6.9–10.3) | p = 0.0036 | *a–c, *a–d |
| Class-switched memory | 2.2 (0.2–3.7) | 0.6 (0.3–1.3) | 18.4 (8.6–28.2) | 17.6 (12.7–22.8) | p < 0.0001 | *a–c, *a–d, *b–c, *b–d |
| Plasmablasts | 0.5 (0.1–1.4) | 0.5 (0.1–1.1) | 3.9 (1.3–7.8) | 1.4 (0.8–1.6) | p = 0.0004 | *a–c, *b–c |
| CD21low B cells | 2.2 (0.9–6.7) | 2.5 (0.8–6.5) | 1.3 (0.9–1.9) | 0.6 (0.4–0.9) | p = 0.0005 | *a–d, *b–d |
| Transitional B | 4.3 (1.9–8.4) | 5.5 (2.4–21.6) | 2.1 (1.0–6.2) | 1.8 (1.4–2.3) | p = 0.0149 | *b–d |
| Naïve B | 56.3 (24.6–71.5) | 54.8 (48.8–81.3) | 51.0 (46.0–73.4) | 68.0 (63.5–73.1) | - | - |
| Nonswitched memory | 15.9 (8.9–38.7) | 11.0 (8.8–28.7) | 6.0 (3.7–11.1) | 8.6 (6.9–10.3) | p = 0.0036 | *a–c, *a–d |
| As Median (Q1–Q3) | CVID-C (a) n = 24 | CVID-OI (b) n = 9 | SLE (c) n = 17 | HC (d) n = 20 | p < 0.05 Group a-b-c-d ANOVA, Kruskal–Wallis | p < 0.05 between Groups Post Hoc Test |
|---|---|---|---|---|---|---|
| % of CD4 cells | ||||||
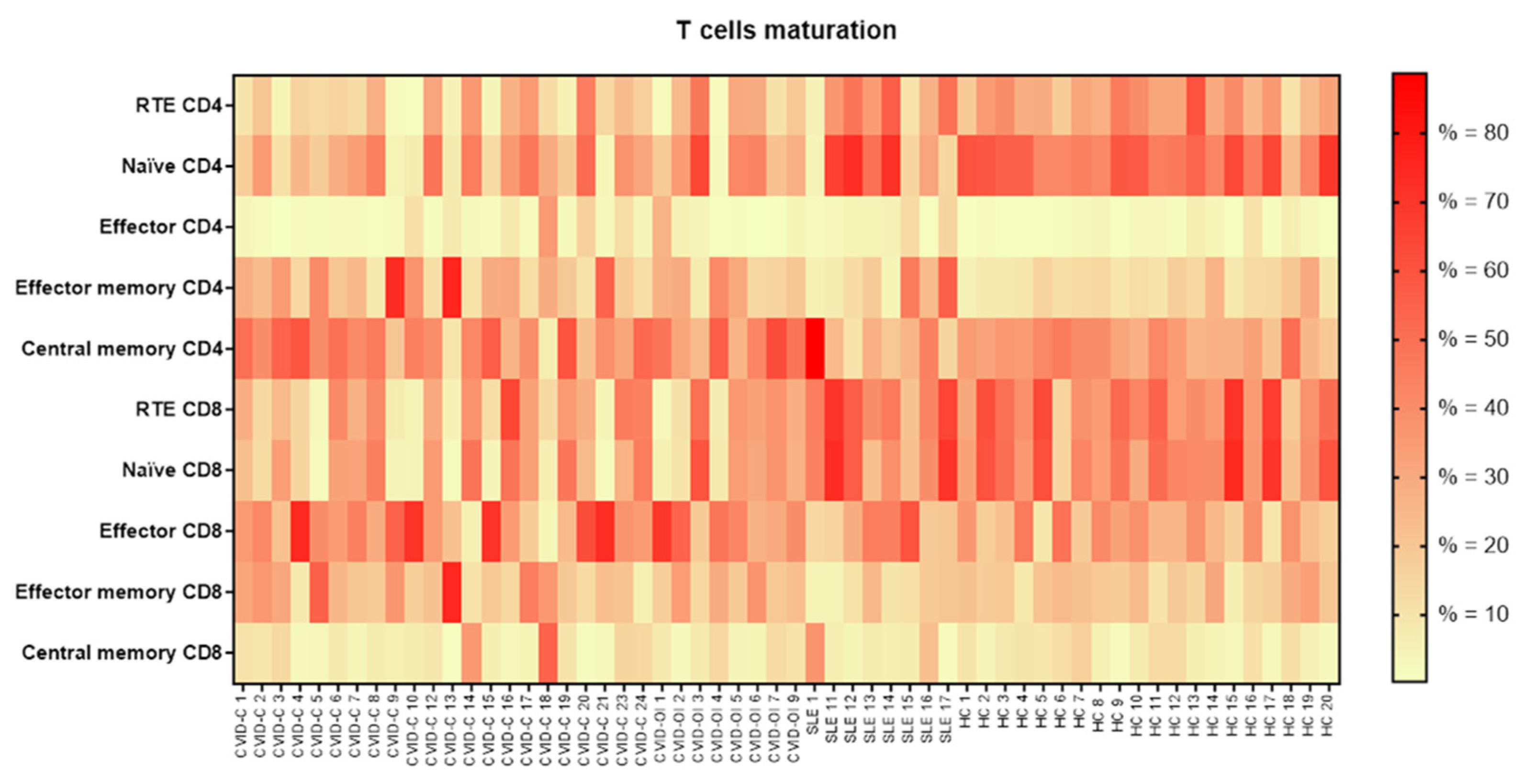
| Recent thymic emigrants (RTE) CD4 | 15.2 (5.2–27.3) | 21.8 (6.3–29.7) | 35.5 (18.5–48.7) | 31.2 (26.3–37.6) | p = 0.0031 | *a–d |
| Naïve CD4 | 28.7 (12.8–37.9) | 31.1 (20.0–42.3) | 40.6 (15.0–68.4) | 50.0 (42.1–58.3) | p = 0.0009 | *a–d |
| Effector CD4 | 2.4 (1.9–7.7) | 2.8 (0.7–4.6) | 4.5 (2.2–8.7) | 1.8 (1.1–3.4) | - | - |
| Effector memory CD4 | 23.9 (12.1–34.8) | 23.6 (15.2–29.7) | 15.4 (6.3–34.9) | 12.5 (9.2–15.0) | p = 0.0126 | *a–d |
| Central memory CD4 | 40.2 (31.2–50.2) | 44.9 (28.8–52.0) | 25.3 (17.4–35.5) | 33.2 (27.2–40.3) | - | - |
| CD21low B cells | 2.2 (0.9–6.7) | 2.5 (0.8–6.5) | 1.3 (0.9–1.9) | 0.6 (0.4–0.9) | p = 0.0005 | *a–d, *b–d |
| Transitional B | 4.3 (1.9–8.4) | 5.5 (2.4–21.6) | 2.1 (1.0–6.2) | 1.8 (1.4–2.3) | p = 0.0149 | *b–d |
| Naïve B | 56.3 (24.6–71.5) | 54.8 (48.8–81.3) | 51.0 (46.0–73.4) | 68.0 (63.5–73.1) | - | - |
| Nonswitched memory | 15.9 (8.9–38.7) | 11.0 (8.8–28.7) | 6.0 (3.7–11.1) | 8.6 (6.9–10.3) | p = 0.0036 | *a–c, *a–d |
| % of CD8 cells | ||||||
| Recent thymic emigrants (RTE) CD8 | 27.6 (12.1–37.5) | 30.4 (8.9–36.5) | 45.6 (41.6–60.4) | 39.5 (34.4–52.9) | p = 0.0006 | *a–c, *a–d |
| Naïve CD8 | 25.4 (4.5–34.7) | 29.3 (9.1–35.9) | 41.6 (30.9–63.3) | 41.3 (34.6–55.2) | p = 0.0019 | *a–d |
| Effector CD8 | 35.3 (23.8–54.3) | 41.1 (28.5–50.7) | 24.8 (17.5–44.7) | 25.5 (18.1–38.2) | - | - |
| Effector memory CD8 | 21.0 (17.2–35.7) | 20.8 (18.2–31.5) | 10.8 (7.4–19.8) | 19.3 (16.2–22.9) | p = 0.0490 | *a–c |
| Central memory CD8 | 7.3 (3.2–10.6) | 7.9 (4.4–11.6) | 6.8 (5.2–14.8) | 7.8 (4.1–11.4) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Więsik-Szewczyk, E.; Rutkowska, E.; Kwiecień, I.; Korzeniowska, M.; Sołdacki, D.; Jahnz-Różyk, K. Patients with Common Variable Immunodeficiency Complicated by Autoimmune Phenomena Have Lymphopenia and Reduced Treg, Th17, and NK Cells. J. Clin. Med. 2021, 10, 3356. https://doi.org/10.3390/jcm10153356
Więsik-Szewczyk E, Rutkowska E, Kwiecień I, Korzeniowska M, Sołdacki D, Jahnz-Różyk K. Patients with Common Variable Immunodeficiency Complicated by Autoimmune Phenomena Have Lymphopenia and Reduced Treg, Th17, and NK Cells. Journal of Clinical Medicine. 2021; 10(15):3356. https://doi.org/10.3390/jcm10153356
Chicago/Turabian StyleWięsik-Szewczyk, Ewa, Elżbieta Rutkowska, Iwona Kwiecień, Marcelina Korzeniowska, Dariusz Sołdacki, and Karina Jahnz-Różyk. 2021. "Patients with Common Variable Immunodeficiency Complicated by Autoimmune Phenomena Have Lymphopenia and Reduced Treg, Th17, and NK Cells" Journal of Clinical Medicine 10, no. 15: 3356. https://doi.org/10.3390/jcm10153356
APA StyleWięsik-Szewczyk, E., Rutkowska, E., Kwiecień, I., Korzeniowska, M., Sołdacki, D., & Jahnz-Różyk, K. (2021). Patients with Common Variable Immunodeficiency Complicated by Autoimmune Phenomena Have Lymphopenia and Reduced Treg, Th17, and NK Cells. Journal of Clinical Medicine, 10(15), 3356. https://doi.org/10.3390/jcm10153356







