Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics
Abstract
:1. Introduction
2. Hemorrhages/Microaneurysms
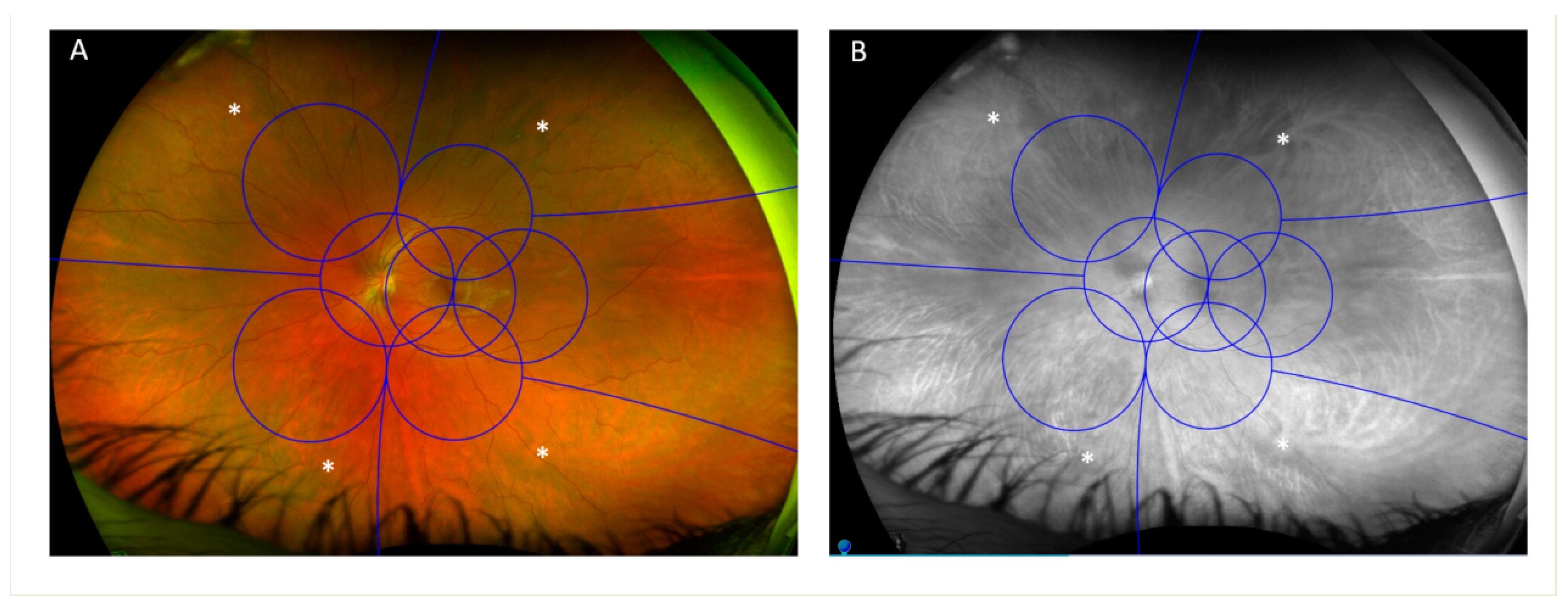
3. Visible Retinal Area
4. Arteriolar and Venular Diameters
5. Retinal Nonperfusion
6. Retinal Vascular Bed Area
7. Wide Field Optical Coherence Tomography Angiography (WF-OCTA) Quantitative Metrics
7.1. Macular OCTA (3 × 3 mm)
7.2. Widefield OCTA (WF-OCTA)
8. Artificial Intelligence
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choudhry, N.; Duker, J.S.; Freund, K.B.; Kiss, S.; Querques, G.; Rosen, R.; Sarraf, D.; Souied, E.H.; Stanga, P.E.; Staurenghi, G.; et al. Classification and Guidelines for Widefield Imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol. Retin. 2019, 3, 843–849. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Soliman, A.Z.; Aiello, L.M.; Aiello, L.P. Peripheral lesions identified by mydriatic ultrawide field imaging: Distribution and potential impact on diabetic retinopathy severity. Ophthalmology 2013, 120, 2587–2595. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98 (Suppl. 5), 786–806. [Google Scholar] [CrossRef]
- Kernt, M.; Hadi, I.; Pinter, F.; Seidensticker, F.; Hirneiss, C.; Haritoglou, C.; Kampik, A.; Ulbig, M.W.; Neubauer, A.S. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care 2012, 35, 2459–2463. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.K.; Aiello, L.P. The Future of Ultrawide Field Imaging for Diabetic Retinopathy: Pondering the Retinal Periphery. JAMA Ophthalmol. 2016, 134, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.; Odia, I.; Glassman, A.R.; Melia, M.; Jampol, L.M.; Bressler, N.M.; Kiss, S.; Silva, P.S.; Wykoff, C.C.; Sun, J.K.; et al. Comparison of early treatment diabetic retinopathy study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019, 137, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Alagorie, A.R.; Ramasamy, K.; van Hemert, J.; Yadav, N.; Pappuru, R.R.; Tufail, A.; Nittala, M.G.; Sadda, S.R.; Raman, R.; et al. Distribution of peripheral lesions identified by mydriatic ultra-wide field fundus imaging in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Cavallerano, J.D.; Haddad, N.M.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991, 98 (Suppl. 5), 823–833. [Google Scholar] [CrossRef]
- Wilkinson, C.; Ferris, F.L., III; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Sun, J.K.; Aiello, L.P.; Abràmoff, M.D.; Antonetti, T.A.; Dutta, S.; Pragnell, M.; Levine, S.R.; Gardner, T.W. Updating the Staging System for Diabetic Retinal Disease. Ophthalmology 2021, 128, 490–493. [Google Scholar] [CrossRef]
- Antheriou, M.C.; Jacquier, P.; Malclès, A. Fundus Abnormalities in Acute Leukemia. JAMA Ophthalmol. 2020, 138, e190934. [Google Scholar] [CrossRef]
- Kohner, E.M.; Dollery, C.T. The rate of formation and disappearance of microaneurysms in diabetic retinopathy. Trans. Ophthalmol. Soc. UK 1970, 90, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Meuer, S.M.; Moss, S.E.; Klein, B.E. The relationship of retinal microaneurysm counts to the 4-year progression of diabetic retinopathy. Arch. Ophthalmol. 1989, 107, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Meuer, S.M.; Moss, S.E.; Klein, B.E. Retinal microaneurysm counts and 10-year progression of diabetic retinopathy. Arch. Ophthalmol. 1995, 113, 1386–1391. [Google Scholar] [CrossRef]
- Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. The Diabetes Control and Complications. Diabetes 1997, 46, 1829–1839. [Google Scholar] [CrossRef]
- Sjolie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Aldington, S.; Chaturvedi, N. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet. Med. 2011, 28, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.L.; Broe, R.; Frydkjaer-Olsen, U.; Olsen, B.S.; Mortensen, H.B.; Peto, T.; Grauslund, J. Microaneurysm count as a predictor of long-term progression in diabetic retinopathy in young patients with type 1 diabetes: The Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 199–205. [Google Scholar] [CrossRef]
- Ashraf, M.; Sampani, K.; AbdelAl, O.; Fleming, A.; Cavallerano, J.; Souka, A.; Baha, S.M.E.; Silva, P.S.; Sun, J.; Aiello, L.P. Disparity of microaneurysm count between ultrawide field colour imaging and ultrawide field fluorescein angiography in eyes with diabetic retinopathy. Br. J. Ophthalmol. 2020, 104, 1762–1767. [Google Scholar] [CrossRef]
- Sears, C.M.; Nittala, M.G.; Jayadev, C.; Verhoek, M.; Fleming, A.; van Hemert, J.; Tsui, I.; Sadda, S.R. Comparison of Subjective Assessment and Precise Quantitative Assessment of Lesion Distribution in Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, 365–371. [Google Scholar] [CrossRef]
- Sadda, S.R.; Nittala Mphil, M.G.; Taweebanjongsin, W.; Verma, A.; Velaga, S.B.; Alagorie, A.R.; Sears, C.M.; Silva, P.S.; Aiello, L.P. Quantitative assessment of the severity of diabetic retinopathy. Am. J. Ophthalmol. 2020, 218, 342–352. [Google Scholar] [CrossRef]
- Silva, P.S.; El-Rami, H.; Barham, R.; Gupta, A.; Fleming, A.; van Hemert, G.; Cavallerano, G.D.; Sun, J.K.; Aiello, L.P. Hemorrhage and/or Microaneurysm Severity and Count in Ultrawide Field Images and Early Treatment Diabetic Retinopathy Study Photography. Ophthalmology 2017, 124, 970–976. [Google Scholar] [CrossRef]
- Silva, P.S.; Dela Cruz, A.J.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, G.D.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef]
- Son, G.; Kim, Y.J. Analysis of quantitative correlations between microaneurysm, ischaemic index and new vessels in ultrawide-field fluorescein angiography images using automated software. Br. J. Ophthalmol. 2019, 103, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Wang, K.; Vasanji, A.; Hu, M.; Srivastava, S.K. Automated quantitative characterisation of retinal vascular leakage and microaneurysms in ultra-widefield fluorescein angiography. Br. J. Ophthalmol. 2017, 101, 696–699. [Google Scholar] [CrossRef]
- Jiang, A.; Srivastava, S.; Figueiredo, N. Repeatability of automated leakage quantification and microaneurysm identification utilising an analysis platform for ultra-widefield fluorescein angiography. Br. J. Ophthalmol. 2019, 104, 500–503. [Google Scholar] [CrossRef]
- Jiang, A.C.; Srivastava, S.K.; Hu, M.; Figueiredo, N.; Babiuch, A.; Boss, J.D.; Reese, J.L.; Ehlers, J.P. Quantitative Ultra-Widefield Angiographic Features and Associations with Diabetic Macular Edema. Ophthalmol. Retin. 2020, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Jiang, A.C.; Boss, J.D.; Hu, M.; Figueiredo, N.; Babiuch, A.; Talcott, K.; Sharma, S.; Hach, J.; Le, T.; et al. Quantitative Ultra-Widefield Angiography and Diabetic Retinopathy Severity: An Assessment of Panretinal Leakage Index, Ischemic Index and Microaneurysm Count. Ophthalmology 2019, 126, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Babiuch, A.; Wykoff, C.C.; Hach, J.; Srivastava, S.; Talcott, K.E.; Yu, H.J.; Nittala, M.; Sadda, S.; Ip, M.S.; Le, T.; et al. Longitudinal panretinal microaneurysm dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Levenkova, A.; Sowmya, A.; Kalloniatis, M.; Ly, A.; Ho, A. Lesion detection in ultra-wide field retinal images for diabetic retinopathy diagnosis. Int. Soc. Opt. Photonics 2018, 10575, 1057531. [Google Scholar]
- Silva, P.S.; Elmasry, M.; Pisig, A.; Aldairy, Y.; van hemert, J.; Fleming, A.; Sun, J.K.; Aiello, L.P. Automated Hemorrhage and Microaneurysm Counts on Ultrawide Field Images Predict Increased Risk of Diabetic Retinopathy Progression Over 4 Years. Investig. Ophthalmol. Vis. Sci. 2018, 59, 737. [Google Scholar]
- Silva, P.S.; Stanton, R.C.; Elmasry, M.A.; Fleming, A.; Pellegrini, E.; van Hemert, J.; Tolls, D.; Tolson, A.M.; Lewis, D.; Stainback, J.; et al. Association of Systemic Comorbities with Predominantly Peripheral Diabetic Retinopathy Lesions (PPL) Identified on Ultrawide Field (UWF) Retinal Imaging. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4772. [Google Scholar]
- Ashraf, M.; Rageh, A.; Gilbert, M.; Tolls, D.; Fleming, A.; Souka, A.; El-Baha, S.; Cavallerano, J.D.; Sun, J.K.; Aiello, L.P.; et al. Factors Affecting Predominantly Peripheral Lesion Identification and Grading. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Cavallerano, J.; Elmasry, M.A.; Fleming, A.; van Hemert, J.; Lewis, D.; Bhaskar, V.; Tolson, A.; Tolls, D.; Sun, J.K.; et al. Impact of Visible Retinal Area on Diabetic Retinopathy Severity and Detection of Predominantly Peripheral Lesions when Using Ultrawide Field Imaging. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3201. [Google Scholar]
- Klein, R.; Klein, B.E.; Moss, S.E.; Wong, T.Y.; Hubbard, L.; Cruickshanks, K.J.; Palta, M. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch. Ophthalmol. 2004, 122, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Shokrollah, S.; Pisig, A.U.; Sampani, K.; Abdelal, O.; Cavallerano, J.D.; Robertson, G.; Fleming, A.; van Hemert, J.; Pitoc, V.M.; et al. Retinal vascular caliber association with nonperfusion and diabetic retinopathy severity depends on vascular caliber measurement location. Ophthalmol. Retin. 2020, 5, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, K.; Ghasemi Falavarjani, K.; Sagong, M.; Uji, A.; Ip, M.; Wykoff, C.C.; Brown, D.M.; van Hemert, J.; Sadda, S.R. Distribution of Nonperfusion Area on Ultra-widefield Fluorescein Angiography in Eyes with Diabetic Macular Edema: DAVE Study. Am. J. Ophthalmol. 2017, 180, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Uji, A.; Wang, K.; Falavarjani, K.G.; Khalil, J.; Wykoff, C.C.; Brown, D.M.; Van Hemert, J.; Sagong, M.; Sadda, S.R.; et al. Severity of diabetic macular edema correlates with retinal vascular bed area on ultra-wide field fluorescein angiography: Dave study. Retina 2020, 40, 1029–1037. [Google Scholar] [CrossRef]
- Talks, S.J.; Bhatia, D.; Menon, G.; Cole, A.; Eleftheriadis, H.; Downey, L.; Chong, N.V.; Sivaprasad, S. Randomised trial of wide-field guided PRP for diabetic macular oedema treated with ranibizumab. Eye 2019, 33, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.M.; Ou, W.C.; Wong, T.P.; Kim, R.Y.; Croft, D.E.; Wykoff, C.C. Targeted Retinal Photocoagulation for Diabetic Macular Edema with Peripheral Retinal Nonperfusion: Three-Year Randomized DAVE Trial. Ophthalmology 2018, 125, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology 1991, 98 (Suppl. 5), 807–822. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein Angiographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 13. Ophthalmology 1991, 98 (Suppl. 5), 834–840. [Google Scholar] [CrossRef]
- Yu, G.; Aaberg, M.T.; Patel, T.P.; Iyengar, R.S.; Powell, C.; Tran, A.; Miranda, C.; Young, E.; Demetriou, K.; Devisetty, L.; et al. Quantification of Retinal Nonperfusion and Neovascularization with Ultrawidefield Fluorescein Angiography in Patients with Diabetes and Associated Characteristics of Advanced Disease. JAMA Ophthalmol. 2020, 138, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; Ramu, J.; Chan, E.W.; Bainbridge, J.W.; Hykin, P.G.; Talks, S.J.; Sivaprasad, S. Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes with Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.R.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Non-perfusion and Neovascularization on Ultra-Wide Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am. J. Ophthalmol. 2019, 206, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Maram, J.; Alagorie, A.R.; Nittala, M.G.; van Hemert, J.; Keane, D.; Carnevale, J.; Bell, D.; Singer, M.; Sadda, S.R. Distribution and Location of Vortex Vein Ampullae in Healthy Human Eyes as Assessed by Ultra-Widefield Indocyanine Green Angiography. Ophthalmol. Retin. 2020, 4, 530–534. [Google Scholar] [CrossRef]
- Rageh, A.; Elmasry, M.A.; Gilbert, M.; Silva, P.S.; Sun, J.K.; Aiello., L.P. Vortex vein location as an individualized marker of anatomic retinal far periphery using non-steered ultrawide field fluorescein angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 478. [Google Scholar]
- Fan, W.; Uji, A.; Borrelli, E.; Singer, M.; Sagong, M.; Van Hemert, J.; Sadda, S.R. Precise Measurement of Retinal Vascular Bed Area and Density on Ultra-wide Fluorescein Angiography in Normal Subjects. Am. J. Ophthalmol. 2018, 188, 155–163. [Google Scholar] [CrossRef]
- Fan, W.; Uji, A. Retinal vascular bed area on ultra-wide field fluorescein angiography indicates the severity of diabetic retinopathy. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Jia, Y.; Zhang, M.; Su, J.P.; Liu, G.; Hwang, T.S.; Bailey, S.T.; Huang, D. Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT27–OCT36. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Ashraf, M.; Sampani, K.; Clermont, A.; Abu-Qamar, O.; Rhee, J.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Vascular Density of Deep, Intermediate and Superficial Vascular Plexuses Are Differentially Affected by Diabetic Retinopathy Severity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef]
- Ashraf, M.; Sampani, K.; Rageh, A.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Interaction between the Distribution of Diabetic Retinopathy Lesions and the Association of Optical Coherence Tomography Angiography Scans With Diabetic Retinopathy Severity. JAMA Ophthalmol. 2020, 138, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, M.A.; Sampani, K.; Pitoc, C.M.; Fleming, A.; Robertson, G.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Differential Association of Macular Superficial versus Deep Vascular Density with Microaneurysms and Nonperfusion in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5344. [Google Scholar]
- Zhu, Y.; Cui, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Wu, D.M.; Eliott, D.; Vavvas, D.G.; Husain, D.; et al. Different Scan Protocols Affect the Detection Rates of Diabetic Retinopathy Lesions by Wide-Field Swept-Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 215, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.F.; Flynn, H.W.; Sridhar, J.; Townsend, J.H.; Shi, Y.; Fan, K.C.; Scott, N.L.; Hinkle, J.W.; Lyu, C.; Gregori, G.; et al. Distribution of Diabetic Neovascularization on Ultra-Widefield Fluorescein Angiography and on Simulated Widefield OCT Angiography. Am. J. Ophthalmol. 2019, 207, 110–120. [Google Scholar] [CrossRef]
- Sawada, O.; Ichiyama, Y.; Obata, S.; Ito, Y.; Kakinoki, M.; Sawada, T.; Saishin, Y.; Ohji, M. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1275–1280. [Google Scholar] [CrossRef]
- Pichi, F.; Smith, S.D.; Abboud, E.B.; Neri, P.; Woodstock, E.; Hay, S.; Levine, E.; Baumal, C.R. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1901–1909. [Google Scholar] [CrossRef]
- Khalid, H.; Schwartz, R. Widefield optical coherence tomography angiography for early detection and objective evaluation of proliferative diabetic retinopathy. J. Ophthalmol. 2021, 105, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Laíns, I.; Wu, D.M.; Eliott, D.; Vavvas, D.G.; et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef]
- Lu, E.S.; Cui, Y.; Le, R.; Zhu, Y.; Wang, J.C.; Laíns, I.; Katz, R.; Lu, Y.; Zeng, R.; Garg, I.; et al. Detection of neovascularisation in the vitreoretinal interface slab using widefield swept-source optical coherence tomography angiography in diabetic retinopathy. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Al-Khersan, H.; Russell, J.F.; Lazzarini, T.A.; Scott, N.L.; Hinkle, G.W.; Patel, N.A.; Yannuzzi, N.A.; Fowler, B.J.; Hussain, R.M.; Barikian, A.; et al. Comparison Between Graders in Detection of Diabetic Neovascularization with Swept Source OCT Angiography and Fluorescein Angiography. Am. J. Ophthalmol. 2020, 224, 292–300. [Google Scholar] [CrossRef]
- Couturier, A.; Rey, P.A.; Erginay, A.; Lavia, C.; Bonnin, S.; Dupas, B.; Gaudric, A.; Tadayoni, R. Widefield OCT-Angiography and Fluorescein Angiography Assessments of Nonperfusion in Diabetic Retinopathy and Edema Treated with Anti-Vascular Endothelial Growth Factor. Ophthalmology 2019, 126, 1685–1694. [Google Scholar] [CrossRef]
- Russell, J.F.; Al-Khersan, H.; Shi, Y.; Scott, N.L.; Hinkle, J.W.; Fan, K.C.; Lyu, C.; Feuer, W.J.; Gregori, G.; Rosenfeld, P.J. Retinal Nonperfusion in Proliferative Diabetic Retinopathy Before and After Panretinal Photocoagulation Assessed by Widefield OCT Angiography. Am. J. Ophthalmol. 2020, 213, 177–185. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Lu, E.S.; Le, R.; Laíns, I.; Katz, R.; Wang, J.C.; Garg, I.; Lu, Y.; Zeng, R.; et al. Widefield Swept-Source OCT Angiography Metrics Associated with the Development of Diabetic Vitreous Hemorrhage: A Prospective Study. Ophthalmology 2021. [Google Scholar] [CrossRef]
- Krause, J.; Gulshan, V.; Rahimy, E.; Karth, P.; Widner, K.; Corrado, G.S.; Peng, L.; Webster, D.R. Grader Variability and the Importance of Reference Standards for Evaluating Machine Learning Models for Diabetic Retinopathy. Ophthalmology 2018, 125, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Tabuchi, H.; Masumoto, H.; Enno, H.; Niki, M.; Ohara, Z.; Yoshizumi, Y.; Ohsugi, H.; Mitamura, Y. Accuracy of ultrawide-field fundus ophthalmoscopy-assisted deep learning for detecting treatment-naïve proliferative diabetic retinopathy. Int. Ophthalmol. 2019, 39, 2153–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Luenam, P.; Ran, A.R.; Quadeer, A.A.; Raman, R.; Sen, P.; Khan, R.; Giridhar, A.; Haridas, S.; Iglicki, M.; et al. Detection of Diabetic Retinopathy from Ultra-Widefield Scanning Laser Ophthalmoscope Images: A Multicenter Deep Learning Analysis. Ophthalmol. Retin. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.; Cavallerano, J.D.; Sun, J.K.; Silva, P.S.; Aiello, L.P. Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. J. Clin. Med. 2021, 10, 3300. https://doi.org/10.3390/jcm10153300
Ashraf M, Cavallerano JD, Sun JK, Silva PS, Aiello LP. Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. Journal of Clinical Medicine. 2021; 10(15):3300. https://doi.org/10.3390/jcm10153300
Chicago/Turabian StyleAshraf, Mohamed, Jerry D. Cavallerano, Jennifer K. Sun, Paolo S. Silva, and Lloyd Paul Aiello. 2021. "Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics" Journal of Clinical Medicine 10, no. 15: 3300. https://doi.org/10.3390/jcm10153300
APA StyleAshraf, M., Cavallerano, J. D., Sun, J. K., Silva, P. S., & Aiello, L. P. (2021). Ultrawide Field Imaging in Diabetic Retinopathy: Exploring the Role of Quantitative Metrics. Journal of Clinical Medicine, 10(15), 3300. https://doi.org/10.3390/jcm10153300





